Ab interno trabecular bypass surgery with Schlemm´s canal microstent (Hydrus) for open angle glaucoma
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012740.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 09 March 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Eyes and Vision Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Francisco Otarola, Kuang Hu and Catey Bunce wrote the protocol. All authors reviewed and approved the protocol.
Francisco Otarola, Gianni Virgili, Anupa Shah, and Kuang Hu screened the search results, extracted the data from the included studies, and wrote the review. All authors reviewed and approved the review.
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK.
CB acknowledges financial support for her CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology
GG acknowledges support for this research by the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
External sources
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
-
Declarations of interest
FO has no conflict of interest to declare.
GV has no conflict of interest to declare.
AS has no conflict of interest to declare.
KH performs minimally‐invasive glaucoma surgery. He has lectured on 'Constructing clinical trials for MIGS ‐ the lack of evidence and what to do about it' at the Moorfields International Glaucoma Symposium 2016, sponsored by Laboratoires Thea, which is contributing an educational grant to Moorfields Eye Hospital.
CB has no conflict of interest to declare.
GG: In the last five years, GG has received travel funding, and his host organisation has received both educational and unrestricted research funding from pharmaceutical and equipment manufacturers that are involved in the treatment of glaucoma, but none that are otherwise related to (or competing with) the subject of this review.
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Nitin Anand and Jennifer Evans for their comments on the published protocol that forms the template for this one (Hu 2016).
We thank the members of the MIGS Consortium for their input in this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Mar 09 | Ab interno trabecular bypass surgery with Schlemm´s canal microstent (Hydrus) for open angle glaucoma | Review | Francisco Otarola, Gianni Virgili, Anupa Shah, Kuang Hu, Catey Bunce, Gus Gazzard | |
| 2017 Aug 03 | Ab interno trabecular bypass surgery with Schlemm´s Canal Microstent (Hydrus) for open angle glaucoma | Protocol | Francisco Otarola, Kuang Hu, Gus Gazzard, Catey Bunce | |
Differences between protocol and review
-
The follow‐up times for the outcomes were decided after the protocol was published.
-
Two additional co‐authors, A Shah and G Virgili joined the review team.
-
The protocol included combination therapy with phacoemulsification as a separate comparison, and also for subgroup analysis. After discussion within the review team and MIGS Consortium, we opted to include it as a separate comparison, as this is likely to be a different indication.
-
We changed the objectives and removed the restriction to the inclusion of participants with medically uncontrolled glaucoma; explanations are given in the text as appropriate.
-
We added the secondary outcome: rate of visual field progression (DB/time) or proportion of participants whose field loss progressed in the follow‐up period.
-
In the 'Summary of findings' table, intraoperative and postoperative complications were pooled as a single outcome.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
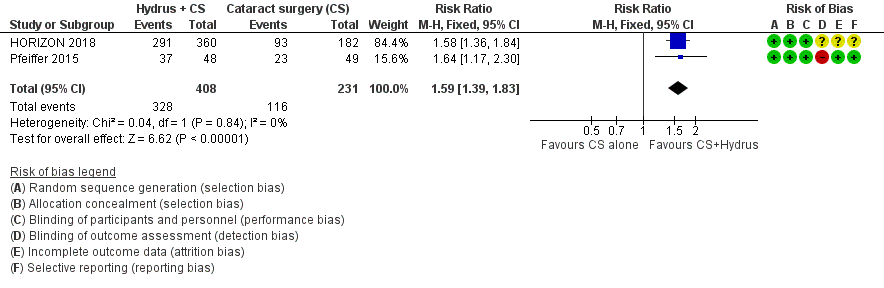
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs. cataract surgery (CS) alone, outcome: 1.1 Proportion drop‐free: short term
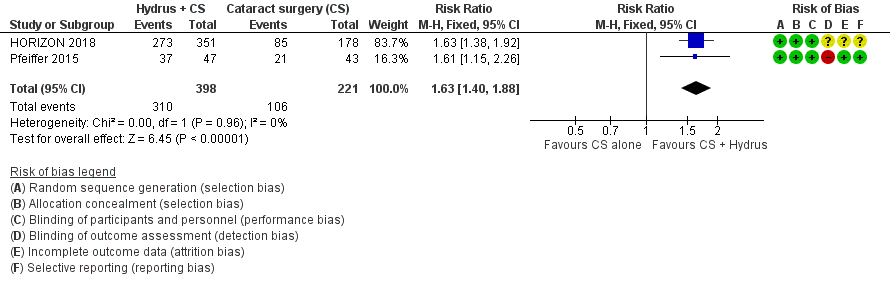
Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.2 Proportion drop‐free: medium term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.4 Mean change in IOP‐lowering drops taken per day: medium term

Forest plot of comparison: 1 Cataract surgery with Hydrus microstent vs cataract surgery (CS) alone, outcome: 1.3 Mean change in IOP measured using Goldmann applanation tonometry: medium term

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 2 Proportion drop‐free: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months).

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 5 Proportion of participants requiring additional glaucoma surgery or laser.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 6 Adverse events: loss of 2+ VA lines.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 7 Adverse events: IOP spike > 10 mmHg.

Comparison 1 Cataract surgery + Hydrus microstent vs cataract surgery (CS) alone, Outcome 8 Adverse events: bleeding.

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 1 Proportion drop‐free: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months).

Comparison 2 Hydrus microstent vs iStent trabecular micro‐bypass stent, Outcome 4 Proportion of participants with IOP < 21 mmHg.
| Cataract surgery with Hydrus microstent compared to cataract surgery alone | ||||||
| Patient or population: people with cataracts and open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with cataract surgery alone | Risk with cataract surgery with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) medium‐term follow‐up at 24 months | Study population | RR 1.63 | 619 | ⊕⊕⊕⊝ | ||
| 480 per 1000 | 782 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry medium‐term follow‐up at 24 months | The mean change in unmedicated IOP in the cataract surgery group was ‐5.95 mmHg | The MD in the cataract surgery plus Hydrus group was 2 mmHg lower | ‐ | 619 | ⊕⊕⊕⊝ | |
| Mean change in the number of IOP‐lowering drops instilled per day medium‐term follow‐up at 24 months | The mean change in the number of IOP‐lowering drops instilled per day in the cataract surgery group was ‐0.76 drops | The MD in the cataract surgery plus Hydrus group was 0.41 drops lower | ‐ | 619 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | RR 0.17 (0.03 to 0.86) | 619 | ⊕⊕⊝⊝ | ||
| 25 per 1000 | 4 per 1000 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications medium‐term follow‐up at 24 months | Intraoperative: device malposition (1.6%) or hyphaema obscuring the surgeons view (1.1%) only occurred with Hydrus implantation Postoperative: Intraocular bleeding, loss of 2 or more VA lines, and IOP spikes of 10 mmHg or more were rare in both groups. There were no cases of endophthalmitis in either group | ⊕⊕⊝⊝ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnclear or high risk of bias for most domains (‐1 for risk of bias) | ||||||
| Hydrus microstent compared to iStent trabecular micro‐bypass stent | ||||||
| Patient or population: people with open angle glaucoma, many of whom had mild or moderate glaucoma, which was well‐controlled with medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with iStent | Risk with Hydrus | |||||
| Proportion of participants who were medication‐free (not using eye drops) short‐term follow‐up at 12 months | Study population | RR 1.94 | 148 | ⊕⊕⊝⊝ | ||
| 240 per 1000 | 466 per 1000 | |||||
| Mean change in unmedicated IOP (after washout) measured using Goldmann applanation tonometry short‐term follow‐up at 12 months | The mean change in unmedicated IOP in the iStent group was ‐5.1 mmHg | The MD in the Hydrus group was 3.1 lower | ‐ | 148 | ⊕⊕⊕⊝ | |
| Mean change in number of IOP‐lowering drops instilled per day short‐term follow‐up at 12 months | The mean change in the number of IOP‐lowering drops instilled per day in the iStent group was 0 | The MD in the Hydrus group was 0.6 lower | ‐ | 148 | ⊕⊕⊝⊝ | |
| Proportion of participants who required further glaucoma surgery, including laser | Study population | not analysed | 148 | ⊕⊝⊝⊝ | ||
| 0/74 | 2/76 | |||||
| Visual field progression | No data available | |||||
| Mean change in health‐related quality of life | No data available | |||||
| Proportion of participants experiencing intraoperative or postoperative complications short‐term follow‐up at 12 months | No intraoperative complications reported. Postoperative: no cases of intraocular bleeding or endophthalmitis in either group. Hydrus: 2/74 cases of VA loss of 2 or more lines, 3/74 IOP spikes > 10 mmHg iStent: 1/76 cases of VA loss of 2 or more lines, 4/76 IOP spikes > 10 mmHg | not analysed | 148 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aUnmasked investigator | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 2 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.39, 1.83] |
| 2 Proportion drop‐free: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.40, 1.88] |
| 3 Mean change in IOP measured using Goldmann applanation tonometry: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.69, ‐1.31] |
| 4 Mean change in IOP‐lowering drops instilled per day: medium‐term (18 to 36 months) Show forest plot | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.56, ‐0.27] |
| 5 Proportion of participants requiring additional glaucoma surgery or laser Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.86] |
| 6 Adverse events: loss of 2+ VA lines Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.50] |
| 7 Adverse events: IOP spike > 10 mmHg Show forest plot | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.24] |
| 8 Adverse events: bleeding Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion drop‐free: short‐term (6 to 18 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in IOP measured using Goldmann applanation tonometry: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in IOP‐lowering drops instilled per day: short‐term (6 to 18 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Proportion of participants with IOP < 21 mmHg Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |