Dispositivos de drenaje subconjuntival mínimamente invasivos para el glaucoma sin control clínico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012742.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 16 diciembre 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Protocol
AK and EN wrote the protocol.
All authors reviewed and approved the protocol.
Review
AS, EN, and RS screened the search results.
RS extracted the data for the ongoing study and AS checked data.
AS and AK wrote the review.
AAZ, CAM, EN, KH and RS commented on the draft.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
-
Declarations of interest
Anthony J King undertook an invited lecture for Allergan on trabeculectomy surgery and role of phasing in glaucoma.
Anupa Shah: none known.
Eleni Nikita: none known.
Kuang Hu performs this and other forms of minimally‐invasive glaucoma surgery. He lectured on "Constructing clinical trials for MIGS ‐ the lack of evidence and what to do about it" at the Moorfields International Glaucoma Symposium 2016, sponsored by Laboratoires Thea, which is contributing an educational grant to Moorfields Eye Hospital.
Caroline A Mulvaney: none known.
Richard Stead: none known.
Augusto Azuara‐Blanco has done unpaid consultancy work for Bayer (2015 and 2016) as a member of an independent panel judging the quality of care of NHS Departments of Ophthalmology. Bayer provided funds to Queen's University Belfast.
Acknowledgements
Cochrane Eyes and Vision (CEV) will created and executed the electronic search strategies. We thank Nitin Anand and Jennifer Evans for their comments on the published protocol that forms the template for this one (Hu 2016) and on the review itself.
We thank the members of the MIGS Consortium for their input in this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Dec 16 | Subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma | Review | Anthony J King, Anupa Shah, Eleni Nikita, Kuang Hu, Caroline A Mulvaney, Richard Stead, Augusto Azuara‐Blanco | |
| 2017 Aug 03 | Subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma | Protocol | Anthony J King, Kuang Hu, Eleni Nikita, Caroline A Mulvaney, Augusto Azuara‐Blanco, Richard Stead | |
Differences between protocol and review
-
The follow‐up times for the outcomes were decided after the protocol was published.
-
An additional co‐author, A Shah joined the review team.
-
The protocol included combination therapy with phacoemulsification as a separate comparison and also for subgroup analysis. After discussion within the review team and MIGS Consortium, we opted to include it as a separate comparison as this is likely to be a different indication.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
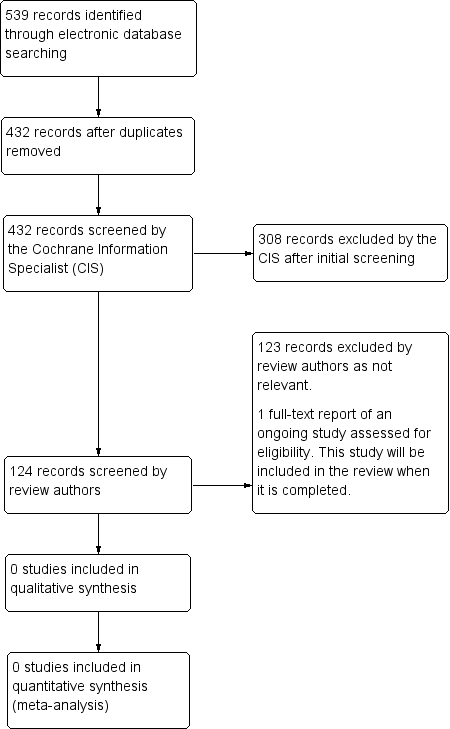
Study flow diagram.

