Fármacos antiinflamatorios no esteroideos orales (AINE) para el dolor por cáncer en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012638.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 julio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
RAM, SD, and PW drafted the protocol, and other authors commented on that draft to produce the final version.
SD and RAM searched for studies, carried out data extraction, and assessed risk of bias. SD, RAM, and PW prepared a draft of the full review, and all authors contributed to the final version.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
PW: none known.
SD: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
EDM: none known.
RFB: none known; RFB is a specialised pain physician and has managed patients with cancer pain.
DBC: none known; DBC is a specialised pain physician and has managed patients with cancer pain.
MM: none known.
BW: none known. BW is a specialist Palliative Medicine Consultant physician and manages patients with advanced life threatening illnesses, including cancer.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Acknowledgements
We thank Ke Deng for timely help with obtaining and screening two Chinese papers (excluded studies).
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Institutional support was provided by the Oxford Pain Relief Trust.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jul 12 | Oral nonsteroidal anti‐inflammatory drugs (NSAIDs) for cancer pain in adults | Review | Sheena Derry, Philip J Wiffen, R Andrew Moore, Ewan D McNicol, Rae Frances Bell, Daniel B Carr, Mairead McIntyre, Bee Wee | |
| 2017 Apr 25 | Oral nonsteroidal anti‐inflammatory drugs (NSAIDs) for cancer pain in adults | Protocol | Sheena Derry, Philip J Wiffen, R Andrew Moore, Ewan D McNicol, Rae F Bell, Daniel B Carr, Mairead McIntyre, Bee Wee | |
Differences between protocol and review
Selective reporting bias added to risk of bias.
Notes
A restricted search in January 2019 identified one potentially relevant study [Liu 2017; 342 participants], but we judged the results were unlikely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Liu et al, Combined application of diclofenac and celecoxib with an opioid yields superior effcacy in metastatic bone cancer pain: a randomized controlled trial. Int J Clin Oncol (2017) 22:980–985. DOI 10.1007/s10147‐017‐1133‐y
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
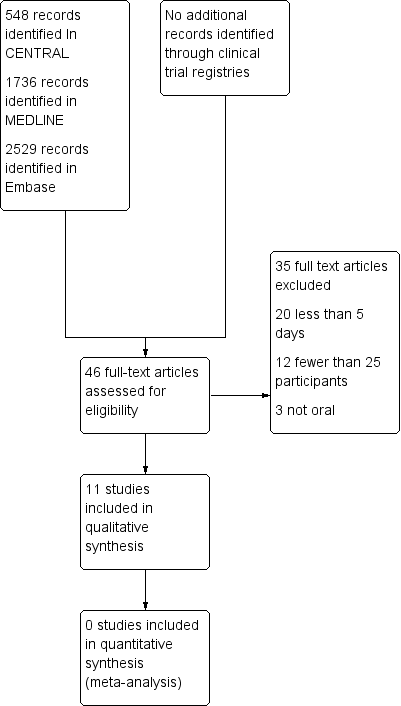
Study flow diagram.
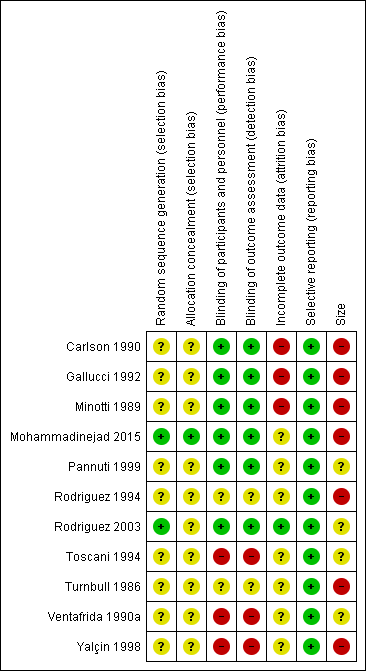
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| NSAID for cancer pain ‐ non‐controlled data | ||||
| Patient or population: people with cancer pain Settings: inpatient or outpatient Intervention: any NSAID, and dose Comparison: no control ‐ cohort of treated participants | ||||
| Outcomes | Probable outcome with NSAID | No of Participants | Quality of the evidence | Comments |
| Participants with at least 30% or at least 50% reduction in pain | No data | No data | Very low | Limited data, several risks of bias |
| PGIC much or very much improved | No data | No data | Very low | Limited data, several risks of bias |
| Pain no worse than mild at one or two weeks (or equivalent) | Range of estimates from 260 in 1000 to 510 in 1000 | 4 studies 415 participants randomised | Very low | Limited data, several risks of bias |
| Serious adverse events | 2 serious adverse events reported | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Adverse events | Dry mouth 10% Loss of appetite 4% Somnolence 9% Dyspepsia 9% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Withdrawals | All cause 23% Lack of efficacy 24% Adverse event 5% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Death | 22 deaths, not clearly related to treatment | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||

