Dispositivos de drenaje subconjuntival mínimamente invasivos para el glaucoma sin control clínico
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Glaucoma, Open‐Angle] explode all trees
#2 MeSH descriptor: [Intraocular Pressure] explode all trees
#3 MeSH descriptor: [Ocular Hypertension] explode all trees
#4 OAG or POAG or IOP or OHT
#5 simple near/3 glaucoma*
#6 open near/2 angle near/2 glaucoma*
#7 chronic near/2 glaucoma*
#8 secondary near/2 glaucoma*
#9 low near/2 tension near/2 glaucoma*
#10 low near/2 pressure near/2 glaucoma*
#11 normal near/2 tension near/2 glaucoma*
#12 normal near/2 pressure near/2 glaucoma*
#13 pigment near/2 glaucoma*
#14 MeSH descriptor: [Exfoliation Syndrome] this term only
#15 exfoliat* near/2 syndrome*
#16 exfoliat* near/2 glaucoma*
#17 pseudoexfoliat* near/2 syndrome*
#18 pseudoexfoliat* near/2 glaucoma*
#19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 Xen*
#21 gel* near/3 (stent* or implant*)
#22 AqueSys
#23 InnFocus or MicroShunt*
#24 poly styrene‐block‐isobutylene‐block‐styrene
#25 #20 or #21 or #22 or #23 or #24
#26 #19 and #25
Appendix 2. MEDLINE Ovid search strategy
1. randomised controlled trial.pt.
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. exp animals/
10. exp humans/
11. 9 not (9 and 10)
12. 8 not 11
13. exp glaucoma open angle/
14. exp intraocular pressure/
15. ocular hypertension/
16. (OAG or POAG or IOP or OHT).tw.
17. (simple$ adj3 glaucoma$).tw.
18. (open adj2 angle adj2 glaucoma$).tw.
19. (primary adj2 glaucoma$).tw.
20. (chronic adj2 glaucoma$).tw.
21. (secondary adj2 glaucoma$).tw.
22. (low adj2 tension adj2 glaucoma$).tw.
23. (low adj2 pressure adj2 glaucoma$).tw.
24. (normal adj2 tension adj2 glaucoma$).tw.
25. (normal adj2 pressure adj2 glaucoma$).tw.
26. (pigment$ adj2 glaucoma$).tw.
27. exfoliation syndrome/
28. (exfoliat$ adj2 syndrome$).tw.
29. (exfoliat$ adj2 glaucoma$).tw.
30. (pseudoexfoliat$ adj2 syndrome$).tw.
31. (pseudoexfoliat$ adj2 glaucoma$).tw.
32. or/13‐31
33. Xen$.tw.
34. (gel$ adj3 (stent$ or implant$)).tw.
35. AqueSys.tw.
36. (InnFocus or MicroShunt$).tw.
37. poly styrene‐block‐isobutylene‐block‐styrene.tw.
38. or/33‐37
39. 32 and 38
40. 12 and 39
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomised controlled trial/
2. exp randomization/
3. exp double blind procedure/
4. exp single blind procedure/
5. random$.tw.
6. or/1‐5
7. (animal or animal experiment).sh.
8. human.sh.
9. 7 and 8
10. 7 not 9
11. 6 not 10
12. exp clinical trial/
13. (clin$ adj3 trial$).tw.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
15. exp placebo/
16. placebo$.tw.
17. random$.tw.
18. exp experimental design/
19. exp crossover procedure/
20. exp control group/
21. exp latin square design/
22. or/12‐21
23. 22 not 10
24. 23 not 11
25. exp comparative study/
26. exp evaluation/
27. exp prospective study/
28. (control$ or prospectiv$ or volunteer$).tw.
29. or/25‐28
30. 29 not 10
31. 30 not (11 or 23)
32. 11 or 24 or 31
33. open angle glaucoma/
34. intraocular pressure/
35. intraocular hypertension/
36. (OAG or POAG or IOP or OHT).tw.
37. (open adj2 angle adj2 glaucoma$).tw.
38. (primary adj2 glaucoma$).tw.
39. (chronic adj2 glaucoma$).tw.
40. (secondary adj2 glaucoma$).tw.
41. (low adj2 tension adj2 glaucoma$).tw.
42. (low adj2 pressure adj2 glaucoma$).tw.
43. (normal adj2 tension adj2 glaucoma$).tw.
44. (normal adj2 pressure adj2 glaucoma$).tw.
45. (pigment$ adj2 glaucoma$).tw.
46. exfoliation syndrome/
47. (exfoliat$ adj2 syndrome$).tw.
48. (exfoliat$ adj2 glaucoma$).tw.
49. (pseudoexfoliat$ adj2 syndrome$).tw.
50. (pseudoexfoliat$ adj2 glaucoma$).tw.
51. or/33‐50
52. Xen$.tw.
53. (gel$ adj3 (stent$ or implant$)).tw.
54. AqueSys.tw.
55. (InnFocus or MicroShunt$).tw.
56. poly styrene‐block‐isobutylene‐block‐styrene.tw.
57. or/52‐56
58. 51 and 57
59. 32 and 58
Appendix 4. ISRCTN search strategy
" Xen OR gelatin implant OR gelatin implant OR AqueSys OR InnFocus OR MicroShunt "
Appendix 5. ClinicalTrials.gov search strategy
Xen OR gelatin implant OR gelatin implant OR AqueSys OR InnFocus OR MicroShunt
Appendix 6. WHO ICTRP search strategy
Xen OR gelatin implant OR gelatin implant OR AqueSys OR InnFocus OR MicroShunt
Appendix 7. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design | Parallel group RCTi.e. people randomised to treatment Within‐person RCTi.e. eyes randomised to treatment Cluster RCTi.e. communities randomised to treatment Cross‐over RCT Other, specify | Number of study arms Method of randomisation Exclusions after randomisation Losses to follow‐up Number randomised/analysed Method of masking How were missing data handled? e.g. available‐case analysis, imputation methods Reported power calculation (Y/N),if yes, sample size and power Unusual study design/issues |
| Eyes Unit of randomisation/ unit of analysis | One eye included in study, specify how eye selected Two eyes included in study, both eyes received same treatment, briefly specify how analysed (best/worst/average/both and adjusted for within person correlation/both and not adjusted for within person correlation) and specify if mixture of one eye and two eyes Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done | |
| Participants | ||
| Country | — | Setting Ethnic group Method of recruitment Participation rate Equivalence of baseline characteristics (Y/N) Diagnostic criteria |
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Mean age and age range | ||
| Inclusion criteria | — | |
| Exclusion criteria | — | |
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 | Number of people randomised to this group Intervention name Comparator name Specify whether phacoemulsification, or other intervention, performed at same time as intervention | Xen/InnFocus Implant surgical parameters, e.g. location of implant under the conjunctive or in the anterior chamber, dose of mitomycin‐C used Comparator parameters, e.g. dosage of drugs |
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 | IOP at baseline IOP at follow‐up Number of glaucoma medications at baseline Number of glaucoma medications at follow‐up Intraoperative complications Postoperative complications Duration of follow‐up Loss to follow‐up Intervals at which outcomes assessed Adverse events reported (Y/N) | Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name: (if applicable) Date of publication Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | — | |
| Declaration of interest See MECIR 69 | — | |
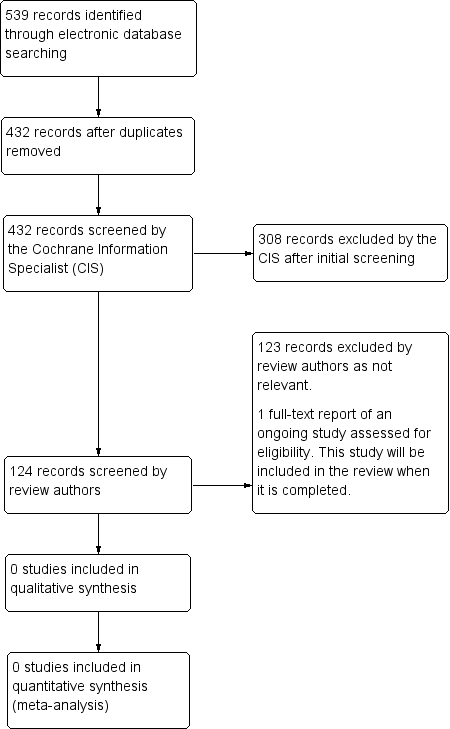
Study flow diagram.

