肌内注射与口服皮质类固醇激素以减少急性哮喘患者急诊部出院后复发
Abstract
研究背景
急性哮喘是一个常见的急诊就诊原因, 虽然大多数病人可以出院, 复发需要额外的医疗护理是常见的。全身性皮质类固醇激素是治疗中度到严重度急性哮喘的重要组成部分,然而没有明确的证据表明最有效的给药途径, 以改善从急性护理出院病人的结果。
研究目的
检查在出院前提供的单次肌内 (IM) 皮质类固醇激素与短期口服皮质类固醇治疗急性哮喘患者出院或同等急性期护理的疗效和安全性设置。
检索策略
Cochrane呼吸道组检索了Cochrane呼吸道试验注册库(Cochrane Airways Group Register of Trials),到2018年3月14日。此外,在2017年4月,我们完成了对九个电子数据库的广泛检索,包括Medline、Embase、EBM ALL、Global Health、International Pharmaceutical Abstracts、CINAHL、SCOPUS、Proquest Disserations and Theses Global和LILACS。 我们也检索了灰色文献以获得更多的研究。
标准/纳入排除标准
我们纳入了随机对照试验和对照临床试验,如果它们将肌内注射与口服皮质类固醇激素的有效性进行比较,以治疗到急诊部或类似的急诊护理设置的儿童或成人急性哮喘患者。两位系统综述评论员评进行了研究的纳入和质量的评价。我们通过第三方解决分歧并应用Cochrane偏倚风险工具评估了原始研究的偏倚风险。我们使用GRADE评估了证据的质量。
数据收集与分析
对于二分变量的结局,我们使用随机效应模型以95%置信区间(CIs)的风险比(RR)计算了个体和汇总统计。我们用随机效应模型以95%置信区间(CIs)的平均差(MD)或标准平均差(SMD)报告了连续变量的结局。我们使用I²和Cochran Q统计量报告了异质性。我们使用Cochrane推荐的标准程序。
主要结果
九项涉及804名受试者的研究 (肌内注射 = 402 名受试者; 口服 = 402 名受试者) 符合我们的系统综述的纳入标准。四项试验纳入儿童 (245名受试者),五项研究纳入成人 (559名受试者)。所有的研究都招募了到急诊部的受试者,只有一项研究招募了到初级保健诊所的受试者。所有的儿科研究都比较肌内注射地塞米松和口服泼尼松龙。在成人研究中,所提供的肌内注射皮质类固醇激素包括甲基强的松龙、倍他米松、地塞米松或曲安奈德,而口服类固醇的方案包括波尼松龙,甲基强的松龙,或地塞米松。只有5项研究是安慰剂对照的。为了本系统综述的目的, 我们没有在分析中涉及皮质类固醇剂量当量。最常见给予受试者的联合干预研究包括短效β‐激动剂 (SABA) 、甲基黄嘌呤和溴化异丙托品。在一些情况下,一些研究报告了在急诊部期间向受试者提供补充口服或静脉注射皮质类固醇,主要有激动剂 (SABA) 、甲基黄嘌呤和溴化异丙托品。在出院时向受试者提供的联合干预主要有激动剂 (SABA) 、甲基黄嘌呤、长效β‐激动剂(LABA)和溴化异丙托品。纳入的研究的质量在各个偏倚风险领域评为不清楚到高风险。主要结局是复发导致额外护理,整个结局指标定义为未提前计划地看医生以期改善哮喘症状,或从急诊部出院后需要后续使用皮质类固醇治疗。
我们发现肌内注射与口服皮质类固醇激素在降低复发风险方面同样有效(RR 0.94,95%CI 0.72至1.24;9项研究,804名受试者;I² = 0%;低质量证据)。我们发现儿科和成年受试者之间的复发率没有亚组差异(P=0.71),出院后10天内或10天后复发(P=0.22),或轻度/中度或重度恶化的受试者(P=0.35)。虽然我们发现接受肌内注射的受试者与口服皮质类固醇在副作用的风险方面没有统计学差异(RR 0.83,95%CI 0.64至1.07;5项研究,404名受试者;I² = 0%; 中等质量的证据),估计在每1000名接受肌内注射皮质类固醇治疗的患者中报告经历不良事件的患者减少了50人(95%从106人减少到21人)。我们发现不同研究对具体不良事件的报告不一致。具体不良事件包括恶心和呕吐、疼痛、肿胀、发红、失眠或人格改变,在频率上没有差异。我们没有寻求额外的不良事件的数据。
接受肌内注射皮质类固醇或口服皮质类固醇治疗的受试者均报告呼气流量峰值降低了(MD−7.78L/min,95%CI−38.83L/min至23.28L/min;4项研究,272名受试者;I=33%;中等质量证据),类似的症状持续性(RR 0.41,95%CI 0.14至1.20;3项研究,80名受试者;I² =44%;低质量证据),和24小时β激动剂使用(RR 0.54,95%CI 0.21‐1.37;2项研究,48名受试者;I² =0%;低质量证据)。
作者结论
没有足够的证据来判断肌内注射皮质类固醇在减少复发方面是否比口服皮质类固醇更有效予急诊部或类似的急诊护理机构出院的儿童或成人。虽然我们没有发现统计学差异,但接受肌内注射皮质类固醇的患者报告较少的不良事件。比较肌内注射与口服皮质类固醇的有效性的进一步研究可以提供进一步的证据明晰化。此外,在考虑剂量和药代动力学特性的情况下,需要对不同肌内注射皮质类固醇(例如,肌内注射地塞米松与肌内注射甲基强泼尼松龙) 和不同的口服皮质类固醇(例如,口服地塞米松与口服强的尼松龙)进行比较的研究,以更好地确定最佳的肌内注射或口服类固醇方案,以改善患者的结局。门诊治疗的其他因素,如患者得偏好和潜在的依从性问题,可能决定医生处方。
PICO
Plain language summary
肌内注射与口服皮质类固醇激素以减少急性哮喘
系统综述问题
我们检查了皮质类固醇注射相比口服皮质类固醇的有效性以改善到急诊部或类似的急诊护理设置的急性哮喘患者的结局。
背景
哮喘发作的原因是呼吸道通道的肺部因炎症而收缩, 导致喘息、咳嗽和呼吸困难。哮喘发作的人通常会去急诊部。皮质类固醇激素是一种功能强大的抗炎药物, 是哮喘恶化的治疗基石, 已被证明能有效改善哮喘患者的肺功能, 减少住院。出院时, 患者通常会得到皮质类固醇激素, 以减少因哮喘症状恶化而返回急诊部的几率。皮质类固醇可以通过一次单一的注射进入肌肉 ("肌内注射") 或作为片剂带回家, 目前还不清楚, 哪些方案的皮质类固醇更有效地改善患者从急诊室出院后的结局。
检索日期
我们在2018年3月进行了最新的检索。
研究特点
我们纳入了九项研究比较了皮质类固醇注射与口服皮质类固醇对治疗到急诊部或类似的急诊护理设置的急性哮喘患者的有效性。这些研究总共招收了804名儿童和成人受试者。大多数研究对可注射皮质类固醇地塞米松或甲基强甲泼尼松龙比较与皮质类固醇药泼尼松片或甲基强做出了研究。
研究资金来源
大多数研究没有报告资金来源 (5 项研究)。两项研究获得了整体健康研究补助金的资助。一项研究是由一家制药公司 (辉瑞) 资助;然而, 报告说, 该公司没有参与任何方面的研究或手稿的准备工作。一项研究报告没有得到资金资助。
证据的主要结果
肌内注射皮质类固醇似乎是和皮质类固醇片剂一样有效与预防复发。我们没有发现在接受肌肉注射和皮质类固醇药片的受试者之间复发的风险有什么不同。虽然并非所有研究都报告它们的研究小组有不良影响, 但我们发现接受肌肉注射和皮质类固醇药片的受试者之间并没有差异。在随访中, 我们发现接受肌肉注射或皮质类固醇药片的参与者在肺功能测试中没有差异。在报告症状评分和持续时间的研究中, 我们没有发现接受注射皮质类固醇或片剂的受试者之间有什么差异。
证据的质量
肌肉注射皮质类固醇激素在改善健康结局方面的的证据的质量从低到中等。我们对肌肉激素对住院的估计影响, 对呼吸功能的改善和复发的评估, 仅有中等把握, 因为纳入的研究存有误差风险。
Authors' conclusions
Summary of findings
| Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma | |||||
| Patient or population: patients with acute asthma | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Oral corticosteroids | Intramuscular corticosteroids | ||||
| Relapse | 201 per 1000 | 12 fewer per 1000 | RR 0.94 | 804 | ⊕⊕⊝⊝ |
| Relapse within 10 days post‐discharge | 154 per 1000 | 40 fewer per 1000 (from 75 fewer to 11 more) | RR 0.74 | 742 | ⊕⊕⊕⊝ |
| Relapse occurring after 10 days post‐discharge | 245 per 1000 | 2 fewer per 1000 | RR 0.99 | 556 | ⊕⊕⊝⊝ |
| Adverse events | 294 per 1000 | 50 fewer per 1000 | RR 0.83 | 404 | ⊕⊕⊝⊝ |
| Pulmonary function: Peak expiratory flow | The mean pulmonary function: peak expiratory flow ranged across control groups from | The mean pulmonary function: peak expiratory flow in the intervention groups was | 272 | ⊕⊕⊕⊝ | |
| Symptom persistence | 537 per 1000 | 317 fewer per 1000 | RR 0.41 | 80 | ⊕⊕⊝⊝ |
| 24‐hour beta agonist use | 375 per 1000 | 172 fewer per 1000 | RR 0.54 | 48 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level for risk of bias. Majority of studies received an unclear risk of bias for random sequence generation and selective outcome reporting | |||||
Background
Description of the condition
Asthma is a chronic inflammatory disease of the airways of the lungs that affects both children and adults. It is estimated that 300 million people worldwide have asthma (Croisant 2014). In the United States, the prevalence of asthma has increased from 7.3% to 8.2% from 2001 to 2009 (Croisant 2014). Acute asthma, characterised by worsening cough, wheezing, shortness of breath, or chest tightness, is a common cause of presentation to the hospital emergency department (ED) or similar acute care settings. Most patients with acute asthma presenting to the ED can be safely managed with interventions including systemic corticosteroids, anticholinergics, or beta₂‐agonists, and discharged home (Rowe 2009). However, approximately 10% to 18% (Emerman 1999; Emerman 2001; Rowe 2015; Topal 2014), and up to 31% (Ducharme 1993), of patients will relapse and return to the ED or other acute care settings with acute exacerbations of asthma within the following four weeks. In the US, it is estimated that the economic cost of asthma in 2007 was approximately USD 56 billion due to medical costs and missed work and school days (CDC 2011). Identifying effective treatment options to help patients manage their symptoms after discharge from acute care and reducing the proportion of patients who relapse are important management issues designed to improve health outcomes for patients with asthma.
Description of the intervention
Systemic corticosteroids are potent general anti‐inflammatory agents for the treatment of asthma (Alangari 2014). When given in the ED, systemic corticosteroids can reduce hospitalisations and improve lung function in patients with acute asthma (Rowe 2001). A Cochrane Review reported significant decreases in symptom scores when patients were given systemic corticosteroids at or following discharge from the ED (i.e. an IM injection at discharge, or oral corticosteroids to be taken at home over the subsequent 3 to 8 days). Heterogeneity in outcome reporting prohibited meaningful pooling (Rowe 2007a). Treatment with systemic corticosteroids at discharge has also been shown to prevent relapse (Rowe 2007a). Current guidelines recommend systemic corticosteroids at ED discharge for all but the mildest presentations of acute asthma in an effort to reduce future relapse (GINA 2017). While systemic corticosteroids can effectively mitigate asthma relapses, the optimal route of administration is less clear.
How the intervention might work
At discharge from the ED or acute care setting, systemic corticosteroids may be administered via intramuscular (IM) or oral routes. A single dose of IM corticosteroids has long‐acting pharmacokinetic properties with fewer side effects associated with nausea/vomiting; however, adverse events associated with the IM injection (i.e. pain and swelling around the injection site) are well known to occur (Lahn 2004). Oral corticosteroids have short‐acting properties, and patients are typically provided with a short course of oral corticosteroids for five to seven days (GINA 2017). While no injection is needed, side effects associated with oral corticosteroids often include nausea and vomiting, and adherence/compliance with oral corticosteroid regimens is often sub‐optimal (Ducharme 2011). Although IM corticosteroids represent an alternative treatment option for patients with intolerance to oral agents or patients where adherence/compliance concerns exist, it is unclear whether IM corticosteroids are as effective as oral corticosteroids in mitigating relapse.
Why it is important to do this review
The effectiveness of systemic corticosteroids is known (Rowe 2007a), and widely accepted by clinicians; however, whether patients benefit more or less from IM or oral corticosteroids is less clear. This review provides supplemental information with another review of different oral corticosteroid regimens for acute asthma (Normansell 2016). An umbrella review reported no differences in relapse events in adults after treatment with IM or oral corticosteroids for acute asthma (Krishnan 2009); however, this review was limited to English‐language studies, and since its publication no other systematic reviews have been conducted that have used an extensive literature search to synthesize all of the available evidence from studies that have compared IM to oral corticosteroids.
Objectives
To examine the effectiveness and safety of a single dose of intramuscular (IM) corticosteroids provided prior to discharge compared to a short course of oral corticosteroids in the treatment of acute asthma patients discharged from an ED or equivalent acute care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT) in this systematic review. We also included studies reported in full text, those published as an abstract only, and unpublished data in our search criteria.
Types of participants
We considered for inclusion in the review studies that included children or adults presenting to a hospital ED or other equivalent acute care setting with an uncomplicated exacerbation of asthma. For the purposes of this review, we defined 'uncomplicated exacerbations' as patients whose acute exacerbation of asthma was the primary reason for presentation to the ED, with no coexisting complications (e.g. no evidence of pneumonia, pneumothorax, etc.). The asthma diagnosis had to be made either using international/national clinical guidelines, or spirometric criteria, or both. We excluded from this review studies that focused on corticosteroid treatment in hospitalised participants. We only included studies that assessed patients with acute asthma who were treated and discharged from the ED or other urgent care/acute care setting. None of the included studies enrolled participants with both chronic obstructive pulmonary disease (COPD) and asthma; however, we had determined a priori that if this were to be the case, we would only include these studies if they provided results of asthma participants separately from COPD participants, or if the study population of COPD participants consisted of less than 20% of the total population.
Types of interventions
We included studies that compared a single dose of IM corticosteroids given prior to discharge versus a short course (one to 14 days) of oral corticosteroids. There were no restrictions on the IM or oral corticosteroids used, or the dosage. The single dose of IM corticosteroids may have been administered to participants at any point prior to discharge from the ED or other equivalent acute care setting. The short course of oral corticosteroids may have been started at any point prior to discharge or have been provided to participants to be taken over a period of a few days (one to 14 days) post‐discharge. We did not set any restrictions on the type of co‐interventions participants could receive (e.g. beta₂‐agonists, systemic or inhaled corticosteroids (ICS), anticholinergics, theophylline compounds, or antihistamines) during their stay in the ED/acute care setting; however, we did not permit other prescriptions of corticosteroids.
Types of outcome measures
Primary outcomes
The primary dichotomous outcome was relapse to additional care defined as an unscheduled visit to a health practitioner for worsening asthma symptoms, or requiring subsequent treatment with corticosteroids, which may have occurred at any time point after discharge from the ED. We also accepted the occurrence of relapse at any point as well as whether the occurrence of relapse was reported via self‐report or verification via health records.
Secondary outcomes
-
The occurrence of serious adverse events (e.g. hospitalisation; intensive care unit (ICU) admission; death; relapse for visits other than worsening symptoms of asthma).
-
Adverse events (e.g. pain, cellulitis/abscess, gastrointestinal bleeding, vomiting, behavioural/mental health exacerbations, abdominal pain, insomnia, hyperphagia/weight gain, skin eruptions, etc.).
-
Continuous data from pulmonary function testing (including peak expiratory flow (PEF), absolute/per cent change in PEF from baseline; forced expiratory volume in one second (FEV₁), and absolute/per cent change in FEV₁ from baseline).
-
Continuous data from symptom scores, measured via validated scales.
-
Duration of symptoms (days until symptom free).
-
Descriptive analysis of compliance/adherence with oral (corticosteroid or placebo) treatment.
-
Quality of life measures measured via validated scale.
-
The number of beta₂‐agonists doses taken by participants within a 24‐hour period of discharge.
Reporting one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register (CATR), which is maintained by the Information Specialist for the Cochrane Airways Group. The CATR contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, through the Cochrane Register of Studies Online (http://crso.cochrane.org/).
-
Weekly searches of MEDLINE Ovid SP.
-
Weekly searches of Embase Ovid SP.
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature).
-
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the CATR are identified through search strategies based on the scope of the Cochrane Airways Group. Details of these strategies, as well as a list of handsearched conference proceedings, are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review. This search was completed on 14 March 2018.
In addition, an expert medical librarian (SC) conducted a supplemental search of nine electronic databases including MEDLINE (Appendix 3), Embase (Appendix 4), CINAHL (Appendix 5), Proquest Dissertation Abstracts and Theses Global (Appendix 6), SCOPUS (Appendix 7), PROSPERO (Appendix 8), the Cochrane Library (Appendix 9), and LILACS (Appendix 10) using controlled vocabulary and key words.
We also conducted an extensive search of the grey literature including ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), Google Scholar, bibliographies of included studies and relevant reviews, and SCOPUS forward search of a sentinel paper; and a handsearch of medical conference abstracts from 2008 to 2017 including the Canadian Journal of Emergency Medicine, Academic Emergency Medicine, and Annals of Emergency Medicine.
We conducted all searches without any restrictions on language or publication status.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched relevant manufacturers' web sites for study information.
We searched for errata or retractions from included studies published in full text on PubMed and planned to report the date this was done within the review.
Data collection and analysis
Selection of studies
Two review authors (SWK and EC) screened the titles and abstracts of the search results independently and coded them as either potentially eligible or ineligible. We then retrieved the full‐text study reports of all potentially eligible studies and two review authors (SWK and EC) independently screened them for inclusion and recorded the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (CVR or BHR). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted on at least one included study in the review. Two review authors (SWK and EC) independently extracted the following study characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number, mean age, age range, sex, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for studies and notable conflicts of interest of study authors.
Two review authors (SWK and EC) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if an included study did not report outcome data in a usable way. We resolved disagreements by consensus or by involving a third review author (CVR or BHR). One review author (SWK) transferred data into the Review Manager 5 (RevMan 5) file (Review Manager 2014). To ensure that the data was entered correctly, a second review author (EC) verified the extracted data for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (SWK and EC) assessed risk of bias independently for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (CVR or BHR). We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We judged each potential source of bias as high risk, low risk, or unclear risk and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias relates to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that may have contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and justify any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Dichotomous data are reported as relative risk (RR) values and continuous data are reported as mean difference (MD) or standardised mean difference (SMD), as appropriate. If we combined data from rating scales in a meta‐analysis, we ensured we entered them with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only when meaningful; that is, if the treatments, participants, outcomes and the underlying interventions were similar enough for pooling to make sense.
We provided a narrative description of skewed data reported as medians and interquartile ranges (IQR).
In individual studies with multiple arms, we included only the study arms assessing IM and oral corticosteroids. If we combined two comparison groups (e.g. IM corticosteroids versus different regimens of oral corticosteroids) in the same meta‐analysis, we either combined the active study arms or halved the control group to avoid double‐counting.
If a study reported outcomes at multiple time points, we used the last time point measured.
We conducted an 'as reported' and intention‐to‐treat (ITT) analysis of the primary outcome. We assessed the secondary outcomes using an 'as reported' analysis.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of individuals who relapsed rather than number of relapses per individual).
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. if we identified a study as an abstract only). Where this was not possible and the missing data had the potential to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity we reported it and explored the possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We were unable to pool more than 10 studies, so we were unable to create a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes including: all relapse, relapse within 10 days post‐discharge, relapse after 10 days post‐discharge, adverse events, PEF/FEV₁, symptom scores, and beta₂‐agonist use in a 24‐hour period. We used the five GRADE considerations (i.e. risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and used GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We examined potential sources of heterogeneity in the primary outcome in the following subgroup analyses.
-
Children (zero to 18 years of age) versus adults (18 years of age and older) to examine any potential age‐specific treatment effects of IM or oral corticosteroids.
-
Relapse occurring within 10 days and over 10 days post‐discharge.
-
Low versus moderate versus high exacerbation severity based on the pulmonary function taken at the time of the participant's presentation to the ED, prior to treatment with a bronchodilator.
-
Co‐interventions received (ICS versus ICS corticosteroids/long‐acting beta₂‐agonists (LABA)).
We used the formal test for subgroup interactions in RevMan 5 (Review Manager 2014). We restricted the subgroup analysis to relapse.
Sensitivity analysis
We carried out the following sensitivity analyses, by removing the following types of studies from the primary outcome analyses.
-
Studies that we consider to be at high risk of bias based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Studies in which the duration of oral corticosteroid treatment was less than five days.
-
The results from fixed‐effect models were compared with the random‐effects models for the main outcome.
-
Studies in which supplemental corticosteroids were provided to the patients in the ED as a co‐intervention.
Results
Description of studies
Please see Characteristics of included studies for additional information on the included studies.
Results of the search
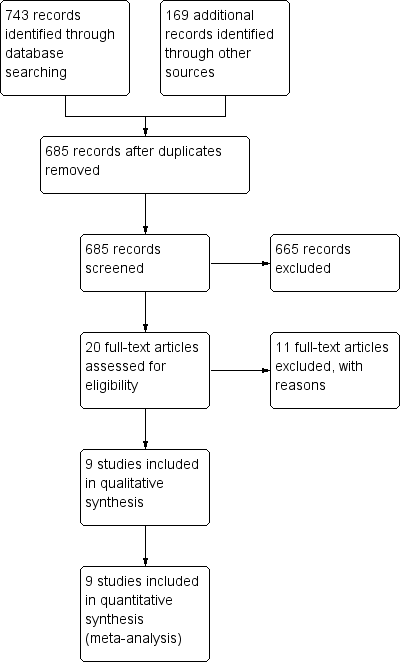
The literature searches identified 912 studies (see Figure 1). Removing duplicates resulted in 685 potentially eligible studies overall. We selected 20 studies for full‐text review, while we excluded the remaining 665 studies, based on lack of relevance. We excluded 11 studies after full‐text review (see Excluded studies). We included the remaining nine studies in the review. We identified one of the eligible studies by grey literature search (Al‐Wahadneh 2006). A search of errata or retractions in September 2017 did not identify any retracted publications. We included insufficient studies in this review for an assessment of publication bias (i.e. by a funnel plot). We made every effort to locate all available studies through an extensive search of the literature, with no limitations set on year of publication or language of publication.

Study flow diagram.
Included studies
Publication
The first study to compare the effectiveness of IM versus oral corticosteroids in participants with acute asthma discharged from an acute care setting was published in 1988 (Hoffman 1988) while the most recent study was published in 2007 (Gordon 2007). The majority of studies were published in the United States (Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Schuckman 1998), while the remaining studies were published in Canada (Chan 2001), Jordan (Al‐Wahadneh 2006), and Taiwan (Lee 1993). All of the studies were RCTs published in peer‐reviewed journals.
Participants/Setting
The nine studies enrolled a total of 804 participants. Overall, the studies were small clinical trials, with a range of 17 to 187 participants across the studies. Five of the studies enrolled fewer than 90 participants each (Al‐Wahadneh 2006; Gries 2000; Hoffman 1988; Klig 1997; Lee 1993). Five of the included studies enrolled adults (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998), while the remaining four enrolled children (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The age of eligible children included in the studies ranged from 9 months to 14 years (Al‐Wahadneh 2006), 18 months to 7 years (Gordon 2007), 6 months to 7 years (Gries 2000), and 3 to 16 years (Klig 1997). Among the adult studies, the mean ages of participants ranged from 31 to 42 years old (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998), while the mean age of children ranged from 3 to 6.8 years old (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). Most of the included studies recruited more male participants, except in three studies where women comprised over 50% of participants (Chan 2001; Lahn 2004; Schuckman 1998). Only three studies reported smoking history (Chan 2001; Lahn 2004; Schuckman 1998). Chan 2001 reported that current smokers made up 31% to 33% of the participants in the IM and oral corticosteroid groups respectively. Tobacco use ranged from 35% to 38% of participants randomised to the IM and oral corticosteroid groups respectively in Lahn 2004, while Schuckman 1998 reported a range of 64% to 74% of participants randomised to receive IM or oral corticosteroids had ever smoked. All of the studies recruited participants with acute asthma presenting to an ED, except one study which recruited participants with acute asthma attending a primary care clinic (Gries 2000). We attempted to estimate and categorize exacerbation severity among the included studies based on the reported baseline pulmonary function of the included studies (Table 1). We considered studies that reported a baseline average PEF of greater than 200 L/min of the groups to be mild/moderate (Chan 2001; Lahn 2004; Lee 1993; Schuckman 1998). We considered the exacerbation severity of patients enrolled in Hoffman 1988 to be severe, as the baseline PEF of included patients was less than 150 L/min. Four studies did not report baseline pulmonary function and so exacerbation severity could not be estimated (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997).
| Studies | Pulmonary function: Eligibility criteria | Exacerbation severity |
| Severity estimated using modified scoring system based on GINA guidelines. Reported to enrolling patients with mild‐moderate exacerbations, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported mean baseline PEF greater than 200 L/min: IM group: 270 L/min (SD: 103); oral group: 261 L/min (SD: 104). | Mild/moderate | |
| Reported to enrolling patients identified as moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Applied adapted exacerbation severity score (unspecified). Reported to enrolling patients rated as mild/moderate, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported baseline mean PEF of enrolled patients of less than 150 L/min: IM group: 129 L/min (SD:14); oral group: 141 L/min (SD: 14). | Severe | |
| Exacerbation severity estimated via pulmonary index score. Study reported to enrolling patients with mild/moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Eligibility criteria required patients to have a PEFR of ≤ 70% predicted with a minimum PEFR of ≥ 40%. Reported PEF of enrolled patients was ≥ 200 L/min: IM group: 205 L/min (SD: 70); oral group: 209 L/min (SD: 72). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 210 L/min (SD: 30); oral group: 208 L/min (SD: 26). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 243.6 L/min (SD: 64); oral group: 244.7 L/min (SD: 83). | Mild/moderate |
Abbreviations:
GINA = Global Initiative for Asthma; PEF = Peak expiratory flow; PEFR = Peak expiratory flow rate; IM = intramuscular; SD = standard deviation
Intervention
All of the paediatric studies provided their participants with a single dose of IM dexamethasone (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997), while the IM corticosteroid provided in adult studies ranged from methylprednisolone (Hoffman 1988; Lahn 2004), to betamethasone (Chan 2001), dexamethasone (Lee 1993), and triamcinolone (Schuckman 1998). All of the participants randomised to receive IM corticosteroids received a single dose of IM corticosteroids prior to discharge. Only five studies were placebo controlled, in which all participants receiving IM corticosteroids were also provided with a course of oral placebo tablets (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). There was significant variation between the IM corticosteroid used, as well as the dosing. We provide additional information on the IM corticosteroids used in Characteristics of included studies and Table 2. We estimated corticosteroid equivalency to methylprednisolone using an equivalence converter (www.medcalc.com/steroid.html). Overall, the median dose was 78 mg with a range of 40 mg to 120 mg (Table 2).
| Studies | Location/setting | Co‐interventions | Corticosteroid doses and durations | Methyprednisolone equivalency | Relapse outcome |
| Jordan, ED | Provided in ED: not stated Provided at discharge: SABA | Dexamethasone (IM) 1.7 mg/kg Mean dose: 24 mg Single dose Prednisolone (oral) 2 mg/kg/day for 5 days Mean dose: 19.2 mg per day Total dose: 96 mg | IM Methylprednisone equivalency: 120 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 76.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/16 Day 21 Oral group 3/14 Day 21 | |
| Canada, ED | Provided in ED: SABA, methylxanthines, supplemental oral/IV corticosteroids Provided at discharge: Methylxanthines, unspecified inhaled beta₂‐agonists, and ICS | Betamethasone (IM) 12 mg Single dose. Received placebo capsules over 7 days. Prednisone (oral) 50 mg a day for 7 days. Received a single placebo injection Total dose: 350 mg | IM Methylprednisone equivalency: 72 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 280 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 12/86 Day 7 Oral group 19/82 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA, ipratropium bromide. IV corticosteroids for patients who vomited oral corticosteroids. Provided at discharge: Inhaled beta₂‐agonists and ICS | Dexamethasone (IM) 0.6 mg/kg (max 16 mg) Single dose Prednisolone (oral) 2 mg/kg (max 50 mg) daily for 5 days Total: 250 mg | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12‐36 hours) Oral Methylprednisone equivalency: 200 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 8/69 Day 4 Oral group 11/73 Day 4 IM group 15/68 Day 14 Oral group 16/73 Day 14 | |
| United States, Tertiary medical center | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) Patients 6 to 12 months old received 16 mg. Patients 13 to 35 months old received 24 mg. Children ≥ 36 months received 36 mg. Single dose Prednisone (oral) 2 mg/kg a day for 5 days Total dose: unclear | IM Methylprednisone equivalency: Unable to assess Oral Methylprednisone equivalency: Unable to assess | IM group 1/15 Day 28 Oral group 3/17 Day 28 | |
| United States, ED | Provided in ED: SABA, epinephrine, methylxanthines, IV corticosteroids Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Methylprednisonlone (IM) 80 mg Single dose. Received placebo capsules for 7 days Methylprednisolone (oral) Tapering dose over 7 days. Total dose: 216 mg Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 216 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/8 day Day 7 Oral group 2/10 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) 0.3 mg/kg Total dose: 15 mg Single dose Prednisone (oral) 2 mg/kg a day for 3 days Total dose: 100 mg | IM Methylprednisone equivalency: 75 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/21 Day 5 Oral group 0/21 Day 5 | |
| United States, ED | Provided in ED: inhaled beta₂‐agonists, IV corticosteroids Provided at discharge: SABA | Methylprednisolone (IM) 160 mg Single dose. Received placebo capsules for 8 days Methylprednisolone (oral) Tapering dose over 8 days. Total dose: 160 mg (tapering dose 32 mg day 1). Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 13/92 Day 10 Oral group 12/88 Day 10 IM group 17/92 Day 21 Oral group 20/88 Day 21 | |
| Taiwan, ED | Provided in ED: SABA, methylxanthines Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Dexamethasone (IM) 10 mg Single dose. Received placebo capsules for 7 days Dexamethasone (oral) Tapering dose over 7 days. 11.75 mg total. Received placebo injection | IM Methylprednisone equivalency: 50 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 58.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/17 Day 7 Oral group 0/19 Day 7 | |
| United States, ED | Provided in ED: SABA, oral/IV corticosteroids Provided at discharge: SABA, antibiotics, ICS, cromolyn sodium, ipratropium bromide | Triamcinolone (IM) 40 mg Single dose. Received placebo capsules for 5 days Prednisone (oral) 40 mg a day for 5 days. Total dose: 160 mg Received placebo injection | IM Methylprednisone equivalency: 40 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 160 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 7/78 Day 7 Oral group 11/76 Day 7 |
ED = emergency department; SABA = short‐acting beta₂‐agonists; LABA = long‐acting beta₂‐agonists; IV = intravenous; IM = intramuscular; ICS = inhaled corticosteroids
Comparison
All of the paediatric studies compared IM dexamethasone to oral prednisone/prednisolone (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The oral corticosteroid regimen provided in the adult studies consisted of either prednisone (Chan 2001; Schuckman 1998), methylprednisolone (Hoffman 1988; Lahn 2004), or dexamethasone (Lee 1993). The paediatric studies tended to provide participants a shorter course of oral corticosteroids, lasting from three (Klig 1997) to five days (Al‐Wahadneh 2006; Gordon 2007; Gries 2000). The course of oral corticosteroids tended to last slightly longer in the adult studies, ranging from five (Schuckman 1998), seven (Hoffman 1988; Lee 1993), to eight days (Lahn 2004). Six studies provided a consistent dose of oral corticosteroids (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Klig 1997; Schuckman 1998) while three studies provided a tapering dose (Hoffman 1988; Lahn 2004; Lee 1993). All participants randomised to receive oral corticosteroids were provided with their medications prior to discharge which was to be taken at home over the next 3 to 8 days. Only five studies were placebo controlled, in which all participants receiving oral corticosteroids were provided with IM placebo (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). We present additional information on the oral corticosteroids provided in Characteristics of included studies and Table 2. We estimated corticosteroid equivalency to methylprednisolone using an equivalence converter (www.medcalc.com/steroid.html). Overall, the median dose was 116 mg with a range of 58.8 mg to 280 mg (Table 2).
Co‐interventions
The co‐interventions provided varied among the included studies (see Characteristics of included studies and Table 2). In some cases, all participants received standardised co‐interventions (Al‐Wahadneh 2006; Gordon 2007; Hoffman 1988; Gries 2000; Klig 1997; Lee 1993; Schuckman 1998), while another study reported that participants received non‐standardized co‐interventions at the discretion of the attending physicians (Chan 2001). The most common co‐interventions provided to participants during the acute care visit included: SABA (Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998), methylxanthines (Chan 2001; Hoffman 1988; Lee 1993), and ipratropium bromide (Gordon 2007). In some instances, some studies reported providing participants with supplemental oral (Chan 2001; Schuckman 1998) or IV (Chan 2001; Hoffman 1988; Schuckman 1998) corticosteroids during their stay in the ED. Gordon 2007 provided participants with IV corticosteroids if they vomited the oral corticosteroids while in the ED. Co‐interventions provided to participants at discharge consisted primarily of SABA (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997; Lahn 2004; Schuckman 1998), methylxanthine (Chan 2001; Hoffman 1988; Lee 1993), LABA (Chan 2001), and unspecified inhaled beta₂‐agonists (Hoffman 1988). Supplemental corticosteroids provided at discharge were limited across the included studies. Chan 2001 and Gordon 2007 reported that the attending ED physician decided whether or not to provide participants with ICS at discharge. Participants in Lahn 2004 received ICS at discharge if they were using them previously.
Outcomes
All included studies reported relapse to additional care, with a measurement range of four to 28 days after discharge. Seven studies assessed relapse within 10 days post‐discharge (Chan 2001; Gordon 2007; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998), while five studies measured relapse occurring more than 10 days post‐discharge (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Lahn 2004). Three studies assessed relapse at two different time points after discharge (Chan 2001; Gordon 2007; Lahn 2004). In comparison to relapse, we found infrequent reporting and inconsistent measurement of our proposed secondary outcomes.
Excluded studies
We excluded 11 studies from this review, mostly due to inappropriate study design (not an RCT) (Andrews 2012; Droszcz 1981; Droszcz 1985; Ducharme 2004; Green 1995; Hofmann 2008; Kelso 2014; Ozpenpe 2011; Razi 2006; Watnick 2016; White 2010) (See Characteristics of excluded studies).
Risk of bias in included studies
Overall, the risk of bias of the included studies ranged from unclear to high and none of the studies had an overall low risk of bias (see Figure 2; Figure 3). Of the nine studies assessed, we rated four as having high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997), while we assessed the remaining studies as having an unclear risk of bias (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies provided adequate information on randomisation to allow for an assessment of low risk of bias for sequence generation (Lahn 2004; Lee 1993; Schuckman 1998). We considered the remaining studies to be at unclear risk of bias due to missing information on randomisation of participants (Al‐Wahadneh 2006; Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997). Five studies had a low risk of bias with regard to allocation concealment (Chan 2001; Gordon 2007; Klig 1997; Lahn 2004; Schuckman 1998), while the remaining studies did not provide enough information to make a clear judgement on the risk of bias on allocation concealment (Al‐Wahadneh 2006; Gries 2000; Hoffman 1988; Lee 1993).
Blinding
Given the feasibility of employing a placebo‐controlled study design, and that many outcomes of interest were collected via participant self‐report, we considered the primary and secondary outcomes of interest may have been susceptible to potential performance and detection bias. We considered three studies to have adequately blinded participants and personnel (Chan 2001; Lahn 2004; Schuckman 1998). Four studies did not blind participants or personnel, and we judged them to be at high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). We considered two studies to be at unclear risk of bias due to a lack of information provided (Hoffman 1988; Lee 1993). Five studies reported adequate blinding of outcome assessors (Chan 2001; Gordon 2007; Gries 2000; Klig 1997; Schuckman 1998). We considered one study to be at high risk of bias because they did not blind outcome assessors (Al‐Wahadneh 2006), while we considered three studies to be at unclear risk of bias due to a lack of available information on the blinding of outcome assessors (Hoffman 1988; Lahn 2004; Lee 1993).
Incomplete outcome data
We assessed two studies as having an unclear risk of bias due to a lack of information on attrition (Al‐Wahadneh 2006; Lee 1993). We assessed the remaining studies as having a low risk of bias (Chan 2001; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Schuckman 1998).
Selective reporting
We considered only one study to be at low risk of bias for reporting bias; we received a study protocol upon request from the study authors (Chan 2001). We considered the remaining studies at be at unclear risk of reporting bias due to the lack of an available protocol (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Hoffman 1988; Klig 1997; Lahn 2004; Lee 1993; Schuckman 1998).
Other potential sources of bias
We considered four studies to be at low risk of bias for other potential sources of bias (Chan 2001; Klig 1997; Lahn 2004; Schuckman 1998). Three studies reported their source of funding in the included article (Chan 2001; Lahn 2004; Schuckman 1998), while we found additional information regarding study funding in one study (Klig 1997). Two studies received funding from general health research grants (Chan 2001; Schuckman 1998). One study received pharmaceutical company (Pfizer) sponsorship but reported that Pfizer was not involved in any aspect of the study or manuscript preparation (Lahn 2004). One study was unfunded (Klig 1997). We considered five studies to be at unclear risk of bias because the studies did not state their source of funding (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Hoffman 1988; Lee 1993). We did not consider any study to be at high risk of bias for other potential sources of bias.
Effects of interventions
See summary of findings Table for the main comparison for a summary of the main comparisons. Insufficient reporting prevented meaningful meta‐analysis of several proposed secondary outcomes, including symptom scores; relapse requiring hospitalisation; and quality of life; and specific adverse events such as pain; swelling; redness/bruising; personality changes; and insomnia.
Primary Outcome
Relapse
We detected no difference in the proportion of patients who relapsed between participants receiving IM versus oral corticosteroids (RR 0.94, 95% CI 0.72 to 1.24; 9 studies, 804 participants = 804; I² = 0%; Analysis 1.1) with low heterogeneity (I² = 0%). An ITT analysis revealed similar results (RR 0.95, 95% CI 0.72 to 1.26; 9 studies, 821 participants; I² = 0%; Analysis 1.2).
Subgroup analysis
A subgroup analysis comparing the effectiveness of IM versus oral corticosteroids to reduce relapse in paediatric (RR 0.86, 95% CI 0.48 to 1.53; 4 studies, 245 participants; I² = 0%) or adult (RR 0.97, 95% CI 0.71 to 1.33; 5 studies, 559 participants; I² = 0%) participants revealed no subgroup differences (P = 0.71; Analysis 1.3). In addition, we found no subgroup differences between studies assessing relapse within 10 days (RR 0.74, 95% CI 0.51 to 1.07; 7 studies, 742 participants; I² = 0%) or greater than 10 days post‐discharge (RR 0.99, 95% CI 0.74 to 1.33; 5 studies, 556 participants; I² = 0%) (P = 0.22; Analysis 1.4). It is important to note, however, that for the relapse greater than 10 days post‐discharge subgroup, it is unclear whether this includes relapses occurring within 10 days. We found no subgroup differences (P = 0.35; Analysis 1.5) in relapse between studies estimated to have enrolled participants with mild/moderate (RR 0.98, 95% CI 0.71 to 1.34; 4 studies, 539 participants; I² = 0%) versus severe exacerbations (RR 0.24, 95% CI 0.01 to 4.47; 1 study, 18 participants; I² = 100%). We could not complete the proposed co‐interventions subgroup comparing the effects of ICS versus ICS/LABA on relapse as planned due to a lack of studies providing information on the use of ICS and ICS/LABA agents as co‐interventions.
Sensitivity analysis
We conducted a sensitivity analysis in which we removed from the meta‐analysis the four studies considered to be at high risk of bias (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). We identified no difference in relapse proportions between participants receiving IM or oral corticosteroids (RR 0.97, 95% CI 0.71 to 1.33; 5 studies, 559 participants; I² = 0%; Analysis 1.6). An additional sensitivity analysis, in which studies providing participants with an oral corticosteroid regimen of less than five days (Klig 1997), revealed no difference in relapse rates between participants receiving IM or oral corticosteroids (RR 0.94, 95% CI 0.72 to 1.24; 8 studies, 762 participants; I² = 0%; Analysis 1.7). Finally, fixed effects revealed similar findings to that of random effects (RR 0.91, 95% CI 0.69 to 1.19; 9 studies, 804 participants; I² = 0%; Analysis 1.8). Removing studies in which patients received additional corticosteroids as a co‐intervention during the patients' stay in the ED revealed no differences in relapse rates between participants receiving IM or oral corticosteroids (RR 0.76, 95% CI 0.45 to 1.29; 5 studies, 320 participants; I² = 0%; Analysis 1.9).
Secondary outcomes
Serious adverse effects
Insufficient reporting of serious adverse events prohibited any meaningful meta‐analysis. Only one study reported on participants relapsing and requiring hospitalisation or ICU admission by 14‐day follow‐up; however, the effect estimate is imprecise (RR 1.43, 95% CI 0.33 to 6.16; 1 study, 141 participants; Analysis 1.10) (Gordon 2007).
Adverse events
Across the included studies we found poor documentation and inconsistent measurement of adverse events associated with the use of IM and oral corticosteroids, resulting in few meaningful comparisons. Overall, a meta‐analysis did not reveal whether participants receiving IM corticosteroids are more or less likely to experience any adverse events compared to participants receiving oral corticosteroids (RR 0.83, 95% CI 0.64 to 1.07; 5 studies, 404 participants; I² = 0%; Analysis 1.11). In some cases, the occurrence of specific adverse events was reported in greater detail. We found no differences in the frequency of nausea and vomiting among participants receiving either IM or oral corticosteroids (RR 0.56, 95% CI 0.09 to 3.59; 3 studies, 320 participants; I² = 58%; Analysis 1.12). We found insufficient evidence to conclusively state whether there is a difference in the occurrence of insomnia (RR 1.74, 95% CI 0.61 to 4.97; 1 study, 171 participants; I² = 0%; Analysis 1.13) or personality changes (RR 0.82, 95% CI 0.56 to 1.19; 1 study, 30 participants; I² = 0%; Analysis 1.14) among participants receiving IM or oral corticosteroids. Some studies reported the occurrence of side effects associated with the IM corticosteroid injection. Two studies strictly reported on the occurrence of pain, swelling, and redness at the injection site among participants receiving IM corticosteroids only, as these studies did not utilize a IM placebo control group (Gordon 2007; Gries 2000). Gordon 2007 reported the proportion of paediatric participants experiencing discomfort, swelling, and redness following the IM corticosteroid injection was 1.6%, 6.5%, and 1.6% respectively. Gries 2000 reported that no paediatric participants receiving IM corticosteroids experienced any other side effects besides personality changes. Two placebo‐controlled studies compared the occurrence of pain, swelling, and redness between participants receiving IM corticosteroids compared to participants receiving oral corticosteroids who received an IM placebo injection. Overall, the imprecision of the estimate precluded identifying a difference in the occurrence of pain (RR 1.75, 95% CI 0.32 to 9.66; 2 studies, 181 participants; I² = 8%; Analysis 1.15), swelling (RR 4.76, 95% CI 0.57 to 39.84; 2 studies, 180 participants; I² = 0%; Analysis 1.16), or redness (RR 13.50, 95% CI 0.77 to 235.63; 2 studies, 175 participants; I² = 0%; Analysis 1.17) between participants receiving IM corticosteroids versus IM placebo.
Pulmonary function
None of the included studies assessing a paediatric population collected PEF or FEV₁ measures. Four studies assessed PEF among an adult population following discharge from the ED. We found no differences in PEF between participants receiving IM versus oral corticosteroids at follow‐up (MD −7.78 L/min, 95% CI −38.83 L/min to 23.28 L/min; 4 studies, 272 participants; I² = 33%; Analysis 1.18). Only one study reported on FEV₁/FVC (%), and it reported no significant differences between participants receiving IM versus oral corticosteroids (MD −1.00, 95% CI −12.44 to 10.44; 1 study, 36 participants; I² = 0%; Analysis 1.19). No studies assessed per cent change in baseline PEF following discharge from the ED.
Symptom scores
None of the included studies assessed symptom scores following discharge from the ED using validated scales. One study reported symptom scores for shortness of breath, cough, wheeze, activity limitation, and sleep disturbance at days 7 and 21 post‐discharge using an unspecified tool, in which participants were asked to give a score between 1 (indicating 'not present') to 10 (indicating 'most severe') for each symptom (Chan 2001). At day 21 post‐discharge, symptom scores between participants receiving IM versus oral corticosteroids were similar for each symptom including: shortness of breath (IM 3.8 (SD 2.4) n = 48; oral: 3.8 (SD 2.4) n = 58), cough (IM 2.8 (SD 2.2) n = 48; oral 3.4 (SD 2.4) n = 58), wheeze (IM 3.4 (SD 2.5) n = 48; oral 3.4 (SD 2.3) n = 58), activity limitation (IM 2.1 (SD 2.3) n = 48; oral 2.2 (SD 2.1) n = 58), and sleep disturbance (IM 2.9 (SD 2.7) n = 48; oral 2.8 (SD 2.7) n = 58). The study reported no significant differences in symptoms' scores between patients receiving IM or oral corticosteroids (P value not reported).
Duration of symptoms
None of the included studies reported the duration for which symptoms occurred following discharge from the ED. Three studies reported whether participants were still experiencing non‐specific symptoms at the time of follow‐up, which was assessed at day 5 (Al‐Wahadneh 2006; Gries 2000) and between 5 to 7 days post‐discharge (Hoffman 1988). We found no significant differences in the presence of symptoms at follow‐up between participants receiving IM or oral corticosteroids (RR 0.41, 95% CI 0.14 to 1.20; 3 studies, 80 participants; I² = 44%; Analysis 1.20). Three studies reported whether participants were still experiencing cough (RR 0.69, 95% CI 0.17 to 2.73; 3 studies, 178 participants; I² = 50%; Analysis 1.21) or wheezing (RR 0.59, 95% CI 0.14 to 2.52; 3 studies, 177 participants; I² = 53%; Analysis 1.22) at the time of follow‐up but no differences between participants receiving IM versus oral corticosteroids were found. As previously noted, while Chan 2001 reported symptom scores for shortness of breath, cough, wheeze, activity limitation, and sleep disturbances at days 7 and 21 post‐discharge, the study did not assess whether there was a significant difference in symptom scores between the IM and oral corticosteroid groups at days 7 and 21.
Compliance with oral medications
Three adult studies reported compliance with the oral corticosteroid treatment regimens. Although the overall compliance with the prescribed oral corticosteroid regimen varied, the reported adherence was high, with a low of 66.7% (n = 6/9; Hoffman 1988), to 94% (n = 64/68; Schuckman 1998), and 100% (n = 84/84; Chan 2001). Only two paediatric studies examined compliance with oral corticosteroids. Al‐Wahadneh 2006 reported that eight parents reported difficulties in providing their children with the oral corticosteroid tablets and four children (n = 4/14) missed between 50% and 75% of their doses. Gries 2000 reported that 41% (n = 7/17) of patients did not comply with their prescribed oral prednisone regimen, with three participants missing more than 75% of their oral prednisone doses, while another four children did not take one‐third of their medication.
Beta agonist use
Two studies reported the use of beta₂‐agonists among participants 24 hours after discharge from the ED. We found no differences in beta₂‐agonists use 24 hours after discharge from the ED among participants receiving either IM or oral corticosteroids (RR 0.54, 95% CI 0.21 to 1.37; 2 studies, 48 participants; I² = 0%; Analysis 1.23).
Discussion
Summary of main results
Systemic corticosteroids are an effective treatment in reducing relapses in patients with acute asthma after discharge from an emergency department (ED) or equivalent acute care setting (Krishnan 2009; Rowe 2007a). While systemic corticosteroids are commonly provided to prevent admission and reduce the risk of relapse, the optimal route of administration is unclear.
This systematic review set out to compare the effectiveness of a single dose of intramuscular (IM) corticosteroids to a regimen of oral corticosteroids to prevent relapse among children and adult patients discharged from an ED or equivalent acute care setting for acute asthma. Utilizing methods to reduce publication and selection bias, we identified nine trials involving 804 participants comparing a single dose of IM to oral corticosteroids in adult and paediatric participants with acute asthma.
Overall, this review could not identify a statistically significant difference in relapses among participants receiving IM versus oral corticosteroids. Furthermore, we identified no subgroup differences between children and adults, relapse occurring within or after 10 days post‐discharge, or participants presenting to the ED with a mild/moderate versus severe exacerbation. The effectiveness of IM versus oral corticosteroids to mitigate relapse remained consistent following several sensitivity analyses, including fixed versus random effects, variations in study quality, and variations in duration of oral corticosteroid regimens. Regarding other important clinical outcomes, the risk for adverse events among participants receiving IM corticosteroids ranged from a 36% decrease up to a 7% increase. Neither corticosteroid regimen demonstrated superiority with respect to pulmonary function measures, symptom persistence, or need for beta₂‐agonists following discharge.
With a similar risk for relapse, the decision to provide patients with either IM or oral corticosteroids at discharge will likely involve other considerations such as patient preference, palatability, socio‐economic conditions, ability to afford prescriptions, side‐effect profile, and clinician estimation of patient adherence. This may vary between paediatric and adult patients. For patients who may have difficulties complying with an oral corticosteroid or patients who have previously experienced nausea or vomiting with oral corticosteroids, IM corticosteroids appear to provide physicians with an effective alternative. Unfortunately, a lack of reporting of many secondary outcomes of interest severely limited the number of studies that could be included in each meta‐analysis. While all of the included studies reported on relapse outcomes, the majority of secondary outcomes were reported sparingly across the included studies, and this limited the ability of this review to make any meaningful conclusions regarding the overall effectiveness of IM corticosteroids.
Overall completeness and applicability of evidence
We believe the overall completeness of the review to be moderate. We conducted an extensive search of the literature which identified nine studies including 804 participants. Among the nine studies, five enrolled adults (n = 559) (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998) while four studies involved children (n = 245) (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997).
Overall, the paediatric studies tended to focus on young children, with the mean ages of the IM and oral corticosteroid groups ranging from 3 to 7 years old. The age of eligible children included in the studies ranged from 9 months to 14 years (Al‐Wahadneh 2006), 18 months to 7 years (Gordon 2007), 6 months to 7 years (Gries 2000), and 3 to 16 years (Klig 1997). It is important for future studies assessing the effectiveness of corticosteroids in children with acute asthma to attempt to ensure that the included patients had a true diagnosis of asthma: among the included children studies, only two of the studies reported only enrolling children with prior episodes of respiratory illness treated with bronchodilators (Gordon 2007; Klig 1997).
Within the paediatric studies, there is a paucity of studies on systemic corticosteroids in children aged 7 to 17 years old. Given the evidence that pre‐school‐aged children with acute asthma/wheezing may not respond to corticosteroids due to fewer airway eosinophils (Castro‐Rodriguez 2016), it is possible that the effectiveness of the IM or oral corticosteroid treatments may have been impacted by the age of the enrolled patients, which would affect the generalisability of this review. To our knowledge, efforts were made to avoid the inclusion of patients with a diagnosis of bronchiolitis in the included paediatric studies. This contamination, however, would have a conservative effect by decreasing the chance of detecting a difference.
In the adult studies, the age of eligible participants ranged from over the age of 18 (Chan 2001), 15 to 55 (Hoffman 1988), 18 to 45 (Lahn 2004), 16 to 60 (Lee 1993), and 18 to 50 years old (Schuckman 1998). While the majority of studies included young and middle‐aged adults, only one study included patients over the age of 60 (Chan 2001). As a result, the results of this review may not be applicable to the teenage (up to age 14) and older (> 60 years) patients with acute asthma. In addition, the effectiveness of corticosteroids in the paediatric studies may be underestimated due to the age of the participants included in the paediatric studies. All of the included studies enrolled patients presenting with acute asthma to the ED, except Gries 2000 which enrolled patients in an paediatric medical clinic. Overall, the applicability of our review focuses primarily on very young (0 to 7 years old) and young/middle‐aged adults with acute asthma presenting to the ED with acute asthma in North America (United States/Canada). More research is needed in acute care centres across the world. In particular, additional paediatric (enrolling school aged children) or elderly studies are needed to better understand the effectiveness of IM versus oral corticosteroids in those patient populations.
The type of IM agents used, as well as the doses employed, varied considerably across studies. For example, IM agents included methylprednisolone (Hoffman 1988; Lahn 2004), betamethasone (Chan 2001), dexamethasone (Lee 1993), and triamcinolone (Schuckman 1998); they also varied in dose. It is possible that the variation in the dosing and IM agents used could have impacted the effectiveness of this corticosteroid comparison. Moreover, the co‐interventions were poorly reported; and, given the age of some of the studies, the use of these agents in clinical practice likely has been stopped (e.g. metaproterenol, aminophylline, etc.).
Some newer agents, which are now the mainstay of chronic asthma treatment such as ICS and ICS/LABA agents, were either not prescribed or not reported in sufficient detail among the included studies. A recent study found that patients receiving ICS/LABA at presentation to the ED were at the highest risk of relapse following discharge (Rowe 2015) and there is evidence that ICS agents, when added to oral (and presumably IM) systemic corticosteroids at discharge, may reduce relapse (Rowe 1999). Among patients presenting with acute asthma who are already receiving ICS agents, fewer relapses occurred when ICS/LABA was substituted for ICS agents in addition to oral (and presumably IM) systemic corticosteroids at discharge (Rowe 2007b). A lack of reporting on the use of ICS and ICS/LABA agents limited the ability of this review to estimate the impact of these agents on the efficacy of IM or oral corticosteroids.
This review did not attempt to control for corticosteroid equivalency of the varying corticosteroids regimens used across the studies. It is possible that the dose and pharmacokinetic properties of various prescribed corticosteroids could have impacted the effectiveness of the IM or oral corticosteroids. Other studies have compared the effectiveness of oral prednisone compared to oral dexamethasone (which has a longer half‐life) to improve outcomes in children with acute asthma (Paniagua 2017; Normansell 2016). A recently published Cochrane Review reported insufficient evidence to identify whether lower or shorter doses of oral corticosteroids were any less effective than longer or higher doses and recommended additional large high‐quality clinical trials (Normansell 2016). Additional studies comparing the effectiveness of varying IM corticosteroids (e.g. IM dexamethasone versus IM methylprednisone) and the impact of dosing/pharmacokinetics are needed.
Quality of the evidence
The overall risk of bias of the included studies ranged from unclear to high, with no studies being assessed as having a low risk of bias. Four high risk of bias studies were not placebo controlled trials (Al‐Wahadneh 2006; Gordon 2007; Gries 2000; Klig 1997). The remaining studies had an overall unclear risk of bias (Chan 2001; Hoffman 1988; Lahn 2004; Lee 1993; Schuckman 1998). The majority of studies did not adequately describe their method of randomisation and all but Chan 2001 had an unclear risk of bias for selective outcome reporting due to a lack of an available protocol. Using GRADE, the overall quality of the outcomes ranged from low to moderate. We reduced to low quality the primary outcomes of relapse as well as relapse after 10 days due to overall unclear to high risk of bias of the studies, and imprecision due to wide confidence intervals including both benefit, harm, and no effect. We assessed the quality of the subgroup analyses for relapse to be low due to the low number of available patients and wide confidence intervals. We judged the quality of the outcome adverse events to be low quality due to the overall unclear to high risk of bias of the included studies and imprecision due to few events. We considered both 'symptom persistence' and '24 hour beta₂‐agonists use' to be low quality due the overall unclear to high risk of bias of the included studies, as well as few events. We assessed the 'outcome of relapse within 10 days' as being of moderate quality due to the overall unclear to high risk of bias of the studies. We reduced 'peak expiratory flow' to moderate quality due to imprecision of the results.
Potential biases in the review process
While there is always a potential for screening and study selection bias, we took several steps to attenuate this risk. An expert health sciences librarian conducted a comprehensive search of the literature, including the CATR, a separate search of nine electronic databases, and a detailed search of the grey literature. Indeed, we identified one study from the grey literature (Al‐Wahadneh 2006). We placed no limits with regard to language, date of publication, or status of publication on any of the searches. We translated and screened all foreign language articles identified. If the information provided in the text did not allow for a clear inclusion/exclusion decision, we attempted to contact study authors to clarify the information provided in the text. Two review authors independently completed study selection, including both title and abstract screening and full‐text review. Despite our efforts, we recognize that some studies could have been missed. Unfortunately, due to an insufficient number of included studies, publication bias could not be assessed as planned (Higgins 2011). As such, the risk of publication bias should be considered unclear.
Agreements and disagreements with other studies or reviews
Only two reviews have been published comparing the effectiveness of IM versus oral corticosteroids in patients presenting to an acute care centre with acute asthma (Krishnan 2009; Rowe 2017). The results of this review are similar to a previously published umbrella review which compared the effectiveness of IM versus oral corticosteroids in adults (Krishnan 2009). The review identified five adult studies (all five of which were included in this review) and reported no significant difference in relapse rates in adult participants receiving IM versus oral corticosteroids for acute asthma after discharge from an ED. Unlike the current review, Krishnan 2009 did not include paediatric studies. In addition, a recently published review compared the effectiveness of various regimens of systemic corticosteroids to reduce relapse in adults with acute asthma (Rowe 2017). Unlike the current review which strictly included studies comparing IM versus oral corticosteroids, Rowe 2017 included any studies comparing IM or oral corticosteroids to other oral corticosteroid or placebo regimens and conducted indirect comparisons using a Bayesian network analysis. The review reported that both IM and long‐course oral corticosteroids were effective in reducing relapse, with a risk of bias ability of best analysis identifying IM corticosteroids as having the highest risk of bias of being the most effective treatment option compared to short‐course or long‐course oral corticosteroids. Unlike the current review, Rowe 2017 did not exclusively compare the effectiveness of IM versus oral corticosteroids to mitigate relapse.
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 1 Relapse.
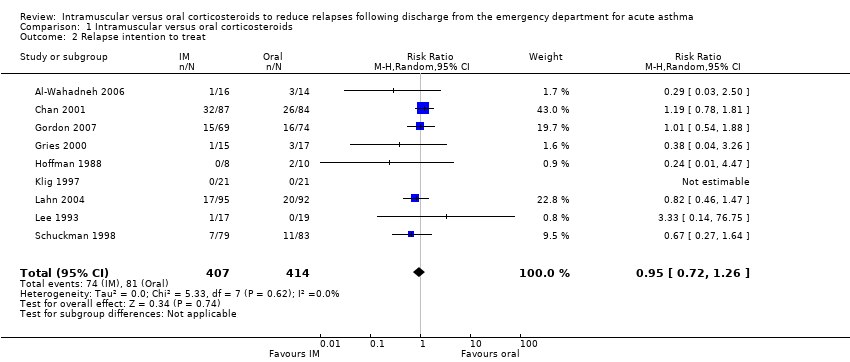
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 2 Relapse intention to treat.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 3 Subgroup analysis: children versus adults.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 5 Subgroup analysis: mild/moderate versus severe exacerbations.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 6 Sensitivity analysis: risk of bias.
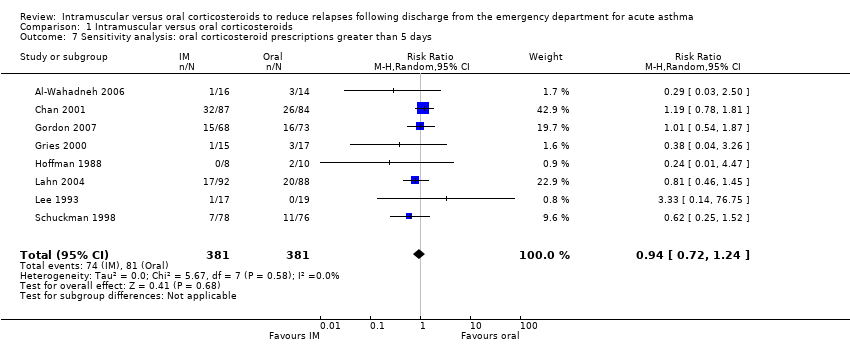
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 8 Sensitivity analysis: fixed effects.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 9 Sensitivity analysis: corticosteroids in ED.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 10 Serious adverse events; hospitalization following discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 11 Adverse events.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 12 Adverse events: nausea/vomiting/GI distress.
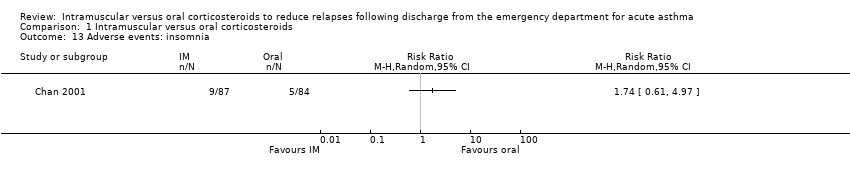
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 13 Adverse events: insomnia.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 14 Adverse events: personality changes.
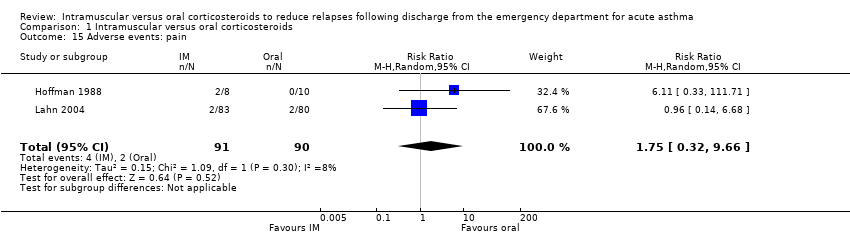
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 15 Adverse events: pain.
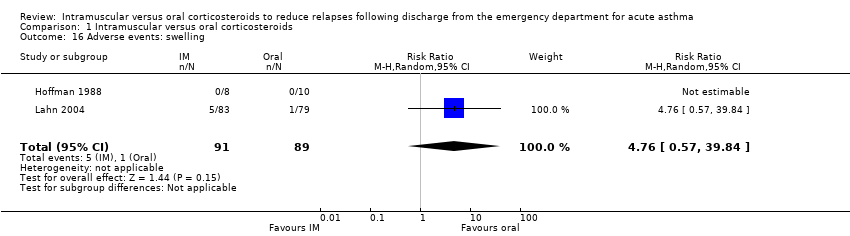
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 16 Adverse events: swelling.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 17 Adverse events: redness.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 18 Pulmonary function: peak expiratory flow (L/min).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 19 Pulmonary function: FEV₁/FVC (%).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 20 Symptom persistence.
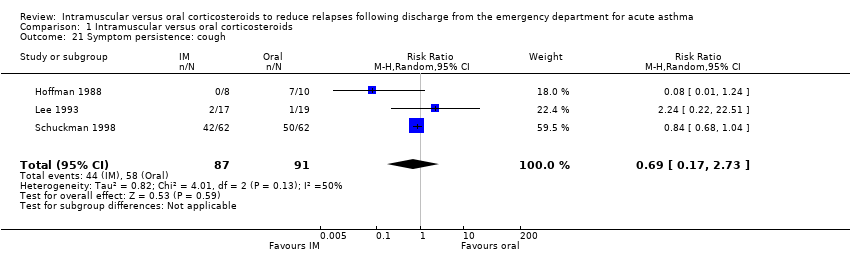
Comparison 1 Intramuscular versus oral corticosteroids, Outcome 21 Symptom persistence: cough.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 22 Symptom persistence: wheezing.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 23 24‐hour beta agonist use.
| Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma | |||||
| Patient or population: patients with acute asthma | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Oral corticosteroids | Intramuscular corticosteroids | ||||
| Relapse | 201 per 1000 | 12 fewer per 1000 | RR 0.94 | 804 | ⊕⊕⊝⊝ |
| Relapse within 10 days post‐discharge | 154 per 1000 | 40 fewer per 1000 (from 75 fewer to 11 more) | RR 0.74 | 742 | ⊕⊕⊕⊝ |
| Relapse occurring after 10 days post‐discharge | 245 per 1000 | 2 fewer per 1000 | RR 0.99 | 556 | ⊕⊕⊝⊝ |
| Adverse events | 294 per 1000 | 50 fewer per 1000 | RR 0.83 | 404 | ⊕⊕⊝⊝ |
| Pulmonary function: Peak expiratory flow | The mean pulmonary function: peak expiratory flow ranged across control groups from | The mean pulmonary function: peak expiratory flow in the intervention groups was | 272 | ⊕⊕⊕⊝ | |
| Symptom persistence | 537 per 1000 | 317 fewer per 1000 | RR 0.41 | 80 | ⊕⊕⊝⊝ |
| 24‐hour beta agonist use | 375 per 1000 | 172 fewer per 1000 | RR 0.54 | 48 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level for risk of bias. Majority of studies received an unclear risk of bias for random sequence generation and selective outcome reporting | |||||
| Studies | Pulmonary function: Eligibility criteria | Exacerbation severity |
| Severity estimated using modified scoring system based on GINA guidelines. Reported to enrolling patients with mild‐moderate exacerbations, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported mean baseline PEF greater than 200 L/min: IM group: 270 L/min (SD: 103); oral group: 261 L/min (SD: 104). | Mild/moderate | |
| Reported to enrolling patients identified as moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Applied adapted exacerbation severity score (unspecified). Reported to enrolling patients rated as mild/moderate, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported baseline mean PEF of enrolled patients of less than 150 L/min: IM group: 129 L/min (SD:14); oral group: 141 L/min (SD: 14). | Severe | |
| Exacerbation severity estimated via pulmonary index score. Study reported to enrolling patients with mild/moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Eligibility criteria required patients to have a PEFR of ≤ 70% predicted with a minimum PEFR of ≥ 40%. Reported PEF of enrolled patients was ≥ 200 L/min: IM group: 205 L/min (SD: 70); oral group: 209 L/min (SD: 72). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 210 L/min (SD: 30); oral group: 208 L/min (SD: 26). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 243.6 L/min (SD: 64); oral group: 244.7 L/min (SD: 83). | Mild/moderate | |
| Abbreviations: GINA = Global Initiative for Asthma; PEF = Peak expiratory flow; PEFR = Peak expiratory flow rate; IM = intramuscular; SD = standard deviation | ||
| Studies | Location/setting | Co‐interventions | Corticosteroid doses and durations | Methyprednisolone equivalency | Relapse outcome |
| Jordan, ED | Provided in ED: not stated Provided at discharge: SABA | Dexamethasone (IM) 1.7 mg/kg Mean dose: 24 mg Single dose Prednisolone (oral) 2 mg/kg/day for 5 days Mean dose: 19.2 mg per day Total dose: 96 mg | IM Methylprednisone equivalency: 120 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 76.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/16 Day 21 Oral group 3/14 Day 21 | |
| Canada, ED | Provided in ED: SABA, methylxanthines, supplemental oral/IV corticosteroids Provided at discharge: Methylxanthines, unspecified inhaled beta₂‐agonists, and ICS | Betamethasone (IM) 12 mg Single dose. Received placebo capsules over 7 days. Prednisone (oral) 50 mg a day for 7 days. Received a single placebo injection Total dose: 350 mg | IM Methylprednisone equivalency: 72 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 280 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 12/86 Day 7 Oral group 19/82 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA, ipratropium bromide. IV corticosteroids for patients who vomited oral corticosteroids. Provided at discharge: Inhaled beta₂‐agonists and ICS | Dexamethasone (IM) 0.6 mg/kg (max 16 mg) Single dose Prednisolone (oral) 2 mg/kg (max 50 mg) daily for 5 days Total: 250 mg | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12‐36 hours) Oral Methylprednisone equivalency: 200 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 8/69 Day 4 Oral group 11/73 Day 4 IM group 15/68 Day 14 Oral group 16/73 Day 14 | |
| United States, Tertiary medical center | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) Patients 6 to 12 months old received 16 mg. Patients 13 to 35 months old received 24 mg. Children ≥ 36 months received 36 mg. Single dose Prednisone (oral) 2 mg/kg a day for 5 days Total dose: unclear | IM Methylprednisone equivalency: Unable to assess Oral Methylprednisone equivalency: Unable to assess | IM group 1/15 Day 28 Oral group 3/17 Day 28 | |
| United States, ED | Provided in ED: SABA, epinephrine, methylxanthines, IV corticosteroids Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Methylprednisonlone (IM) 80 mg Single dose. Received placebo capsules for 7 days Methylprednisolone (oral) Tapering dose over 7 days. Total dose: 216 mg Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 216 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/8 day Day 7 Oral group 2/10 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) 0.3 mg/kg Total dose: 15 mg Single dose Prednisone (oral) 2 mg/kg a day for 3 days Total dose: 100 mg | IM Methylprednisone equivalency: 75 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/21 Day 5 Oral group 0/21 Day 5 | |
| United States, ED | Provided in ED: inhaled beta₂‐agonists, IV corticosteroids Provided at discharge: SABA | Methylprednisolone (IM) 160 mg Single dose. Received placebo capsules for 8 days Methylprednisolone (oral) Tapering dose over 8 days. Total dose: 160 mg (tapering dose 32 mg day 1). Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 13/92 Day 10 Oral group 12/88 Day 10 IM group 17/92 Day 21 Oral group 20/88 Day 21 | |
| Taiwan, ED | Provided in ED: SABA, methylxanthines Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Dexamethasone (IM) 10 mg Single dose. Received placebo capsules for 7 days Dexamethasone (oral) Tapering dose over 7 days. 11.75 mg total. Received placebo injection | IM Methylprednisone equivalency: 50 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 58.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/17 Day 7 Oral group 0/19 Day 7 | |
| United States, ED | Provided in ED: SABA, oral/IV corticosteroids Provided at discharge: SABA, antibiotics, ICS, cromolyn sodium, ipratropium bromide | Triamcinolone (IM) 40 mg Single dose. Received placebo capsules for 5 days Prednisone (oral) 40 mg a day for 5 days. Total dose: 160 mg Received placebo injection | IM Methylprednisone equivalency: 40 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 160 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 7/78 Day 7 Oral group 11/76 Day 7 | |
| ED = emergency department; SABA = short‐acting beta₂‐agonists; LABA = long‐acting beta₂‐agonists; IV = intravenous; IM = intramuscular; ICS = inhaled corticosteroids | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 2 Relapse intention to treat Show forest plot | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 3 Subgroup analysis: children versus adults Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations Show forest plot | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias Show forest plot | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days Show forest plot | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 8 Sensitivity analysis: fixed effects Show forest plot | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| 9 Sensitivity analysis: corticosteroids in ED Show forest plot | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
| 10 Serious adverse events; hospitalization following discharge Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events Show forest plot | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 12 Adverse events: nausea/vomiting/GI distress Show forest plot | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
| 13 Adverse events: insomnia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Adverse events: personality changes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Adverse events: pain Show forest plot | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
| 16 Adverse events: swelling Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
| 17 Adverse events: redness Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
| 18 Pulmonary function: peak expiratory flow (L/min) Show forest plot | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
| 19 Pulmonary function: FEV₁/FVC (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Symptom persistence Show forest plot | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 21 Symptom persistence: cough Show forest plot | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
| 22 Symptom persistence: wheezing Show forest plot | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
| 23 24‐hour beta agonist use Show forest plot | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |

