Intervenciones para ayudar a apoyar a los cuidadores de pacientes con tumores cerebrales o de la médula espinal
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: patients in follow‐up for pituitary disease in a stable medical situation and their partners Exclusion criteria: aged < 18 or > 75 years, receiving intensive medical treatment, or have psychiatric illness Number randomised: 188 patients (174 included in analysis). Number of caregivers randomised not specified, but 63 were included in the analysis (25 intervention group, 38 control group). Follow‐up: baseline, 8 weeks, 6 months Setting: 2 university medical centres in The Netherlands | |
| Interventions | Intervention group: self‐management programme drawing on techniques from CBT consisting of 8 weekly sessions of 90 minutes, guided by psychologists and medical social workers. Patients and caregivers participated in separate groups of 5–7 participants. Sessions were named: 1. information; 2. self‐monitoring; 3. health promotion; 4. stress management; 5. management of anxiety and depression/caregivers' challenge; 6. social competence; 7. social support; 8. evaluation. Control group: single (optional) information meeting in week 4 or 5 | |
| Outcomes | Caregiver outcomes
Patient outcomes
| |
| Notes | 22% neuro‐oncology (63 caregivers, 25 randomised to the intervention, and 38 to the control group. 14 caregivers of patients with brain tumours participated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described in the report. Patients were randomised using a computer‐generated scheme. Caregivers were assigned to the same group. Confirmed via correspondence. |
| Allocation concealment (selection bias) | Low risk | Not described in the report. Computer random number generator used (confirmed by authors). |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Not described in the report. Analyses were not performed blind (confirmed via correspondence). |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not described in the report. Level of missing data was 35% in caregivers (confirmed via correspondence), analysed with linear mixed models. 48% of caregivers did not complete all intervention sessions. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. All caregiver outcomes in the manuscript were reported on. |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: informal caregivers (i.e. a spouse or significant other providing at least 21 hours of care a week) of patients with a high‐grade (WHO grade III or IV) glioma; aged > 18 years; providing written informed consent Exclusion criteria: patient life expectancy < 3 months; caregiver was unable to complete questionnaires due to insufficient mastery of the Dutch language or severe visual impairments; caregiver was unable to understand or apply the skills taught in the intervention due to physical or mental condition(s). Number randomised: 56: intervention group 31; control group 25 Follow‐up: 8 months Setting: 3 tertiary referral centres for neuro‐oncology patients in The Netherlands | |
| Interventions | Intervention group: 6 × 1‐hour face‐to‐face sessions with a psychologist, on a fortnightly basis, based on CBT and psychoeducation principles. First, the patient's symptoms and the caregiver's involvement were reviewed, and based on a prioritisation of the need for help to assist with patient symptoms, the psychologist and caregiver drew upon a predefined set of strategies. During the first session, patient and caregiver history and current functioning was documented. During the second session, an introduction of the intervention and rationale behind CBT was given. For the next 4 sessions, caregivers could make a selection of topics they wanted to discuss. Options were: 1. contact with the patient; 2. the direct environment (contact with family, friends, and others); 3. epilepsy; 4. changes in behaviour, character, and cognition; 5. time for yourself; 6. children (what and how to tell them); 7. practical and emotional care in the end of life phase. Control group: care as usual | |
| Outcomes | Caregiver outcomes
Outcomes not reported on (confirmed via correspondence)
Patient outcomes (by proxy):
| |
| Notes | 100% neuro‐oncology (56 participants, intervention group 31; control group 25) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used concealed randomisation technique – drawing a ticket from a concealed box (confirmed by authors). |
| Allocation concealment (selection bias) | High risk | Tickets were not numbered (confirmed by authors). |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Analyses were not performed blind (confirmed by authors). |
| Incomplete outcome data (attrition bias) | Unclear risk | High dropout rates (52% in intervention group; 32% in control group at 8 months' follow‐up). Analysed with last observation carried forward method. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol. All outcomes in the manuscript were reported on. Caregiver burden data were collected but deemed unreliable (confirmed by authors). |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: family caregiver ('a person who knows you well and is involved in your medical care') of a patient who: was aged > 18 years; had new diagnosis, recurrence, or progression of an advanced‐stage cancer within about 30–60 days of the date the patient was informed of the diagnosis by his or her oncology clinician; and had an oncologist‐determined prognosis of 6–24 months; was English speaking; was able to complete baseline questionnaires. Exclusion criteria: scored < 4 on the Callahan Cognitive Screen; had an untreated axis 1 psychiatric condition or an active substance‐use disorder; or had uncorrectable hearing disorder or unreliable telephone service. No additional exclusion criteria for caregivers. Number randomised: 124 dyads (63 early intervention; 61 delayed intervention) Follow‐up: by telephone, once every 6 weeks until 24 weeks; then every 3 months until end of study or patient death. Not all were followed up after 24 weeks. Setting: participants were recruited from a cancer centre (and affiliated outreach clinics) and a medical centre in the US. | |
| Interventions | Intervention group: 3 structured 1‐to‐1 telephone sessions (with guidebook; once a week) between an advanced‐practice palliative care nurse coach and a caregiver. Session 1 addressed taking on the caregiver role, defined palliative and supportive care, and introduced problem‐solving using the framework of the COPE attitude. Session 2 covered caregiver self‐care and effective partnering in patient symptoms assessment and management. Session 3 addressed the building of a support team, decision making, decision support, and advance care planning. Sessions lasted on average 23 minutes, and the same nurse coaches followed up with participants monthly until end of study or patient death. Control group: no treatment but could take part in the intervention after 3 months. | |
| Outcomes | Caregiver outcomes
Outcomes reported elsewhere (Dionne‐Odom 2016):
Outcomes not reported on (confirmed via correspondence):
Patient outcomes (Bakitas 2015):
| |
| Notes | Published and unpublished data. 22% neuro‐oncology (124 participants, 63 to the intervention group and 61 to the control group. In total, 27 caregivers of patients with brain tumour were included (3 primary brain tumour; 24 secondary brain tumour; confirmed via correspondence). ENABLE III (Educate, Nurture, Advise, Before Life Ends III) trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described in the report. Patients were randomised 1:1 using a computer‐generated scheme. Caregivers were assigned to the same group. Confirmed via correspondence. |
| Allocation concealment (selection bias) | Low risk | Not described in the report. Computer‐generated randomisation after enrolment, confirmed by authors. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Analyses were not performed blind (confirmed via correspondence). |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout: about 32% of caregivers did not complete all follow‐up assessments; analyses revealed no significant associations between attrition and measured caregiver characteristics or outcome. Maximum likelihood methods were used to estimate missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol. All outcomes specified in the manuscript were reported on; 1 additional outcome (complicated grief) was reported in 2016 publication; personality was assessed but not reported on. Authors confirmed no other outcomes were collected. |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: parents with a child aged 2–7 years diagnosed with a primary malignancy; English was primary language; no prior experience with external beam radiotherapy; who were functioning at the level in which the children could tolerate radiotherapy intervention. Exclusion criteria: not specified. Number randomised: 80 (41 intervention group, 39 control group). Follow‐up: until the final day of radiotherapy simulation (not further specified). Communication with authors revealed that this was, in most cases, approximately a 90‐minute interval between baseline and post assessment. Setting: radiotherapy outpatient clinic in the US. | |
| Interventions | Intervention group: CBT package that included exposure to an interactive‐educational ActiMates Barney, an educational video in the clinic room including filmed modelling, and passive auditory distraction via Barney‐narrated stories delivered during the simulation procedure. Modified control group: similar intervention composed of exposure to non‐interactive children's control character (similar size, colour, and shape to Barney), an age‐appropriate cartoon video, and storied delivered via cassette tape during treatment. | |
| Outcomes | Caregiver outcomes
Patient outcomes
| |
| Notes | Primary child outcomes were published previously (Klosky 2004; Klosky 2007b; Tyc 2002). 67% neuro‐oncology (80 participants, 41 intervention, 39 control. In total, 53 caregivers of people with brain tumours were included). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described in the report. Computer random number generator used (confirmed by authors). |
| Allocation concealment (selection bias) | Low risk | Not described in the report. Computer random number generator used (confirmed by authors). |
| Blinding of participants and personnel (performance bias) | High risk | Not described in the report. The authors explained that participants were not fully aware of study goals and would, therefore, not know whether they were assigned to the intervention or modified control group. However, they were not formally blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Not described in the report. The authors could not remember exactly how data analysis was performed. |
| Incomplete outcome data (attrition bias) | Low risk | Not described in the report, authors confirmed that there was no attrition and all participants completed the outcome measures. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol. All outcomes in the manuscript were reported on; other (patient‐focused) outcomes were published elsewhere. |
| Methods | Quasi‐randomised controlled trial | |
| Participants | Inclusion criteria: people with newly diagnosed primary brain tumour eligible for radiotherapy and aged > 18 years; have mild‐to‐moderate cognitive impairment; have a prognosis of ≥ 6 months and ability to attend sessions at the medical centre for 2 weeks. All patients were required to have a designated caregiver available to attend all sessions. Exclusion criteria: none specified. Number randomised: 16 (9 intervention group, 7 control group). 3 more were not randomised but allocated to the intervention group. Follow‐up: baseline, 2 weeks, 3 months Setting: tertiary medical centre in US | |
| Interventions | Intervention group: received cognitive rehabilitation and problem solving. Cognitive rehabilitation: dyads were taught to use a calendar that had a specific format as an external aid to compensate for cognitive symptoms. 6 × 50‐minute sessions over 2‐week period. Specific goals were developed for each session. Problem solving: teaching dyads a model of stress and a specific problem‐solving technique for its management. 6 × 50‐minute sessions over 2‐week period, delivered concurrently with the cognitive rehabilitation intervention. Control group: standard medical care | |
| Outcomes | Caregiver outcomes
Patient outcomes:
| |
| Notes | 100% neuro‐oncology (16 participants, 9 intervention group, 7 control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation not described. Last 3 participants enrolled were not randomly allocated. |
| Allocation concealment (selection bias) | High risk | Last 3 participants enrolled were not randomly allocated. |
| Blinding of participants and personnel (performance bias) | High risk | Not described in the report, but blinding was likely not possible due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the report. |
| Incomplete outcome data (attrition bias) | High risk | Dropout rates: 26% at postintervention; increased to 32% at 3 months' follow‐up. 33% did not complete the intervention. Not clear how missing data were handled, presumably not included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol. All outcomes in the manuscript were reported on. |
| Methods | Randomised controlled trial (pilot) | |
| Participants | Inclusion criteria: identified as the person who provided the most care for an adult diagnosed with primary malignant brain tumour; English speaking and reading; having an email address; aged >18 years Exclusion criteria: none specified. Authors clarified there were no specific exclusion criteria, other than not meeting inclusion criteria. Number randomised: 40; 30 intervention group, 10 control group Follow‐up: baseline, 3 weeks, 6 weeks Setting: National Cancer Institute‐designated comprehensive cancer centre in the US | |
| Interventions | Intervention group: eSNAP, a web‐based application which takes 10–15 minutes to help caregivers list people or groups who could help within 6 categories of support: 1. hands‐on; 2. informational; 3. communication; 4. financial; 5. emotional; and 6. self‐care. A network visualisation was provided to caregivers in PDF/print. Control group: care as usual | |
| Outcomes | Primary outcome (confirmed by authors)
Secondary outcomes:
| |
| Notes | 100% neuro‐oncology (40 participants; 30 intervention group, 10 control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation not described in the report. Computer random number generator used (confirmed by authors). |
| Allocation concealment (selection bias) | Low risk | Not described in the report. Computer random number generator used (confirmed by authors). |
| Blinding of participants and personnel (performance bias) | High risk | Not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Not described in the report. Analysis were performed blind (confirmed by authors). |
| Incomplete outcome data (attrition bias) | Unclear risk | 20% attrition at 6 weeks. No information provided on reasons for dropout or how missing data were handled in the report. Authors confirmed that within those who completed assessments, < 10% of data were missing and no data imputation was done. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: primary caregivers of a child with active cancer were eligible if: they were residents of Tehran; aged > 18 years; patient started treatment process; child aged < 14 years; had access to a telephone at home; and were willing to participate and provide written consent. Exclusion criteria: none specified Number randomised: 65 randomised, 3 withdrew due to patient death; 31 intervention group, 31 control group. Follow‐up: baseline, postintervention, 30 days Setting: hospital and rehabilitation complex in Tehran, Iran | |
| Interventions | Intervention group: the Brief Psychosocial Intervention plus usual support services. Caregivers were provided with information and support through individual counselling sessions delivered by a trained social worker in 60–90 minutes. Specified sessions goals were: 1. engage and motivate caregivers to participate and develop open communication with social worker; 2. develop optimistic attitude, help maintain hope and focus on achievable short‐term goals; 3. provide information about treatments and medication, help caregivers learn to live with uncertainty; 4. help caregivers cope with stress and teach stress‐relieving techniques, coping strategies, and healthy lifestyle behaviours; and 5. educate self‐care strategies. After each session, caregivers received a homework assignment. Every session was followed up with a telephone call (30–45 minutes). Control group: usual services, including counselling and financial support | |
| Outcomes |
| |
| Notes | 48% neuro‐oncology (65 participants randomised, 31 intervention group, 31 control group. In total, 31 caregivers of patients with brain tumours were included). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation not described in the report. Authors clarified that a member of the team would alternate group allocation in consecutive participants (i.e. sequence was not random). |
| Allocation concealment (selection bias) | High risk | Not described in the report ('centrally randomised'). Based on the author's explanations, we concluded that allocation concealment was not realistically possible. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if statistician was blinded. Authors explained that statistician was not in contact with cases, and would only see study case numbers. |
| Incomplete outcome data (attrition bias) | Low risk | 3 study participants dropped out before the baseline assessment. No further information on attrition or how missing data were handled in the report. Authors confirmed that there was no missing data (all questionnaires were checked directly following completion) and that no further attrition took place. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. |
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: parents of children who had finished cancer treatment were eligible if they: had a child aged ≤ 15 who had completed cancer treatment with curative intent in the past 5 years; were able to read English; were able to access the Internet in a private location. Exclusion criteria: parents could not participate if they: had insufficient English skills; were experiencing extreme anxiety or depression; endorsed current symptoms of psychosis or substance abuse; had a child who was on active treatment, had relapsed, or was in palliative care Number randomised: 56 consented and randomised (before baseline), 9 dropped out before baseline assessment. 25 intervention group; 22 control group. Follow‐up: baseline, 2 weeks, 6 months Setting: children's hospital in Australia | |
| Interventions | Intervention group: Cascade is a manualised programme consisting of 3 weekly 120‐minute online sessions delivered by a psychologist through WebEx. Driven by the theoretical models, Cascade targets intra‐ and interpersonal psychological processes important to adaptation in the context of illness (e.g. acceptance of uncertainty; practical problem solving; mobilising social support resources). CBT strategies were used to target these core mechanisms of change. Topic areas were derived from a previous study (but not specified in paper). After each session, parents would get homework assignments to practice. Control group: waiting list control group, parents could participate in Cascade intervention after 6 months. | |
| Outcomes |
| |
| Notes | 28% neuro‐oncology (47 participants; 25 intervention group, 22 control group. In total, 13 caregivers of brain tumour patients were included). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent personnel used an electronic randomiser to allocate participants to Cascade or waiting list. |
| Allocation concealment (selection bias) | Low risk | Independent personnel used an electronic randomiser to allocate participants to Cascade or waiting list. |
| Blinding of participants and personnel (performance bias) | High risk | Not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | The statistician remained blinded until all analyses were completed. |
| Incomplete outcome data (attrition bias) | High risk | Only participants with all 3 assessments completed were included in analysis of psychosocial outcomes (17% dropout at 6 months in intervention group; 27% dropout in waiting list control group). Unclear what reasons were. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol. All outcomes in the manuscript were reported on. |
BPI: Brief Psychological Intervention; CBT: cognitive behavioural therapy; COPE: Creativity, Optimism, Planning, Expert Information; EORTC BN20: European Organization for Research and Treatment of Cancer Brain Cancer Module; EQ‐5D: EuroQol; MOS: Medical Outcome Study; NEO: Neuroticism‐Extraversion‐Openness; QoL: quality of life; SF‐36: 36‐item Short Form; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| < 20% of the study sample were neuro‐oncology family caregivers (15%). | |
| Unknown percentage of neuro‐oncology caregivers. | |
| Unknown percentage of neuro‐oncology caregivers. | |
| < 20% of the study sample were neuro‐oncology family caregivers (1%). | |
| < 20% of the study sample were neuro‐oncology family caregivers (no exact percentage). | |
| Unknown percentage of neuro‐oncology caregivers. | |
| < 20% of the study sample were neuro‐oncology family caregivers (9%). |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Care‐IS trial |
| Methods | Multicentre prospective phase III randomised controlled trial |
| Participants | Adult primary carers of a patient with high‐grade glioma who 1. is currently undergoing active treatment and is within 2 months of diagnosis; and 2. is currently attending the outpatient departments of 1 of the participating sites. The caregiver should furthermore 1. aged >18 years; 2. understand and speak English; 3. have no mental, cognitive, or functional disability; 4. be willing and able to comply with study requirements; 5. have no familial, sociological, or geographical condition that might hamper compliance; 6. have no severe intercurrent medical or psychotic disease that would hinder the ability to participation in the study. |
| Interventions | The Care‐IS intervention is guided by a nurse and will consist of: 1. a telephone needs assessment; 2. access to a tailored resource file for the caregiver based on the needs that had been identified; 3. an educational and supportive home visit from the nurse; 4. monthly telephone check‐ups. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 2015 |
| Contact information | |
| Notes |
| Trial name or title | Online psychoeducational intervention for family caregivers of high‐grade primary brain tumour patients |
| Methods | Under development. Expected to be tested in a randomised controlled setting following pilot work (commencing 2019). Phase I: qualitative evaluation of acceptability of the intervention and make modifications Phase II: single‐arm pre–post study to evaluate usability, feasibility, and acceptability |
| Participants | Family caregivers of people with high‐grade glioma |
| Interventions | Online psychoeducational intervention based on social cognitive theory. |
| Outcomes | Not yet specified. |
| Starting date | 2019 |
| Contact information | |
| Notes | Confirmed as accurate 24 January 2019 |
| Trial name or title | Improving palliative care of caregivers of patients with glioblastoma |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria Phase I: focus group with GBM caregivers 1. English‐speaking, due to the focus groups being managed in English and the use of certain validated questionnaires only being available in English; 2. aged > 18 years; 3. caregiver to patient with GBM who died ≥ 1 year ago. Phase II: randomised intervention of GBM caregivers 1. English‐speaking; 2. current caregiver to a patient with GBM; 3. aged > 18 years; 4. score > 4 on the Distress Thermometer and indication that this distress is related in some way to the caregiving role per self‐report. Exclusion criteria 1. In the judgement of the consenting professional, clinician or principal investigator, or as per medical record, severe psychopathology or cognitive impairment likely to interfere with the participation or completion of the protocol or ability to provide meaningful information; 2. another family member or caregiver to the same patient is currently enrolled in the study. |
| Interventions | Intervention: MCP‐C. MCP‐C is based on the principles of Viktor Frankl’s Logotherapy. It is designed to help caregivers of patients with advanced cancer sustain or enhance a sense of meaning, peace, and purpose in their lives. MCP‐C is structured as a 7‐session (1‐hour weekly or biweekly sessions) individual intervention that utilises a mixture of didactics, discussion, and experiential exercises that focus around particular themes related to meaning and cancer caregiving. Control: enhanced usual care. The 'enhancement' to usual care in this study involves the inclusion of screening and targeted referral components. Research study assistants conducting the screening and providing feedback and referrals will be trained in the National Comprehensive Cancer Network guidelines for distress management and will discuss the screening results and associated recommendations with the study principal investigator. |
| Outcomes | Aim 1: determine the feasibility, acceptability, and preliminary effects of MCP‐C delivered to caregivers of patients with GBM. Aim 2: to customise the content and format of the MCP‐C to address the unique existential and psychosocial needs of caregivers of patients with GBM |
| Starting date | 12 February 2018 |
| Contact information | |
| Notes | Confirmed as accurate 21 January 2019. Phase I is completed, phase II has thus far recruited 10 caregivers. |
| Trial name or title | Making sense of brain tumor program |
| Methods | Randomised wait‐list controlled trial |
| Participants | Aged ≥ 18 years; diagnosed with a primary brain tumour; living within a 1‐hour drive of Brisbane; adequate communication skills; able to provide informed consent. Approximately 60% of the 27 participants in the intervention group and 23 participants in the wait‐list control group had a family member involved in their programme. |
| Interventions | 10 × 1‐hour weekly sessions for people with brain tumours, guided by a therapist. Their family members were encouraged to be involved as well. During the first 2 sessions, participants described their diagnosis, treatment, and functional changes and set 3–5 goals to focus on. Treatment modules included psychoeducation, neuropsychological feedback, cognitive rehabilitation, psychotherapy, and couple and family support. The last session is used to reflect on the progress and making plans for maintaining and ongoing gains. |
| Outcomes | Patient outcomes
Caregiver outcomes
|
| Starting date | July 2010 |
| Contact information | |
| Notes | Confirmed as accurate 29 January 2019. Authors have indicated it is unlikely that caregiver outcomes will be published separately. |
| Trial name or title | SmartCare: innovations in caregiving interventions |
| Methods | Randomised controlled trial |
| Participants | Family caregivers (i.e. the primary, non‐professional, non‐paid caregiver as identified by the patient) of patients (aged > 21 years; newly diagnosed with a primary malignant brain tumour) could participate if they 1. were aged > 21 years; 2. had telephone access; 3. could read and speak English; 4. were not currently the primary caregiver for anyone else other than children aged < 21 years; 5. obtained a score > 6 on the shortened CES‐D; 6. were currently not receiving any type of formal counselling for depressive symptoms. |
| Interventions | The online SmartCare programme takes 8 weeks to complete. Every 2 weeks, participants complete a needs screening to identify issues that cause distress and are asked to select 1 or 2 issues to work on. Participants explore the issue in more detail and are encouraged to review past attempts to deal with the issue and any challenges faced. They are asked to set small, realistic goals related to the issue. Subsequently, the nurse interventionist provides telephone support to individualise strategies and teach them how to best use the programme to meet their needs. After 1 week of implementing the plan, the caregiver and nurse review if the chosen strategies worked adequately and if needed, revise the plan. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 1 March 2014 |
| Contact information | |
| Notes | Confirmed as accurate 28 January 2019. |
CES‐D: Center for Epidemiological Studies Depression Scale; GBM: glioblastoma multiforme; MCP‐C: Meaning‐Centered Psychotherapy for Cancer Caregivers; QoL: quality of life.

Study flow diagram. RCT: randomised controlled trial.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
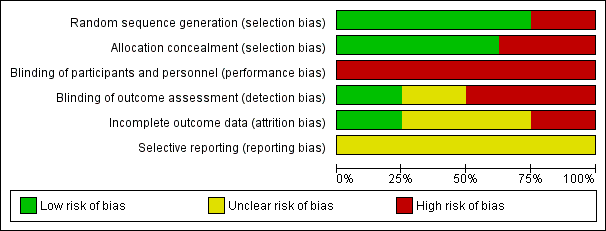
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Interventions to help support caregivers of people with a brain or spinal cord tumour | |||
| Patient or population: caregiver well‐being | |||
| Outcomes | Impact | № of participants | Certainty of the evidence |
| Caregiver psychological distress Follow‐up: range 1 days to 8 months | 4 studies found improvements after the intervention (early palliative care; interactive‐educational programme; electronic social network intervention; self‐management programme); 1 found no significant effects (e‐mental health); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊕⊝⊝ |
| Caregiver burden Follow‐up: range 6 weeks to 3 months | 2 studies found no statistically significant differences in burden scales between the intervention and control groups (early vs delayed palliative care; electronic social network intervention). | (2 RCTs) | ⊕⊕⊝⊝ |
| Caregiver mastery Follow‐up: range 6 months to 8 months | 1 study found improvements in mastery after the intervention (psychoeducation and cognitive behavioural therapy) compared to care‐as‐usual, corrected for changes in patient functioning. 1 study found no improvements in self‐efficacy or coping strategies (self‐management programme). | (2 RCTs) | ⊕⊝⊝⊝ |
| Quality of patient–caregiver relationship | 1 study found no statistically significant differences in family functioning between the intervention and control groups (e‐mental health vs waiting list). | (1 RCT) | ⊕⊝⊝⊝ |
| Caregiver quality of life Follow‐up: range 30 days to 8 months | 2 studies found improvements in QoL over time in the intervention group (psychosocial intervention; self‐management programme) compared to the control group; 1 study found stable QoL in the intervention group vs decline in the control group (no longer statistically significant after controlling for patient functioning); 2 studies found no statistically significant improvements after the intervention (e‐mental health; early palliative care); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊝⊝⊝ |
| Caregiver physical functioning – not measured | None of the included studies assessed caregiver physical functioning. | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CQOLC: Caregiver QoL Index – Cancer; CES‐D: Center for Epidemiological Studies Depression Scale; CI: confidence interval; DASS‐21: Depression, Anxiety and Stress Scales; HADS: Hospital Anxiety and Depression Scale; LASA: Linear Analogue Self‐Assessment; MBCB: Montgomery‐Borgatta Caregiver Burden; POMS: Profile of Mood States; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; STAI: State‐Trait Anxiety Inventory. | |||
| GRADE Working Group grades of evidence | |||
| aDifferent populations (e.g. mixed cancer caregiver samples, paediatric or adult (or both) caregiver samples). | |||

