Pontage trabéculaire ab interno avec microstent canalaire de Schlemm (Hydrus) pour glaucome à angle ouvert
Résumé scientifique
Contexte
Le glaucome est l'une des principales causes de cécité irréversible. Un certain nombre de techniques chirurgicales peu invasives ont été introduites comme traitement pour empêcher la progression du glaucome ; le pontage trabéculaire ab interno avec microstent Hydrus du canal de Schlemm est l'une d'entre elles.
Objectifs
Évaluer l'efficacité et l’innocuité du pontage trabéculaire ab interno avec microstent Hydrus dans le traitement des personnes atteintes de glaucome à angle ouvert (GAO).
Stratégie de recherche documentaire
Le 7 mai 2019, nous avons effectué une recherche dans CENTRAL (2019, numéro 5), qui contient le registre des essais contrôlés du groupe Cochrane sur les yeux et la vision ; Ovid MEDLINE ; Ovid Embase ; le registre ISRCTN ; ClinicalTrials.gov ; et le Système d’enregistrement international des essais cliniques (ICTRP) de l'OMS.
Critères de sélection
Nous avons recherché des essais contrôlés randomisés (ECR) du microstent Hydrus, seul ou avec chirurgie de la cataracte, par rapport à d'autres traitements chirurgicaux (chirurgie de la cataracte seule, autres techniques avec dispositifs minimalement invasifs de glaucome, trabéculectomie), au traitement laser ou au traitement médical.
Recueil et analyse des données
Un minimum de trois auteurs ont indépendamment extrait les données des rapports des études incluses, en utilisant un formulaire de collecte de données et analysé les données, en fonction des méthodes exigées par Cochrane.
Résultats principaux
Nous avons inclus trois études publiées, avec 808 personnes randomisées. Deux études avaient de multiples centres de recrutement internationaux aux États‐Unis et dans d'autres pays. La troisième étude portait sur plusieurs sites basés en Europe. Ces trois études étaient sponsorisées par le fabricant d'Hydrus, Ivantis Inc. Toutes les études incluaient des participants présentant principalement un GAO léger ou modéré (écart moyen entre ‐3,6 dB (décibel) et ‐8,4 dB dans tous les groupes d'étude), contrôlé par des médicaments chez de nombreux participants (pression intraoculaire (PIO) moyenne sous médicament de 17,9 mmHg à 19,1 mmHg). Le biais de dissimulation de l’allocation n’a pas posé de problème, mais la mise en aveugle des évaluateurs des critères de jugement était à risque élevé ou peu clair dans toutes les études ; la mise en aveugle des participants a été réalisée, et les pertes de suivi n'étaient pas source de préoccupations.
Deux études ont comparé le microstent Hydrus combiné à la chirurgie de la cataracte à la chirurgie de la cataracte seule, chez des participants présentant des cataractes visuellement significatives et des GAO.
Nous avons trouvé des données probantes de certitude modérée indiquant que l'ajout du microstent Hydrus à la chirurgie de la cataracte a fait passer la proportion de participants sans médicament d'environ la moitié à plus des trois quarts à 12 mois, suivi à court terme (rapport de risque (RR) 1,59, intervalle de confiance (IC) à 95% 1,39 à 1,83 ; 2 études, 639 participants ; I² = 0%) ; et à 24 mois, suivi à moyen terme (RR 1,63, IC à 95% 1,40 à 1,88 ; 2 études, 619 participants ; I² = 0%).
Le microstent Hydrus combiné à la chirurgie de la cataracte a réduit la variation moyenne à moyen terme de la PIO non médicamentée (après lavage) de 2 mmHg de plus que la chirurgie de la cataracte seule (différence moyenne [mean difference, MD] ‐2,00, IC à 95 % ‐2.69 à ‐1,31 ; 2 études, 619 participants ; I² = 0 % ; preuves de certitude modérée), et le changement moyen des baisses de PIO (MD ‐0,41, 95 % IC ‐0,56 à ‐0,27 ; 2 études, 619 participants ; I² = 0 % ; données probantes de faible certitude). Nous avons également trouvé des données probantes de faible certitude indiquant que l'ajout d'un microstent Hydrus à la chirurgie de la cataracte réduisait la nécessité d'une chirurgie secondaire du glaucome d'environ 2,5 % à moins de 1 % (RR 0,17, IC à 95 % 0,03 à 0,86 ; 2 études, 653 participants ; I² = 27 % ; données probantes de faible certitude).
Les saignements intraoculaires, la perte de deux lignes ou plus d'acuité visuelle (AV) et les pics de PIO de 10 mmHg ou plus étaient rares dans les deux groupes ; les estimations étaient imprécises et incluaient à la fois des effets bénéfiques et des effets nocifs. Aucun cas d'endophtalmite n'a été constaté dans les deux groupes.
Aucune donnée n'était disponible sur la proportion de participants atteignant une PIO inférieure à 21 mmHg, 17 mmHg ou 14 mmHg, sur la qualité de vie liée à la santé (QVLS) ou sur la progression du champ visuel.
Une étude a fourni des données à court terme pour le microstent Hydrus comparé au stent microbypass trabéculaire iStent (iStent : implantation de deux dispositifs en une seule procédure) chez 152 participants avec GAO (148 dans les analyses). L'utilisation de l'Hydrus a augmenté la proportion de participants sans médicament d'environ un quart à environ la moitié par rapport à ceux ayant reçu iStent, mais cette estimation était imprécise (RR 1,94, IC à 95% 1,21 à 3,11 ; données probantes de faible certitude). L'utilisation du microstent Hydrus a réduit la PIO non médicamentée (après lavage) d'environ 3 mmHg de plus que l’iStent (MD ‐3,10, IC à 95 % ‐4,17 à ‐2,03 ; données probantes de certitude modérée), et l'utilisation de médicaments abaissant la PIO (MD ‐0,60, IC à 95 % ‐0,99 à ‐0,21 ; données probantes de faible certitude). Les deux dispositifs ont atteint une PIO finale < 21 mmHg chez la plupart des participants (microstent Hydrus : 91.8% ; iStent: 84 % ; RR 1,09, IC à 95 % 0,97 à 1,23 ; données probantes de faible certitude).
Aucun des participants ayant reçu le microstent Hydrus (N = 74) n'a eu besoin d'une chirurgie supplémentaire pour le glaucome ; deux participants ayant reçu l'iStent (N = 76) en ont eu besoin.
Peu d'événements indésirables ont été constatés dans les deux groupes.
Aucune donnée n'était disponible sur la proportion de participants atteignant une PIO inférieure à 17 mmHg ou 14 mmHg, ou sur la QVLS.
Conclusions des auteurs
Chez les personnes souffrant de cataracte et de glaucome à angle ouvert (GAO), généralement léger à modéré, il existe des données probantes de certitude modérée indiquant que le microstent Hydrus avec chirurgie de la cataracte, comparé à la chirurgie de la cataracte seule, augmente probablement la proportion de participants n’ayant pas besoin de médicaments abaissant la pression intraoculaire (PIO), et pourrait encore réduire la PIO lors du suivi à court et moyen terme.
Il existe des preuves de certitude modérée que le microstent Hydrus est probablement plus efficace que l'iStent pour abaisser la PIO des personnes atteintes de GAO à court terme.
Peu d'études étaient disponibles sur les effets du microstent Hydrus, par conséquent les résultats de cette revue pourraient ne pas être applicables à toutes les personnes atteintes de GAO, en particulier chez certaines personnes atteintes de glaucome non contrôlé médicalement, puisque la PIO était contrôlée par des médicaments chez de nombreux participants des études incluses. Les complications pourraient être rares avec le microstent Hydrus, ainsi qu'avec le comparateur iStent, mais des études plus importantes sont nécessaires pour en étudier l’innocuité.
PICOs
Résumé simplifié
Pontage trabéculaire ab interno avec microstent canalaire de Schlemm Hydrus pour glaucome à angle ouvert
Quel était le but de cette revue ?
L'objectif de cette revue Cochrane était de déterminer si le pontage trabéculaire ab interno avec le microstent Hydrus réduit la pression dans l'œil (pression intraoculaire) des personnes atteintes de glaucome à angle ouvert (GAO). Les auteurs de cette revue Cochrane ont rassemblé et analysé toutes les études pertinentes pour répondre à cette question, et ont trouvé trois études achevées.
Messages principaux
Chez les personnes atteintes de cataracte et de glaucome, le traitement combinant la chirurgie de la cataracte et un implant Hydrus pourrait augmenter le nombre de personnes n’ayant pas besoin de médicaments pour abaisser la pression intraoculaire (PIO), et pourrait davantage réduire la PIO par rapport à la chirurgie de la cataracte seule, à court et moyen terme. Lorsque le microstent Hydrus était comparé à iStent, le microstent était probablement plus efficace chez les personnes atteintes de GAO. Ces preuves proviennent d'études portant sur des personnes chez qui la PIO était souvent bien contrôlée par des médicaments, et leur GAO était principalement léger ou modéré.
Quel était le sujet de la revue ?
Le glaucome est une affection oculaire courante qui peut mener à la cécité si elle n'est pas traitée. Dans le glaucome, le nerf optique (qui relie l'œil au cerveau) est endommagé, souvent à cause d’une augmentation de la pression dans l'œil due à l'accumulation de liquide. Le pontage trabéculaire ab interno avec microstent Hydrus est un type de chirurgie durant laquelle les médecins implantent l'Hydrus (un petit dispositif qui ouvre un canal dans le canal principal de fluide appelé canal de Schlemm) et améliore l'écoulement du fluide dans ce canal. Cela pourrait entraîner une baisse de la pression oculaire et une diminution des risques d'endommagement du nerf optique. Ce type de chirurgie est moins invasif et pourrait entraîner moins de complications et une guérison plus rapide que les autres types de chirurgie du glaucome.
Quels ont été les principaux résultats de la revue ?
Deux études (653 participants atteints de cataracte et de glaucome à angle ouvert) ont montré que la proportion de personnes n'utilisant pas de médicaments abaissant la PIO à deux ans était d'environ la moitié pour celles qui avaient été opérées de la cataracte uniquement, et de plus des trois quarts si le microstent Hydrus était également implanté lors de l'opération de la cataracte ; ces données étaient de certitude modérée en raison de problèmes dans la qualité des études. Environ un participant sur 30 ou 50 a eu besoin d'une nouvelle opération du glaucome après la seule chirurgie de la cataracte, contre un sur 100 ou moins lorsque le microstent Hydrus était ajouté ; ces données étaient de faible certitude, en raison de problèmes dans la qualité des études et du petit nombre d'opérations du glaucome.
Une autre étude (152 participants atteints de glaucome à angle ouvert) a comparé un implant Hydrus à un implant iStent (un petit tube implanté dans le système de drainage de l'œil, connu sous le nom de maillage trabéculaire, permettant au fluide de s'écouler dans le canal de Schlemm) à un an. L'étude a révélé que le microstent Hydrus doublait presque le nombre de personnes n'utilisant pas de médicaments abaissant la PIO à un an, passant d'environ un quart à près de la moitié ; ces données étaient de faible certitude, en raison de problèmes dans la qualité de l'étude et du petit nombre de participants. Dans les deux groupes, il était très rare qu'une autre opération du glaucome soit nécessaire.
Toutes les études incluses étaient sponsorisées par le fabricant Hydrus Ivantis Inc.
L'utilisation de l'implant Hydrus était probablement sans danger dans ces études, mais des études plus importantes et un suivi plus long pourraient être nécessaires pour étudier des événements indésirables très rares ou à long terme. Ces données probantes proviennent d'études portant sur des personnes chez qui la PIO était souvent bien contrôlée par des médicaments, et d'autres essais sont nécessaires avec des participants atteints de glaucome non contrôlé.
Dans quelle mesure cette revue est‐elle à jour ?
Les auteurs de la revue Cochrane ont recherché les études publiées jusqu'au 7 mai 2019.
Authors' conclusions
Summary of findings
| Cataract surgery with Hydrus microstent compared to cataract surgery alone | ||||||
| Patient or population: people with cataracts and open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with cataract surgery alone | Risk with cataract surgery with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) medium‐term follow‐up at 24 months | Study population | RR 1.63 | 619 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 782 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry medium‐term follow‐up at 24 months | The mean change in unmedicated IOP in the cataract surgery group was ‐5.95 mmHg | The MD in the cataract surgery plus Hydrus group was 2 mmHg lower | ‐ | 619 | ⊕⊕⊕⊝ | |
| Mean change in the number of IOP‐lowering drops instilled per day medium‐term follow‐up at 24 months | The mean change in the number of IOP‐lowering drops instilled per day in the cataract surgery group was ‐0.76 drops | The MD in the cataract surgery plus Hydrus group was 0.41 drops lower | ‐ | 619 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | RR 0.17 (0.03 to 0.86) | 619 | ⊕⊕⊝⊝ | ||
| 25 per 1000 | 4 per 1000 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications medium‐term follow‐up at 24 months | Intraoperative: device malposition (1.6%) or hyphaema obscuring the surgeons view (1.1%) only occurred with Hydrus implantation Postoperative: Intraocular bleeding, loss of 2 or more VA lines, and IOP spikes of 10 mmHg or more were rare in both groups. There were no cases of endophthalmitis in either group | ⊕⊕⊝⊝ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnclear or high risk of bias for most domains (‐1 for risk of bias) | ||||||
| Hydrus microstent compared to iStent trabecular micro‐bypass stent | ||||||
| Patient or population: people with open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with iStent | Risk with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) short‐term follow‐up at 12 months | Study population | RR 1.94 | 148 | ⊕⊕⊝⊝ | ||
| 240 per 1000 | 466 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry short‐term follow‐up at 12 months | The mean change in unmedicated IOP in the iStent group was ‐5.1 mmHg | The MD in the Hydrus group was 3.1 lower | ‐ | 148 | ⊕⊕⊕⊝ | |
| Mean change in number of IOP‐lowering drops instilled per day short‐term follow‐up at 12 months | The mean change in the number of IOP‐lowering drops instilled per day in the iStent group was 0 | The MD in the Hydrus group was 0.6 lower | ‐ | 148 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | not analysed | 148 | ⊕⊝⊝⊝ | ||
| 0/74 | 2/76 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications short‐term follow‐up at 12 months | No intraoperative complications reported. Postoperative: no cases of intraocular bleeding or endophthalmitis in either group. Hydrus: 2/74 cases of VA loss of 2 or more lines, 3/74 IOP spikes > 10 mmHg iStent: 1/76 cases of VA loss of 2 or more lines, 4/76 IOP spikes > 10 mmHg | not analysed | 148 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnmasked investigator | ||||||
Background
Description of the condition
Glaucoma is a chronic progressive optic neuropathy, affecting up to 4% of people by the age of 80 years (Burr 2007). It is the leading cause of irreversible blindness, affecting 60 million people globally (Quigley 2006). This figure is expected to increase to 80 million people by 2020. Open angle glaucoma (OAG) is the most common type, accounting for three‐quarters of cases (Quigley 2006). In one large population cohort, one in six patients with OAG became bilaterally blind (Peters 2013). The only proven way to prevent vision loss is to reduce the pressure inside the eye (intraocular pressure) over the long term (AGIS 2000; CNTG Study Group 1998; Heijl 2002; Kass 2002; UKGTS 2015). Approaches to reducing intraocular pressure (IOP) include medical therapy, laser treatments, and surgery. Because commercially available eye‐drop preparations have a short‐lasting effect, medical therapy requires that eye‐drops are instilled one or more times daily for life. Adherence is very poor, even if use is monitored (Friedman 2009; Okeke 2009). Conventional surgical techniques, such as trabeculectomy, are associated with significant risks, with more than 40% of patients developing perioperative complications (Kirwan 2013; Lichter 2001); reoperation is needed in 7% to 18% (Gedde 2012; Kirwan 2013). Therefore, surgery is often reserved for disease that is progressing despite other treatments (King 2013).
Description of the intervention
Recently, a number of minimally‐invasive surgical techniques have been developed, with the aim of achieving long‐term reduction of IOP, with a better safety profile than conventional surgery (Francis 2011). Among them, ab interno trabecular bypass surgery with a Schlemm´s canal Hydrus microstent (Ivantis Inc., Irvine, California) is marketed worldwide.
How the intervention might work
The trabecular meshwork is the main site of resistance to the outflow of aqueous humour from the eye (Overby 2009). The Hydrus microstent is an 8‐mm long crescent‐shaped open structure, curved to match the shape of the Schlemm’s canal. This is intended to promote outflow of aqueous humour, and thereby reduce IOP. The microstent is implanted ab interno, through a clear corneal incision into the Schlemm’s canal, using a preloaded hand‐held injector. After being implanted, the microstent bypasses the trabecular meshwork and dilates the Schlemm’s canal over three clock hours, to provide direct aqueous access from the anterior chamber to multiple collector channels (Pfeiffer 2015).
Why it is important to do this review
Consultation with patients and healthcare professionals has identified a need for better treatments for glaucoma (James Lind Alliance 2013). Minimally‐invasive glaucoma procedures carry the possibility of safe and effective long‐term reduction of IOP, removing concerns about permanent vision loss due to non‐adherence with eye‐drops. A single treatment may also be more acceptable to patients than daily and indefinite self‐administration of eye‐drops. To date, approximately 17,000 treatments have been performed worldwide in either feasibility studies, randomised controlled trials, or data registries (Otarola 2019 [pers comm]). In light of the potential benefits for patients and the widespread uptake of the technique, it is important to critically evaluate the evidence for the efficacy and safety of treatment with the Hydrus microstent. Importantly, Hydrus microstent implantation surgery may be combined with phacoemulsification (cataract surgery, a sight‐restoring operation to remove the natural lens of the eye when it has lost clarity). Since cataract surgery itself reduces IOP (Mansberger 2012), we will specifically examine the evidence for efficacy of Hydrus microstent treatment in people who have concomitant cataract surgery in comparison to those who do not have concomitant cataract surgery.
This Cochrane Review was conducted in parallel with other reviews undertaken by the Cochrane Eyes and Vision MIGS (minimally‐invasive glaucoma surgery) Consortium, which includes MIGS techniques and devices, such as the Trabectome (NeoMedix, Tustin, CA, USA (Hu 2016)), XEN Glaucoma Implant (AqueSys Implant, Aliso Viejo, CA, USA (King 2018)), endoscopic cytophotocoagulation (Endo Optiks, Waltham, MA, USA (Tóth 2019)), iStent and iStent inject (Glaukos Corporation, Laguna Hills, CA, USA (Le 2019)), and supraciliary microstent surgery (Sandhu 2017).
Objectives
To evaluate the efficacy and safety of ab interno trabecular bypass surgery with the Hydrus microstent in treating people with open angle glaucoma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We included reports of RCTs prepared in any language, regardless of their publication status.
Types of participants
Participants had open angle glaucoma (OAG) of any type, including primary and secondary OAG. As there are no universally‐accepted criteria by which glaucoma may be defined, we permitted studies to use their own definitions of glaucoma, provided these were clearly stated. We also included participants with ocular hypertension, normal tension glaucoma, or possible glaucoma (suspects for glaucoma).
We excluded trials with participants with closed angle glaucoma.
We did not apply any restrictions regarding location, setting, or demographics.
Types of interventions
We compared ab interno trabecular bypass surgery with the Hydrus microstent (Ivantis Inc., Irvine, California) to:
-
laser treatment (selective laser trabeculoplasty or argon laser trabeculoplasty);
-
other minimally‐invasive glaucoma surgery (MIGS) techniques;
-
conventional glaucoma surgery (trabeculectomy);
-
medical therapy.
Types of outcome measures
We did not use the reporting of particular outcomes as a criterion for eligibility for the review. We did not exclude studies from the review solely on the grounds of not reporting an outcome of interest.
We reported outcomes in the short‐term (six to 18 months), medium‐term (18 to 36 months), and long‐term (longer than 36 months).
Primary outcomes
-
Proportion of participants who were medication‐free (not using eye drops)
Several different glaucoma outcome measures have been specified as primary outcomes in other Cochrane Reviews and protocols (Ismail 2015). A recent study classified intraocular pressure (IOP), visual field, safety, and anatomic outcomes as being highly important to glaucoma experts (Ismail 2016). A panel of patients from the Patient and Public Involvement Group of the National Institute for Health Research (NIHR) Biomedical Research Centre for Ophthalmology identified drop‐free disease control as a highly valued outcome (unpublished). We chose a participant‐centred primary outcome.
Secondary outcomes
-
Mean change in IOP, measured using Goldmann applanation tonometry
-
Mean change in number of IOP‐lowering drops taken per day
-
Proportion of participants who achieved an IOP of 21 mmHg or less
-
Proportion of participants who achieved an IOP of 17 mmHg or less
-
Proportion of participants who achieved an IOP of 14 mmHg or less
-
Proportion of participants who required further glaucoma surgery, including laser, as recorded by the investigators of the included trial
-
Rate of visual field progression (decibels (dB)/time) or proportion of participants whose field loss progressed in the follow‐up period
-
Mean change in health‐related quality of life (HRQoL)
Adverse effects
-
Proportion of participants experiencing intra‐ and postoperative complications, including, but not restricted to, the following:
-
-
loss of visual acuity (more than 2 Snellen lines, or more than 0.3 logMAR, according to the method of recording visual acuity; or loss of light perception);
-
bleeding, as recorded by the investigators;
-
endophthalmitis, as recorded by the investigators;
-
IOP spikes (postoperative rise in IOP, measured using Goldmann applanation tonometry, of more than 10 mmHg compared to the previous assessment, including measurements taken during the first postoperative month).
-
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following electronic databases for RCTs and controlled clinical trials. There were no restrictions to language or year of publication. The date of the search was 7 May 2019.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5 (which contains the Cochrane Eyes and Vision Trials Register)) in the Cochrane Library (searched 7 May 2019; Appendix 1;
-
MEDLINE Ovid (1946 to 7 May 2019; Appendix 2);
-
Embase Ovid (1980 to 7 May 2019; Appendix 3);
-
International Standard Research Clinical Trial Number (ISRCTN) registry (www.isrctn.com/editAdvancedSearch; searched 7 May 2019; Appendix 4);
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 7 May 2019; Appendix 5);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp; searched 7 May 2019; Appendix 6).
Searching other resources
We searched the reference lists of included studies for other possible studies, and contacted any individuals or organisations who conducted relevant RCTs. We also searched the website of the manufacturer (Ivantis Inc., Irvine, California; www.ivantisinc.com) for any information on forthcoming trials. We are awaiting additional data on included studies from the manufacturer.
Data collection and analysis
Selection of studies
Four review authors independently screened titles and abstracts of all articles identified by the search, using web‐based online review management software (Covidence). If abstracts were not available, we screened full‐text articles. Two review authors independently assessed the full‐text reports of all potentially eligible studies. If there was disagreement regarding eligibility, a third review author arbitrated. If any full‐text reports were rejected, we recorded the reasons for this.
Data extraction and management
We extracted data from reports of included studies using a data collection form, which was developed, but not piloted on the first five studies included as planned. Three review authors independently extracted study characteristics from reports of each study, and two review authors (AS, GV) entered the data for the studies into Review Manager 5 (RevMan 5 (Review Manager 2014)). Two review authors (GV, FO) independently extracted data for the analyses, and one review author (GV) checked the data, and then entered it into RevMan 5. If there was disagreement, a third review author arbitrated.
Data collected in Appendix 7 were presented in the 'Characteristics of included studies' table. Where data on included studies (or ongoing studies) were missing or unclear, we contacted the individuals or organisations involved to obtain clarification. We collected and used the most detailed numerical data available to facilitate analyses of included studies. We obtained these data from individuals or organisations in preference to less precise methods, such as extracting numeric data from graphs, as indicated.
Assessment of risk of bias in included studies
We used the latest version of the Cochrane 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess and judge the risk of bias for included studies (Higgins 2017).
Measures of treatment effect
We calculated the risk ratio for the following outcomes: proportion of participants who were medication‐free (not using eye drops); proportion of participants who achieved an IOP of no more than 21 mmHg, 17 mmHg, and 14 mmHg; proportion of participants who required further glaucoma surgery; and proportion of participants who experienced intra‐ and postoperative complications.
When data were available, we calculated the mean difference for the following continuous outcomes: mean change in IOP; mean change in number of IOP‐lowering drops instilled per day; and mean change in quality of life.
Where possible, we checked for the skewness of continuous data (Altman 1996).
Unit of analysis issues
We noted whether studies included one or two eyes from each participant, and whether randomisation was conducted at the level of the participant or the eye. There is a potential for medical treatments, such as topical beta blockers used for one eye, to influence the outcome in the other eye (Piltz 2000). Surgery to lower IOP in one eye may also affect the IOP of the fellow eye (Radcliffe 2010). Therefore, we excluded studies that had adopted a paired design.
Dealing with missing data
We tried to minimise missing outcome data by contacting individuals and organisations to obtain them. Because the level of missing data in each group and reasons for missing data in each group were similar, we analysed available case data. We are waiting for the manufacturer of the Hydrus microstent to provide us with unpublished data, which we will use in the update of this review.
Assessment of heterogeneity
We assessed the heterogeneity between trials by carefully examining the study reports, assessing forest plots, and examining the I² value. We considered I² values greater than 50% to be indicative of substantial heterogeneity, suggesting that meta‐analysis might not be wise. We also considered the consistency of the effect estimates. If all estimates were in the same direction, we pooled the data, even when heterogeneity was evident; we commented on any heterogeneity in the Discussion section.
Assessment of reporting biases
We planned to develop a funnel plot to assess the risk of publication bias if there were more than 10 trials in our review.
Data synthesis
We undertook a meta‐analysis when data appeared clinically, methodologically, and statistically homogeneous. We checked that participants, interventions, comparators, and outcomes were sufficiently similar to give a clinically meaningful result, and that our I² result did not indicate considerable inconsistency (i.e. I² less than 50%). In future updates of this review, we will pool heterogenous data if all estimates are in the same direction. We used a fixed‐effect model, as there were fewer than three trials included in the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We do not plan to conduct subgroup analyses in future updates of the review.
Sensitivity analysis
We planned to assess the impact of including studies at high risk of bias for an outcome in one or more key domains. However, there were too few included studies to conduct such analyses.
Summary of findings and assessment of the certainty of the evidence
We prepared tables to summarise the findings of the review, including the assessment of the certainty of evidence for all outcomes, using the GRADE approach (GRADEpro GDT).
We reported the following outcomes at medium‐term follow‐up (18 to 36 months) in the 'Summary of findings' table for each comparison listed in the Types of interventions: Ab interno trabecular bypass surgery with Schlemm's canal Hydrus microstent compared with laser treatment, other MIGS techniques, conventional glaucoma surgery (trabeculectomy), or medical therapy.
-
Proportion of participants who were medication‐free (not using eye drops).
-
Mean change in IOP, measured using Goldmann applanation tonometry.
-
Mean change in number of IOP‐lowering drops taken per day.
-
Proportion of participants who required further glaucoma surgery, including laser.
-
Rate of visual field progression (decibels (dB)/time) or proportion of participants whose field loss progressed in the follow up period.
-
Mean change in health‐related quality of life.
-
Proportion of participants experiencing intraoperative and postoperative complications (any time point).
Results
Description of studies
Results of the search
The electronic searches yielded 713 records (Figure 1). After removing 115 duplicates, the Cochrane Information Specialist (CIS) screened the remaining 598 records, and removed 389 records that were not relevant to the scope of the review. We screened the remaining 209 records, and obtained the full‐text reports of six records for further assessment. We included four reports of three studies (COMPARE 2019; HORIZON 2018; Pfeiffer 2015). We identified one ongoing study that met the inclusion criteria, and this will be assessed for inclusion in the review when data become available (NCT02024464). One study was a conference abstract (Altafini 2014). It was not clear whether this study collected data for outcomes of interest to this review. We have contacted the trial investigators and are awaiting a response.
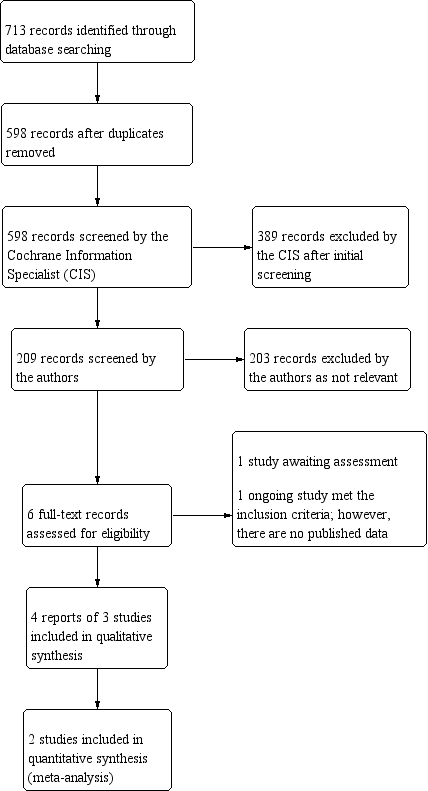
Study flow diagram
Included studies
We identified three studies that met our inclusion criteria. Two studies compared the Hydrus microstent with cataract surgery to cataract surgery alone in people with concurrent cataract and open angle glaucoma (OAG) (HORIZON 2018; Pfeiffer 2015). HORIZON 2018 was conducted at 26 sites in the United States and 12 international sites, and included 369 participants. Pfeiffer 2015 was a single‐masked, multicentred randomised controlled trial (RCT) with 100 participants, based at several sites (Germany, Italy, Spain, and the Netherlands). COMPARE 2019 was a single‐masked, multicentred RCT conducted at 12 sites in the United States and 8 international sites, which compared stand alone Hydrus microstent surgery to stand alone iStent (n.2 implants used in a single procedure) surgery in 152 participants.
All three studies were sponsored by the Hydrus manufacturer (Ivantis, Inc., Irvine, California).
In all studies, in the opinion of the investigators, participants had to be capable of safely undergoing medication wash‐out. Pfeiffer 2015 included 100 participants, taking an average of two medications at baseline. At baseline, the Hydrus microstent with cataract surgery group had a mean medicated intraocular pressure (IOP) of 18.9 (SD 3.3) mmHg and mean deviation (MD) of ‐5.6 (SD 5.4) dB; the cataract surgery alone group had a mean medicated IOP of 18.6 (SD 3.8) mmHg, and a MD of ‐8.4 (SD 7.8) dB.
HORIZON 2018 included participants with visually significant cataracts. At baseline, the Hydrus microstent with cataract surgery group had a mean medicated IOP of 17.9 (SD 3.1) mmHg, took an average of 1.7 (SD 0.9) medications, and had a MD of ‐3.61 (SD 2.49) dB; the cataract surgery alone group had a mean medicated IOP of 18.1 (SD 3.1) mmHg, and a MD of ‐3.61 (SD 2.60) dB.
COMPARE 2019 included participants with phakic and pseudophakic (about 35%) eyes with mostly mild or moderate OAG. At baseline, the Hydrus microstent group had a mean medicated IOP of 19.0 (SD 3.9) mmHg, were on an average of 2.5 (SD 0.7) medications, and had a MD of 6.2 (SD 5.4) dB; the iStent group had a mean medicated IOP of 19.1 (SD 3.6) mmHg, were on an average of 2.7 (SD 0.8) medications, and had a MD of 6.2 (SD 6.5) dB.
The type of participants included in the trials suggests that many of these participants had medically‐controlled glaucoma. See Characteristics of included studies for further information.
Ongoing studies
We identified one ongoing study that met our inclusion criteria, which compares the Hydrus microstent with the iStent trabecular micro‐bypass stent (NCT02024464). See Characteristics of ongoing studies table for further information.
Studies awaiting classification
We have placed one conference abstract (Altafini 2014), in Studies awaiting classification as it is not clear whether this study collected data for outcomes of interest to this review. We have contacted the trial investigators and are awaiting a response.
Excluded studies
We did not exclude any studies.
Risk of bias in included studies
See Figure 2

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation and allocation concealment were adequate in all studies.
Blinding
Participants and personnel were masked to intervention assignment, but masking of outcome assessors was unclear or high risk in all studies.
Incomplete outcome data
HORIZON 2018 reported that 5% of participants were lost at 24 months, but the proportion and causes in each study arm was not reported. Pfeiffer 2015 reported that at 24 months, 3 out of 50 participants from the Hydrus microstent plus cataract surgery group and 7 out of 50 participants from the cataract surgery group were missing; for those with unmedicated IOP, 6 out of 50 from the Hydrus microstent plus cataract surgery group and 16 out of 50 from the cataract surgery group were missing. We considered both studies at unclear risk of bias for this domain. Only two participants in each group were lost to follow‐up in COMPARE 2019.
Selective reporting
We could not obtain a study protocol to check extensively for selection bias, but there seemed to be no major difference compared to the information found on ClinicalTrials.gov.
Other potential sources of bias
No other sources of bias were identified.
Effects of interventions
See: Summary of findings for the main comparison Cataract surgery with Hydrus microstent compared to cataract surgery alone; Summary of findings 2 Hydrus microstent compared to iStent trabecular micro‐bypass stent
Hydrus microstent with cataract surgery versus cataract surgery alone
See summary of findings Table for the main comparison for a summary of all available results.
Proportion of participants who were medication‐free (not using eye drops)
The Hydrus microstent with cataract surgery increased the proportion of participants who were medication‐free, both at short‐term follow‐up (risk ratio (RR) 1.59, 95% confidence interval (CI) 1.39 to 1.83; 2 studies, 639 participants; I² = 0%; moderate‐certainty evidence, due to risk of bias; Analysis 1.1; Figure 3), and at medium‐term follow‐up (RR 1.63, 95% CI 1.40 to 1.88; 2 studies, 619 participants; moderate‐certainty evidence, due to risk of bias; Analysis 1.2; Figure 4).
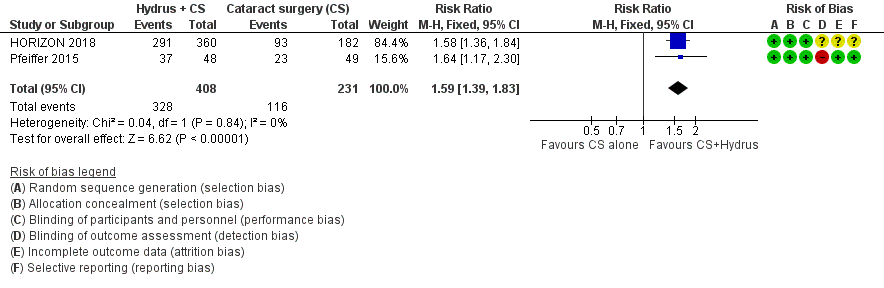
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs. cataract surgery (CS) alone, outcome: 1.1 Proportion drop‐free: short term
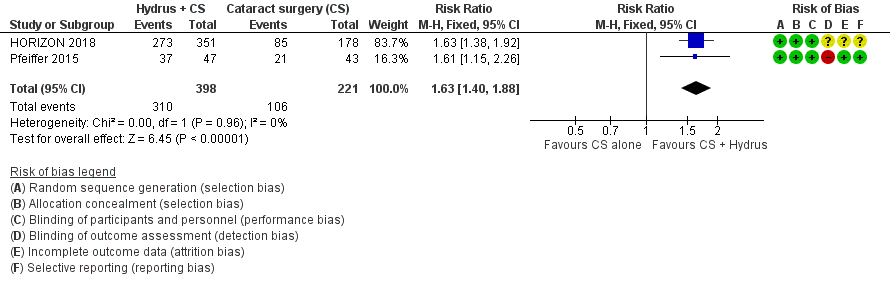
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.2 Proportion drop‐free: medium term
Mean change in intraocular pressure (IOP) measured using Goldmann applanation tonometry
The Hydrus microstent with cataract surgery reduced unmedicated IOP by an additional 2 mmHg (mean difference (MD) ‐2.00 mmHg, 95% CI ‐2.69 to ‐1.31 mmHg; 2 studies, 619 participants; I² = 0%; moderate‐certainty evidence, due to risk of bias; Analysis 1.3; Figure 5). Not all participants in Pfeiffer 2015 underwent washout, but given their small number and the small weight of the study in the analysis, we did not downgrade this evidence further.

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.4 Mean change in IOP‐lowering drops taken per day: medium term
We were only able to obtain medium‐term data on unmedicated IOP (after washout); there were no data available at 12 months, or for medicated IOP (medication needed) at any follow‐up. We expected the difference in medicated IOP to be smaller between intervention groups, because the number of medications during follow‐up was higher in the cataract surgery only group in both studies. The standard deviation of IOP change for Pfeiffer 2015 was not reported, so we imputed it from HORIZON 2018.
Mean change in number of IOP‐lowering medications taken per day
The Hydrus microstent combined with cataract surgery increased the proportion of participants (MD ‐0.41, 95% CI ‐0.56 to ‐0.27; 2 studies, 619 participants; I² = 0%; Analysis 1.4; Figure 6; low‐certainty of evidence due to risk of bias and indirectness).

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.3 Mean change in IOP measured using Goldmann applanation tonometry: medium term
We were only able to obtain medium‐term data. Not all participants in Pfeiffer 2015 underwent washout; in HORIZON 2018, about half of the participants were taking two to four medications at baseline, which led us to downgrade this evidence for indirectness, since this was the largest trial in the analysis.
Proportion of participants who achieved an IOP of 21 mmHg, 17 mmHg, and 14 mmHg or less
There were no data for this outcome.
Proportion of participants who required further glaucoma surgery, including laser, as recorded by the investigators of the included trial
Fewer surgeries were needed for the Hydrus microstent combined with cataract surgery group compared with the cataract surgery alone group, but this analysis was based on only seven events (RR 0.17, 95% CI 0.03 to 0.86; 2 studies, 653 participants; I² = 27%; low‐certainty evidence, due to risk of bias and imprecision; Analysis 1.5).
Rate of visual field progression (decibel (dB)/time), or proportion of participants whose field loss progressed in the follow‐up period
There were no data for this outcome.
Mean change in health‐related quality of life (HRQoL)
None of the studies measured health‐related quality of life.
Proportion of participants experiencing intra‐ and postoperative complications
Only HORIZON 2018 reported intraoperative complications. Device malposition (1.6%) or hyphaema obscuring the surgeons' view (1.1%) occurred only with Hydrus microstent implantation. We judged this evidence to be very low‐certainty, due to risk of bias (‐1) and imprecision (‐2).
Among postoperative complications, intraocular bleeding, loss of 2 or more visual acuity (VA) lines, IOP spikes of 10 mmHg or more were rare in both groups, and estimates were very imprecise (Analysis 1.6; Analysis 1.7; Analysis 1.8). There were no cases of endophthalmitis in either group. We judged this evidence to be very low‐certainty, due to risk of bias (‐1) and imprecision (‐2).
Hydrus microstent versus iStent trabecular micro‐bypass stent
Only one study provided short‐term data for this comparison (summary of findings Table 2; COMPARE 2019).
Proportion of participants who were medication‐free (not using eye drops)
The Hydrus microstent increased the proportion of medication‐free participants from about 24% to 46.6% compared to the iStent trabecular micro‐bypass stent, but this estimate was imprecise (RR 1.94, 95% CI 1.21 to 3.11; 1 study, 146 participants; low‐certainty evidence, due to risk of bias and imprecision; Analysis 2.1).
Mean change in IOP measured using Goldmann applanation tonometry
COMPARE 2019 did not provide data on medicated IOP (eye drops needed); we expected the difference in medicated IOP to be smaller between intervention groups, because the number of medications during follow‐up was higher in the iStent group. The Hydrus microstent reduced unmedicated IOP (after wash‐out) by about 3 mmHg more than the iStent trabecular micro‐bypass stent (MD ‐3.10, 95% CI ‐4.17 to ‐2.03; 1 study, 148 participants; moderate‐certainty of evidence, due to risk of bias; Analysis 2.2); the latter achieved a reduction of about 5 mmHg.
Mean change in number of IOP‐lowering drops taken per day
The Hydrus microstent reduced IOP‐lowering medication by one daily medication compared to the iStent trabecular micro‐bypass stent (MD ‐0.60, 95% CI ‐0.99 to ‐0.21; 1 study, 148 participants; low‐certainty of evidence due to risk of bias and imprecision; Analysis 2.3).
Proportion of participants who achieved an IOP of 21 mmHg, 17 mmHg, and 14 mmHg or less
We extracted the proportion of participants achieving IOP < 21 mmHg, which was high for both the Hydrus microstent (91.8%) and the iStent trabecular micro‐bypass stent (84%); no evidence of difference was found (RR: 1.09, 95% CI 0.97 to 1.23; 1 study, 148 participants; low‐certainty of evidence, due to risk of bias and imprecision; Analysis 2.4).
Proportion of participants who required further glaucoma surgery, including laser, as recorded by the investigators of the included trial
None of the 74 participants with the Hydrus microstent needed further surgery compared to 2 out of 76 with the iStent trabecular micro‐bypass stent. We did not conduct a formal comparison due to sparse data.
Rate of visual field progression (dB/time) or proportion of participants whose field loss progressed in the follow‐up period
There were no data for this outcome.
Mean change in health‐related quality of life
There were no data for this outcome.
Proportion of participants experiencing intra‐ and postoperative complications
Few adverse events were seen in either group in COMPARE 2019. The Hydrus microstent group reported 2/74 cases of VA loss of 2 or more lines and 3/74 IOP spikes > 10 mmHg, while the iStent trabecular micro‐bypass stent group reported 1/76 cases of VA loss of 2 or more lines, and 4/76 IOP spikes > 10 mmHg. There were no cases of bleeding or endophthalmitis in either group. We did not conduct a formal comparison due to sparse data.
Discussion
Summary of main results
We found moderate‐certainty evidence at short‐ and medium‐term follow‐up that in people with cataracts and mainly mild or moderate open angle glaucoma (OAG), which was often well‐controlled with medication, the Hydrus microstent combined with cataract surgery may increase the proportion of people who are medication‐free, and decrease the average unmedicated intraocular pressure (IOP) by about 2 mmHg compared to cataract surgery alone. We found low‐certainty evidence that the Hydrus microstent may also decrease the number of medications and the need for secondary glaucoma surgery, without increasing postoperative complications.
We found low‐certainty evidence from a single trial that compared to the insertion of the iStent trabecular micro‐bypass stent, the Hydrus microstent may increase the proportion of medication‐free participants from about a quarter to about a half, and moderate‐certainty evidence that the Hydrus microstent may further reduce the unmedicated IOP by about 3 mmHg, while decreasing the number of medications. Participants included in this study were also often affected by medically‐controlled, mild or moderate glaucoma.
Overall completeness and applicability of evidence
Because we only included three studies, with specific inclusion criteria, the results of our review may not be applicable to different glaucoma populations, especially to people with medically uncontrolled or severe glaucoma. Furthermore, the included studies did not provide data on long‐term efficacy, or visual field progression.
Quality of the evidence
The certainty of the evidence was generally moderate or low, due to risk of bias and imprecision of many of the estimates. Risk of bias was also due to lack of masking of the treating physician and outcome assessor, which could influence the decision to prescribe medications or further surgery. Finally, we remark that unmedicated IOP is a measure of efficacy with respect to medicated IOP, as measured in practice, which is a measure of effectiveness. As stated in the 'Effects of interventions' section, we expected the difference in medicated IOP to be smaller between intervention groups, because the number of medications during follow‐up was higher in the control group, due to the fact that more eye drops are used when target IOP is not achieved.
The protocol for this review aimed to include studies on participants with medically uncontrolled OAG (Otarola 2017). However, all trials were conducted on a mixed population, including many participants with mild to moderate medically controlled OAG. We did not downgrade the certainty of the evidence for indirectness, since this evidence was still useful to evaluate the efficacy and safety of ab interno trabecular bypass surgery with a Hydrus microstent in people with OAG. Of interest, a post‐hoc analysis of HORIZON 2018 was conducted on data from the USA sites (about 60% of the total sample size), which found that unmedicated IOP reduction was achieved, and was possibly greater in participants with a baseline IOP over 26 mmHg, compared to 24 mmHg or less.
All studies obtained visual field testing at baseline, but this was not reported at one or two years.
Potential biases in the review process
Our literature search was systematic, and we contacted the authors of the included studies to obtain additional information, which is still outstanding. However, we decided to publish the review with the available published evidence, and will update it when further data are received from authors and study sponsors.
Agreements and disagreements with other studies or reviews
Lavia 2017 and Agrawal 2018 conducted systematic reviews on several minimally‐invasive glaucoma surgery (MIGS) devices, which included both randomised and non‐randomised studies. Both reviews included Pfeiffer 2015 and concluded that insufficient evidence from RCTs was available on the Hydrus microstent.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs. cataract surgery (CS) alone, outcome: 1.1 Proportion drop‐free: short term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.2 Proportion drop‐free: medium term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.4 Mean change in IOP‐lowering drops taken per day: medium term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.3 Mean change in IOP measured using Goldmann applanation tonometry: medium term

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 2 Proportion drop‐free: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 5 Proportion of participants requiring additional glaucoma surgery or laser.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 6 Adverse events: loss of 2+ VA lines.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 7 Adverse events: IOP spike > 10 mmHg.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 8 Adverse events: bleeding.

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 4 Proportion of participants with IOP < 21 mmHg.
| Cataract surgery with Hydrus microstent compared to cataract surgery alone | ||||||
| Patient or population: people with cataracts and open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with cataract surgery alone | Risk with cataract surgery with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) medium‐term follow‐up at 24 months | Study population | RR 1.63 | 619 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 782 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry medium‐term follow‐up at 24 months | The mean change in unmedicated IOP in the cataract surgery group was ‐5.95 mmHg | The MD in the cataract surgery plus Hydrus group was 2 mmHg lower | ‐ | 619 | ⊕⊕⊕⊝ | |
| Mean change in the number of IOP‐lowering drops instilled per day medium‐term follow‐up at 24 months | The mean change in the number of IOP‐lowering drops instilled per day in the cataract surgery group was ‐0.76 drops | The MD in the cataract surgery plus Hydrus group was 0.41 drops lower | ‐ | 619 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | RR 0.17 (0.03 to 0.86) | 619 | ⊕⊕⊝⊝ | ||
| 25 per 1000 | 4 per 1000 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications medium‐term follow‐up at 24 months | Intraoperative: device malposition (1.6%) or hyphaema obscuring the surgeons view (1.1%) only occurred with Hydrus implantation Postoperative: Intraocular bleeding, loss of 2 or more VA lines, and IOP spikes of 10 mmHg or more were rare in both groups. There were no cases of endophthalmitis in either group | ⊕⊕⊝⊝ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnclear or high risk of bias for most domains (‐1 for risk of bias) | ||||||
| Hydrus microstent compared to iStent trabecular micro‐bypass stent | ||||||
| Patient or population: people with open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with iStent | Risk with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) short‐term follow‐up at 12 months | Study population | RR 1.94 | 148 | ⊕⊕⊝⊝ | ||
| 240 per 1000 | 466 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry short‐term follow‐up at 12 months | The mean change in unmedicated IOP in the iStent group was ‐5.1 mmHg | The MD in the Hydrus group was 3.1 lower | ‐ | 148 | ⊕⊕⊕⊝ | |
| Mean change in number of IOP‐lowering drops instilled per day short‐term follow‐up at 12 months | The mean change in the number of IOP‐lowering drops instilled per day in the iStent group was 0 | The MD in the Hydrus group was 0.6 lower | ‐ | 148 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | not analysed | 148 | ⊕⊝⊝⊝ | ||
| 0/74 | 2/76 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications short‐term follow‐up at 12 months | No intraoperative complications reported. Postoperative: no cases of intraocular bleeding or endophthalmitis in either group. Hydrus: 2/74 cases of VA loss of 2 or more lines, 3/74 IOP spikes > 10 mmHg iStent: 1/76 cases of VA loss of 2 or more lines, 4/76 IOP spikes > 10 mmHg | not analysed | 148 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnmasked investigator | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 2 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.39, 1.83] |
| 2 Proportion drop‐free: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.40, 1.88] |
| 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.69, ‐1.31] |
| 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.56, ‐0.27] |
| 5 Proportion of participants requiring additional glaucoma surgery or laser Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.86] |
| 6 Adverse events: loss of 2+ VA lines Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.50] |
| 7 Adverse events: IOP spike > 10 mmHg Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.24] |
| 8 Adverse events: bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Proportion of participants with IOP < 21 mmHg Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

