Intervenciones para ayudar a apoyar a los cuidadores de pacientes con tumores cerebrales o de la médula espinal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012582.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 julio 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
FB handsearched journals and conference abstracts.
FB and AGR performed study selection and data extraction independently.
FB and AGR assessed risk of bias.
FB and HB assessed GRADE certainty of evidence.
FB drafted the manuscript.
The other review authors reviewed the manuscript to improve its quality.
Sources of support
Internal sources
-
New Source of support, Other.
External sources
-
Yorkshire Cancer Research University Academic Fellowship, UK.
The lead reviewer is supported by a YCR University Academic Fellowship (L389FB).
Declarations of interest
FB was involved in a randomised controlled trial aimed at supporting informal caregivers of people with high‐grade glioma through psychoeducation and cognitive behavioural therapy.
PS and FB were involved in a trial to support family caregivers of patients diagnosed with a primary brain tumour through a nurse‐guided online programme (not yet published).
HB: none.
AGR: none.
Acknowledgements
We acknowledge Joanne Platt, Gail Quinn, and Dr Robin Grant from the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers (GNOC) for their help with this review.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to GNOC. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
The lead review author was supported by a Yorkshire Cancer Research University Academic Fellowship (L389FB).
The authors and GNOC are grateful to the following peer reviewers for their time and comments: Andy Bryant, Kathy Oliver, and Hanneke Zwinkels.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jul 02 | Interventions to help support caregivers of people with a brain or spinal cord tumour | Review | Florien W Boele, Alasdair G Rooney, Helen Bulbeck, Paula Sherwood | |
| 2017 Mar 10 | Interventions to help support caregivers of people with a brain or spinal cord tumour | Protocol | Florien W Boele, Helen Bulbeck, Catherine Browne, Alasdair G Rooney, Paula Sherwood | |
Differences between protocol and review
-
To carry out study selection, and ensure at least a degree of generalisability, we had to employ a cut‐off for the percentage of neuro‐oncology caregivers in the samples. FB and AR discussed this and set this cut‐off at more than 20%.
-
The protocol was written in the assumption that a meta‐analysis might be possible. After performing the search and study selection it became apparent that this was not possible – hence some references to meta‐analysis methods have been removed.
-
Because of the narrative synthesis methods employed, we could not enter the extracted data into Review Manager as planned. Similarly, we could not assess whether confounding factors influenced study results, and the extent to which these were controlled for in the analysis (e.g. caregiver education, age, sex, income, socioeconomic status, caregiver use of psychotropic medication, nature of relationship with the patient, patient diagnosis, patient age, and patient sex). Therefore, we removed the following paragraph from the 'Data extraction and management' section: "Where possible, we planned to assess the extent to which the following confounding factors may have influenced the results and the extent to which these were controlled for in the analysis: caregiver education, caregiver age, caregiver sex, caregiver income or socioeconomic status, caregiver use of psychotropic medication, nature of the relationship with the patient, patient diagnosis, patient age, patient sex. Extracted data were entered into Review Manager. Again, the two authors mentioned above discussed and any uncertainties were resolved by a third review author."
-
There was a change in the author list: C Browne is no longer involved in this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Adaptation, Psychological;
- *Quality of Life;
- Brain Neoplasms [psychology];
- Caregivers [*psychology];
- Family [psychology];
- Friends [psychology];
- Randomized Controlled Trials as Topic;
- Social Support;
- Spinal Cord Neoplasms [psychology];
- Stress, Psychological [*prevention & control];
- Terminal Care [*psychology];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram. RCT: randomised controlled trial.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
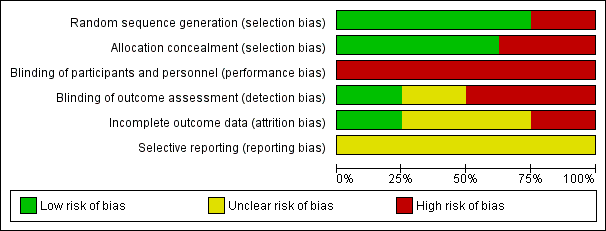
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Interventions to help support caregivers of people with a brain or spinal cord tumour | |||
| Patient or population: caregiver well‐being | |||
| Outcomes | Impact | № of participants | Certainty of the evidence |
| Caregiver psychological distress Follow‐up: range 1 days to 8 months | 4 studies found improvements after the intervention (early palliative care; interactive‐educational programme; electronic social network intervention; self‐management programme); 1 found no significant effects (e‐mental health); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊕⊝⊝ |
| Caregiver burden Follow‐up: range 6 weeks to 3 months | 2 studies found no statistically significant differences in burden scales between the intervention and control groups (early vs delayed palliative care; electronic social network intervention). | (2 RCTs) | ⊕⊕⊝⊝ |
| Caregiver mastery Follow‐up: range 6 months to 8 months | 1 study found improvements in mastery after the intervention (psychoeducation and cognitive behavioural therapy) compared to care‐as‐usual, corrected for changes in patient functioning. 1 study found no improvements in self‐efficacy or coping strategies (self‐management programme). | (2 RCTs) | ⊕⊝⊝⊝ |
| Quality of patient–caregiver relationship | 1 study found no statistically significant differences in family functioning between the intervention and control groups (e‐mental health vs waiting list). | (1 RCT) | ⊕⊝⊝⊝ |
| Caregiver quality of life Follow‐up: range 30 days to 8 months | 2 studies found improvements in QoL over time in the intervention group (psychosocial intervention; self‐management programme) compared to the control group; 1 study found stable QoL in the intervention group vs decline in the control group (no longer statistically significant after controlling for patient functioning); 2 studies found no statistically significant improvements after the intervention (e‐mental health; early palliative care); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊝⊝⊝ |
| Caregiver physical functioning – not measured | None of the included studies assessed caregiver physical functioning. | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CQOLC: Caregiver QoL Index – Cancer; CES‐D: Center for Epidemiological Studies Depression Scale; CI: confidence interval; DASS‐21: Depression, Anxiety and Stress Scales; HADS: Hospital Anxiety and Depression Scale; LASA: Linear Analogue Self‐Assessment; MBCB: Montgomery‐Borgatta Caregiver Burden; POMS: Profile of Mood States; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; STAI: State‐Trait Anxiety Inventory. | |||
| GRADE Working Group grades of evidence | |||
| aDifferent populations (e.g. mixed cancer caregiver samples, paediatric or adult (or both) caregiver samples). | |||

