Dispositivos de drenaje subconjuntival mínimamente invasivos para el glaucoma sin control clínico
Resumen
Antecedentes
El glaucoma es la causa principal de ceguera irreversible. Los dispositivos de drenaje subconjuntival mínimamente invasivos, como el implante de gelatina Xen y el microtubo InnFocus, se han introducido como tratamientos para prevenir la progresión del glaucoma.
Estos implantes generan un canal para permitir que el humor acuoso de la cámara anterior drene en el espacio subconjuntival en la superficie del ojo, y así se reduce la presión intraocular (PIO). Se trata del mismo mecanismo que en la cirugía para el glaucoma realizada habitualmente, es decir, la trabeculectomía.
Objetivos
Evaluar la eficacia y la seguridad de los dispositivos de drenaje subconjuntival mínimamente invasivos para el tratamiento de los pacientes con glaucoma de ángulo abierto e hipertensión ocular, cuya afección no se controla de modo adecuado con gotas.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; que contiene el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión (Cochrane Eyes and Vision Trials Register); 2018, número 6); Ovid MEDLINE; Ovid Embase; el ISRCTN registry; ClinicalTrials.gov y en el WHO ICTRP. La fecha de la búsqueda fue el 10 de julio de 2018.
Criterios de selección
Se buscaron ensayos controlados aleatorios (ECA) del implante de gelatina Xen o el InnFocus MicroShunt comparados con otros tratamientos quirúrgicos (otras técnicas con dispositivos mínimamente invasivos, trabeculectomía), tratamiento con láser o tratamiento médico. También se buscó incluir ensayos cuando estos dispositivos se combinaron con la facoemulsificación en comparación con la facoemulsificación sola.
Obtención y análisis de los datos
Se planeó que dos autores de la revisión extrajeran de forma independiente los datos de los informes de los estudios incluidos mediante un formulario de recopilación de datos y analizaran los datos según los métodos previstos por Cochrane. El resultado primario fue la media del cambio de la PIO. Los resultados secundarios fueron: proporción de participantes que dejaron de utilizar gotas; proporción de participantes que lograron una PIO de 21 mmHg o menos, 17 mmHg o menos o 14 mmHg o menos; y proporción de participantes que presentaron complicaciones intra y posoperatorias. Se programó medir todos los resultados a corto (seis a 18 meses), medio (18 a 36 meses) y largo plazo (a partir de los 36 meses).
Resultados principales
No se encontraron ECA completados que cumplieran los criterios de inclusión. Se encontró un estudio en curso (NCT01881425). El estudio compara el InnFocus MicroShunt con la trabeculectomía en pacientes con glaucoma primario de ángulo abierto. El resultado primario es más de un 20% de reducción de la PIO desde el inicio hasta el seguimiento a los 12 meses. Se ha incluido un total de 889 pacientes de entre 40 y 85 años. La fecha calculada de finalización del estudio es noviembre 2019.
Conclusiones de los autores
No hay evidencia de alta calidad en la actualidad para los efectos de los dispositivos de drenaje subconjuntival mínimamente invasivos para el glaucoma de ángulo abierto sin control médico. Se necesitan ECA de diseño adecuado para evaluar la eficacia y la seguridad a medio y largo plazo de esta técnica.
PICO
Resumen en términos sencillos
Dispositivos de drenaje sobre la superficie del ojo por debajo de la capa superficial para reducir la presión ocular en pacientes con o en riesgo de glaucoma
¿Cuál es el objetivo de la revisión?
El objetivo de esta revisión Cochrane era determinar si los dispositivos de drenaje del compartimiento anterior del ojo (cámara anterior) sobre la superficie del ojo debajo de la capa superficial (espacio subconjuntival), conocidos como dispositivos para el glaucoma mínimamente invasivos, son efectivos en la reducción de la presión ocular de pacientes con glaucoma que se controla de modo adecuado con gotas. Los autores de la revisión Cochrane recopilaron y analizaron todos los estudios relevantes para responder esta pregunta y no encontraron ningún estudio finalizado y sólo hallaron un estudio en curso.
Mensajes clave
No hay ningún estudio publicado relevante que compare los dispositivos de drenaje subconjuntival mínimamente invasivos con otros tratamientos.
¿Qué se estudió en la revisión?
El glaucoma es la principal causa de ceguera irreversible. En el glaucoma, existe daño del nervio óptico en la parte posterior del ojo, en muchos casos porque la presión interna es demasiado alta. Los médicos pueden bajar la presión del ojo mediante una intervención quirúrgica. Los dispositivos de drenaje subconjuntival mínimamente invasivos podrían ayudar a que esta cirugía sea menos traumática, con más seguridad que la cirugía estándar y mayor comodidad para los pacientes con un período más rápido de recuperación visual.
¿Cuáles son los principales resultados de la revisión?
Los autores de la revisión Cochrane no encontraron ningún estudio completo que pudiera incluirse en esta revisión.
¿Cuál es el grado de actualización de la revisión?
Los autores de la revisión Cochrane buscaron estudios publicados hasta el 10 de julio 2018.
Authors' conclusions
Background
The protocol for this review (King 2017) was based on the protocol from the published review on ab interno trabecular bypass surgery with Trabectome for open angle glaucoma (OAG) (Hu 2016).
Description of the condition
Glaucoma is a chronic, progressive optic neuropathy, affecting up to 4% of people by the age of 80 years (Burr 2007). It is the leading cause of irreversible blindness, affecting 60 million people globally (Quigley 2006). This figure is expected to increase to 80 million people by 2020. OAG is the most common type, accounting for three‐quarters of cases (Quigley 2006). In one large population cohort, one in six people with OAG became bilaterally blind (Peters 2013). The only proven way to prevent vision loss is to reduce the pressure inside the eye (intraocular pressure; IOP) over the long term (AGIS 2000; CNTG Study Group 1998; Heijl 2002; Kass 2002). Approaches to reducing IOP include medical therapy, laser treatments, and surgery. Because commercially available eye‐drop preparations have a short‐lasting effect, medical therapy requires eye‐drops to be instilled one or more times daily for life. Adherence is very poor, even if use is monitored (Friedman 2009; Okeke 2009). Conventional surgical techniques such as trabeculectomy are associated with significant risks, with more than 40% of people developing perioperative complications (Kirwan 2013; Lichter 2001), and reoperation being needed in 7% to 18% of people (Gedde 2012; Kirwan 2013). Therefore, they are often reserved for disease that is progressing despite other treatments (King 2013).
Description of the intervention
A number of minimally‐invasive glaucoma surgeries (MIGS) have been developed with the aim of achieving long‐term reduction of IOP with a better safety profile than conventional surgery (Francis 2011). These include Xen gelatin ab interno implant (AqueSys Inc., Aliso Viejo, CA, USA), and InnFocus MicroShunt, ab externo implant (InnFocus Inc., Miami, FL, USA). The Xen has been approved in Europe for the treatment of glaucoma and is a CE marked treatment, but at the time of publishing this review does not have US Food and Drug Administration (FDA) approval. The latter is currently undergoing a phase 3 clinical trial to acquire FDA approval.
How the intervention might work
In people with OAG, there is increased resistance to aqueous humour outflow through the trabecular meshwork. Both the Xen and InnFocus implants bypass this resistance by creating a channel between the anterior chamber of the eye and the subconjunctival space, thus allowing aqueous to bypass the trabecular meshwork into the subconjunctival space and, thereby, reducing IOP. Both devices are routinely augmented with mitomycin‐C, an antimetabolite which is injected subconjunctivally at the time of surgery to reduce postoperative scaring and reduce the risk of surgical failure.
Why it is important to do this review
The increased burden of glaucoma worldwide has generated significant interest in the development of novel surgical treatments for glaucoma. In addition, consultation with patients and healthcare professionals has identified a need for better treatments for glaucoma (James Lind Alliance 2013). These techniques and devices embrace the common theme of being effective in reducing IOP and reducing medication burden, whilst causing minimal tissue trauma, having a very good safety profile, and reduced visual recovery time. Additionally, they have a shorter surgical time, an easily reproducible technique, and a short learning curve, which makes them accessible to all ophthalmologists who manage people with glaucoma, rather than being the territory of glaucoma specialists alone (Batlle 2016; Richter 2016). The literature suggests there is already widespread use of Xen and InnFocus implants in both Europe and the USA (Batlle 2016; Rodriguez‐Una 2016; Sheybani 2015a).
Both devices may be used alone or combined with phacoemulsification (cataract surgery), a sight‐restoring operation to remove the natural lens of the eye when it has lost clarity.
In view of the potential benefits for patients and the widespread uptake of the techniques, it is important to critically evaluate the evidence for the efficacy and safety of the subconjunctival draining minimally‐invasive glaucoma devices when used alone and in combination with phacoemulsification cataract surgery.
As phacoemulsification itself alone is proven to reduce IOP (Mansberger 2012), it is important to establish whether undertaking phacoemulsification in combination with these microshunts is responsible for additional IOP reduction.
This Cochrane Review was conducted in parallel with other reviews currently undertaken by the Cochrane Eyes and Vision MIGS Consortium, which includes MIGS techniques and devices such as the Trabectome (NeoMedix, Tustin, CA, USA) (Hu 2016), Hydrus Schlemm's canal Microstent (Ivantis Inc., Irvine, CA, USA) (Otarola 2017), endoscopic cytophotocoagulation (Endo Optiks, Waltham, MA, USA) (Tóth 2017), and iStent and iStent inject (Glaukos Corporation, Laguna Hills, CA, USA) (Le 2017) and supraciliary microstent surgery (Sandhu 2017).
Objectives
To evaluate the efficacy and safety of subconjunctival draining minimally‐invasive glaucoma devices in treating people with open angle glaucoma and ocular hypertension whose condition is inadequately controlled with drops.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) prepared in any language, irrespective of their publication status.
Types of participants
Participants had OAG of any type, including primary and secondary OAG. We excluded closed angle glaucoma. As there are no universally accepted criteria by which glaucoma may be defined, we permitted studies to use their own definitions of glaucoma. In addition, we included participants with ocular hypertension, normal tension glaucoma, or possible glaucoma (suspects for glaucoma). We did not apply any restrictions regarding location, setting, or demographic factors.
Types of interventions
The intervention was the Xen gelatin ab interno implant (AqueSys Inc., Aliso Viejo, CA, USA), or the InnFocus MicroShunt ab externo implant (InnFocus Inc., Miami, FL, USA).
The Xen Gel Implant is a 6 mm cylinder of collagen‐derived gelatin, cross‐linked with glutaraldehyde. It comes preloaded in an injector and is implanted ab interno, creating a drainage pathway between the anterior chamber and subconjunctival space, creating a bleb (Lewis 2014; Sheybani 2015a; Sheybani 2015b; Sheybani 2016). The procedure is routinely augmented with subconjunctival injection of mitomycin‐C. The InnFocus MicroShunt Device is approximately 70 microns in diameter, with an outer diameter of 350 microns and a length of approximately 8.5 mm (Batlle 2016; Pinchuk 2015; Riss 2015). The surgical procedure involves creating a conjunctival pouch and a small scleral tunnel, through which the shunt enters the anterior chamber. The conjunctiva is sutured at the end of surgery and the aqueous humour flows through the tube in the subconjunctival area and creates a bleb. The procedure is routinely augmented with subconjunctival injection of mitomycin‐C (Batlle 2016: Pinchuk 2008).
We planned to compare subconjunctival draining minimally‐invasive glaucoma devices:
-
in combination with phacoemulsification compared with phacoemulsification alone;
-
to laser treatment (selective laser trabeculoplasty or argon laser trabeculoplasty);
-
to other MIGS techniques;
-
to conventional glaucoma surgery (trabeculectomy);
-
to medical therapy.
Types of outcome measures
We did not use the reporting of particular outcomes as a criterion for eligibility for the review. We did not exclude studies from the review solely on the grounds of an outcome of interest not being reported.
We planned to report outcomes in the short‐term (six to 18 months), medium‐term (18 to 36 months), and long‐term (36 months onwards).
Primary outcomes
-
Mean change in IOP measured with Goldman applanation tonometry.
Several different glaucoma outcome measures have been specified as primary outcomes in other Cochrane Reviews and protocols (Ismail 2015). One study classified IOP, visual field, safety, and anatomic outcomes as being highly important to glaucoma experts (Ismail 2016). A panel of patients from the Patient and Public Involvement Group of the National Institute for Health Research (NIHR) Biomedical Research Centre for Ophthalmology identified drop‐free disease control as a highly valued outcome (unpublished).
Secondary outcomes
-
Proportion of participants who were drop‐free (not using eye drops).
-
Mean change in number of IOP‐lowering drops taken per day.
-
Proportion of participants who achieved an IOP of 21 mmHg or less.
-
Proportion of participants who achieve an IOP of 17 mmHg or less.
-
Proportion of participants who achieve an IOP of 14 mmHg or less.
-
Proportion of participants who required further glaucoma surgery, including laser, as recorded by the investigators of the included trials.
-
Mean change in health‐related quality of life (HRQoL).
Adverse effects
-
Proportion of participants experiencing intra‐ and postoperative complications including, but not restricted to, the following:
-
loss of visual acuity (more than 2 Snellen lines or more than 0.3 logMAR, according to the method of recording visual acuity; or loss of light perception);
-
bleeding, as recorded by the investigators;
-
endophthalmitis, as recorded by the investigators;
-
IOP spikes (postoperative rise in IOP, measured using Goldmann applanation tonometry, of more than 10 mmHg compared to the previous assessment, including during the first postoperative month).
-
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 10 July 2018.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 10 July 2018) (Appendix 1).
-
MEDLINE Ovid (1946 to 10 July 2018) (Appendix 2).
-
Embase Ovid (1980 to 10 July 2018) (Appendix 3).
-
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 10 July 2018) (Appendix 4).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 10 July 2018) (Appendix 5).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 10 July 2018) (Appendix 6).
Searching other resources
We searched the reference lists of included studies for other possible studies. We checked manufacturer's websites (AqueSys Inc., Aliso Viejo, CA, USA; InnFocus Inc, Miami, FL, USA) to ascertain if any new trials are being undertaken but there were no details of any new studies currently being planned or conducted. As we were not able to identify any forthcoming trials from the relevant manufacturers websites we did not contact individuals or organisations for any further follow‐ups.
Data collection and analysis
Selection of studies
Three review authors (AS, EN, RS) independently screened titles and abstracts of all articles identified by the search using web‐based online review management software (Covidence). If abstracts were not available, we planned to screen full‐text articles. We planned to obtain full‐text copies of all reports retained after this initial screening, and two review authors would have assessed them independently for inclusion in the review. If there was disagreement regarding eligibility, a third review author would have arbitrated. If any full‐text reports were rejected, we planned to record the reasons for this in the 'Characteristics of excluded studies' table.
As we only found one ongoing trial and no completed RCTs for inclusion in our review, we were unable to complete the steps for data extraction or analysis. In future updates, if we find any trials that meet our inclusion criteria or if the ongoing trial is completed and results published, we will follow the process outlined below.
Data extraction and management
We will extract data from reports of included studies using a data collection form, which will be developed and piloted on the first five included studies. Two review authors will independently extract study characteristics from reports of each study and enter the data into Review Manager 5 (RevMan 5) (Review Manager 2014). If there is disagreement, a third review author will arbitrate.
Data collected in Appendix 7 will be presented in the 'Characteristics of included studies' table. Where data on included studies are missing or unclear, we will contact the individuals or organisations involved to obtain clarification. We will collect and use the most detailed numerical data available to facilitate analyses of included studies. We intend to obtain these data from individuals or organisations in preference to less precise methods such as extracting numeric data from graphs. If this is necessary, two review authors will independently extract the data and a third review author will arbitrate, in case of disagreement.
Assessment of risk of bias in included studies
We will use the latest version of the Cochrane 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias and assign judgements of this for included studies (Higgins 2017).
Measures of treatment effect
We will report dichotomous data as risk ratios with 95% confidence intervals (CI) and continuous data as mean differences (where studies used the same scale) or standardised mean differences (where studies used different scales) with 95% CI.
We will report HRQoL outcomes as mean differences for continuous data or risk ratios for dichotomous data, depending on how they are reported.
Unit of analysis issues
We will assess whether studies included one or two eyes from each participant and whether or not randomisation was conducted at the level of the participant or the eye.
Dealing with missing data
We will minimise missing outcome data by contacting individuals and organisations to obtain them. If the data are unavailable, but the level of missing data in each group and reasons for missing data in each group are similar, we will analyse available‐case data if an intention‐to‐treat (ITT) analysis has not been performed. If authors have conducted their own ITT analysis despite missing data, we will document whether they provided any justification for the method they used to deal with missing data, and whether they compared their ITT result with an available‐case result.
Assessment of heterogeneity
We will assess heterogeneity between trials by careful examination of the study reports, assessing forest plots, and examining the I2 statistic with its 95% CI. We will consider I2 values greater than 50% as indicative of substantial heterogeneity, suggestive that meta‐analysis might not be wise; however, we will consider the consistency of the effect estimates. If all estimates are in the same direction, we might meta‐analyse, even where heterogeneity is evident and comment on the heterogeneity.
Assessment of reporting biases
We will use a funnel plot to assess the risk of publication bias if there are more than 10 trials in our review.
Data synthesis
We will undertake a meta‐analysis where data appears clinically, methodologically, and statistically homogeneous. We will check that participants, interventions, comparators, and outcomes are sufficiently similar to give a clinically meaningful result and that our I2 result indicates little inconsistency (i.e. I2 less than 50%). We will use a random‐effects model unless there are fewer than three eligible studies, in which case we will use a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We do not plan to conduct subgroup analysis in future updates of the review.
Sensitivity analysis
We planned to assess the impact of including studies at high risk of bias for an outcome in one or more key domains.
'Summary of findings' table
We planned to prepare tables to summarise the findings of the review, including the assessment of the quality of evidence for all primary and secondary outcomes using the GRADE approach (GRADEpro 2015).
We planned to report subconjunctival draining minimally‐invasive devices compared to the following comparison groups described under Types of interventions:
-
in combination with phacoemulsification compared with phacoemulsification alone;
-
laser treatment;
-
other MIGS techniques;
-
conventional glaucoma surgery (trabeculectomy); or
-
medical therapy.
We planned to report the following outcomes at medium‐term follow‐up (18 to 36 months) in the 'Summary of findings' table:
-
Mean change in IOP measured with Goldman applanation tonometry.
-
Proportion of participants who were drop‐free (not using eye drops).
-
Mean change in number of IOP‐lowering drops taken per day.
-
Proportion of participants who required further glaucoma surgery, including laser, as recorded by the investigators of the included trials.
-
Mean change in health‐related quality of life (HRQoL).
-
Proportion of participants experiencing intraoperative complications.
-
Proportion of participants experiencing postoperative complications (any time point).
Results
Description of studies
Results of the search
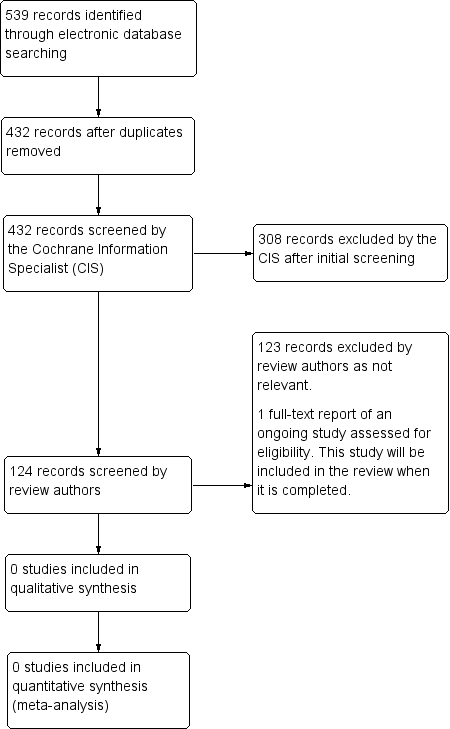
The electronic searches yielded 539 references (Figure 1). After removal of 107 duplicates, the Cochrane Information Specialist screened the remaining 432 records and removed 308 references that were clearly irrelevant. We screened the remaining 124 references and identified one ongoing study that met the inclusion criteria (NCT01881425).

Study flow diagram.
Ongoing studies
The ongoing study should be completed in November 2019. See Ongoing studies for further details.
Risk of bias in included studies
We included no published RCTs that met our inclusion criteria.
Effects of interventions
There were no completed RCTs reporting outcomes of subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma.
Discussion
We found no RCTs reporting the outcomes of subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma.
We found one ongoing RCT for the InnFocus MicroShunt. We will report the outcomes of this trial when they become available should it meet our inclusion criteria.
Summary of main results
There are currently no RCTs reporting the outcomes of subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma.
Overall completeness and applicability of evidence
We performed a thorough search of available evidence as outlined in the published protocol (King 2017).
Quality of the evidence
We found no trials for inclusion in this review.
Potential biases in the review process
While we performed a thorough search of the literature, it is possible that we missed relevant published or ongoing RCTs.
Agreements and disagreements with other studies or reviews
We found no reviews for comparison.
Study flow diagram.

