Intervenciones para ayudar a apoyar a los cuidadores de pacientes con tumores cerebrales o de la médula espinal
Resumen
Antecedentes
El diagnóstico y tratamiento de un tumor cerebral o de la médula espinal puede tener un gran impacto en la vida de los pacientes y sus familias, ya que el cuidado familiar a menudo resulta en una carga y angustia considerables. Satisfacer las necesidades de apoyo de los cuidadores familiares es fundamental para mantener su salud emocional y física. Aunque la disponibilidad del apoyo a los cuidadores es cada vez mayor, la implementación a gran escala se ve obstaculizada por la falta de evidencia de calidad alta sobre su efectividad en la población de cuidadores de neurooncología.
Objetivos
Evaluar la efectividad de las intervenciones de apoyo para mejorar el bienestar de los cuidadores de pacientes con tumores cerebrales o de la médula espinal. Evaluar los efectos de las intervenciones de apoyo para los cuidadores en la mejora del bienestar físico y emocional de los pacientes con un tumor cerebral o de la médula espinal y evaluar los beneficios económicos para la salud de las intervenciones de apoyo para los cuidadores.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (CENTRAL; 2018, Número 7), MEDLINE vía Ovid y en EMBASE vía Ovid. También se realizaron búsquedas manuales en resúmenes de congresos publicados relevantes (cinco años anteriores), publicaciones en las dos revistas del área principales (año anterior), se realizaron búsquedas de ensayos en curso a través de ClinicalTrials.gov y se estableció contacto con grupos de investigación en el área. La búsqueda inicial se realizó en marzo de 2017 con una actualización en agosto de 2018 (las búsquedas manuales se completaron en enero de 2019).
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios (ECA) en que los cuidadores de pacientes de neurooncología constituían más del 20% de la muestra y que evaluaron los cambios en el bienestar de los cuidadores después de cualquier intervención de apoyo.
Obtención y análisis de los datos
Dos autores de revisión de forma independiente seleccionaron los estudios y evaluaron el riesgo de sesgo. Se intentó extraer datos sobre los resultados de la angustia psicológica, la carga, el dominio, la calidad de la relación paciente‐cuidador, la calidad de vida y la funcionalidad física.
Resultados principales
En total, la búsqueda identificó 2102 registros, de los cuales se revisaron 144 en texto completo. Se incluyeron ocho estudios. Cuatro intervenciones se centraron en las parejas paciente‐cuidador y cuatro se dirigieron específicamente al cuidador. La heterogeneidad de las poblaciones y las metodologías impidió realizar el metanálisis. El riesgo de sesgo varió, y todos los estudios incluyeron solo un número pequeño de cuidadores de neurooncología (13 a 56 participantes). Hubo cierta evidencia de efectos positivos del apoyo de los cuidadores sobre la angustia psicológica, el dominio y la calidad de vida (evidencia de certeza baja a muy baja). Ninguno de los estudios informó de los efectos sobre la carga o la calidad de la relación paciente‐cuidador (evidencia de certeza baja a muy baja). Ninguno de los estudios incluidos evaluó la funcionalidad psicosocial del cuidador. Para los resultados secundarios (bienestar emocional o físico del paciente; efectos económicos sobre la salud), se halló muy poca o ninguna evidencia de la efectividad del apoyo del cuidador. Se han identificado cinco ensayos en curso.
Conclusiones de los autores
Los ocho estudios a pequeña escala incluidos emplearon diferentes metodologías en distintas poblaciones, y la evidencia fue en general de certeza baja. Los resultados indican que actualmente no es posible establecer conclusiones definitivas acerca de la efectividad de las intervenciones de apoyo para mejorar el bienestar de los cuidadores de neurooncología. Se necesita más investigación de alta calidad sobre el apoyo a los cuidadores familiares de pacientes diagnosticados y que conviven con un tumor cerebral o de la médula espinal.
PICO
Resumen en términos sencillos
Intervenciones para ayudar a apoyar a los cuidadores de pacientes con tumores cerebrales o de la médula espinal
El problema
Los cuidadores familiares (p.ej. cónyuges, familiares o amigos cercanos) a menudo brindan apoyo físico y emocional a los pacientes con tumores cerebrales o de la columna vertebral (cáncer). Sin embargo, el cuidado familiar está vinculado a una carga y angustia considerables. Por lo tanto, es importante satisfacer las necesidades de apoyo de los cuidadores familiares para mantener su salud emocional y física. Se espera que esto ayude al cuidador, al paciente y a la unidad familiar.
Objetivo de la revisión
Evaluar la efectividad de las intervenciones de apoyo para mejorar el bienestar de los cuidadores de pacientes con tumores cerebrales o de la médula espinal. Evaluar los efectos de las intervenciones de apoyo para los cuidadores en la mejora del bienestar físico y emocional de los pacientes con un tumor cerebral o de la médula espinal y evaluar los beneficios económicos para la salud de las intervenciones de apoyo para los cuidadores.
Características de los estudios
Se incluyeron ocho estudios clínicos. Cuatro estudios informaron sobre las intervenciones para la relación paciente‐cuidador y cuatro estaban orientados específicamente a mejorar el bienestar de los cuidadores. Se encontraron cinco estudios en curso.
¿Cuáles fueron los principales hallazgos?
Las intervenciones estudiadas fueron de naturaleza diversa (p.ej. terapia cognitivo‐conductual [terapia de conversación]; psicoeducación [proporcionar educación e información a las personas que buscan o reciben servicios de salud mental]; entrenamiento en habilidades de afrontamiento; autocuidado; intervención en redes sociales); y prestación de servicios (p.ej. presencial; basada en la web), y todos los estudios fueron relativamente pequeños (incluidos entre 13 y 56 cuidadores de neurooncología). Se encontró cierta evidencia de los efectos positivos del apoyo de los cuidadores sobre la angustia psicológica, los sentimientos de dominio (es decir, la sensación de estar en control de la situación de los cuidadores) y la calidad de vida.
Fiabilidad de la evidencia
Ninguno de los estudios informó de los efectos sobre la carga o la calidad de la relación paciente‐cuidador. Ninguno de los estudios midió el bienestar físico de los cuidadores. En general, la calidad de la evidencia fue baja a muy baja, lo que significa que el efecto verdadero del apoyo a los cuidadores puede ser significativamente diferente.
Conclusions
Los resultados indican que actualmente no es posible establecer conclusiones definitivas acerca de la efectividad de las intervenciones de apoyo para mejorar el bienestar de los cuidadores de neurooncología. Se necesita más investigación de alta calidad sobre el apoyo a los cuidadores familiares de pacientes diagnosticados y que conviven con un tumor cerebral o de la médula espinal.
Authors' conclusions
Summary of findings
| Interventions to help support caregivers of people with a brain or spinal cord tumour | |||
| Patient or population: caregiver well‐being | |||
| Outcomes | Impact | № of participants | Certainty of the evidence |
| Caregiver psychological distress Follow‐up: range 1 days to 8 months | 4 studies found improvements after the intervention (early palliative care; interactive‐educational programme; electronic social network intervention; self‐management programme); 1 found no significant effects (e‐mental health); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊕⊝⊝ |
| Caregiver burden Follow‐up: range 6 weeks to 3 months | 2 studies found no statistically significant differences in burden scales between the intervention and control groups (early vs delayed palliative care; electronic social network intervention). | (2 RCTs) | ⊕⊕⊝⊝ |
| Caregiver mastery Follow‐up: range 6 months to 8 months | 1 study found improvements in mastery after the intervention (psychoeducation and cognitive behavioural therapy) compared to care‐as‐usual, corrected for changes in patient functioning. 1 study found no improvements in self‐efficacy or coping strategies (self‐management programme). | (2 RCTs) | ⊕⊝⊝⊝ |
| Quality of patient–caregiver relationship | 1 study found no statistically significant differences in family functioning between the intervention and control groups (e‐mental health vs waiting list). | (1 RCT) | ⊕⊝⊝⊝ |
| Caregiver quality of life Follow‐up: range 30 days to 8 months | 2 studies found improvements in QoL over time in the intervention group (psychosocial intervention; self‐management programme) compared to the control group; 1 study found stable QoL in the intervention group vs decline in the control group (no longer statistically significant after controlling for patient functioning); 2 studies found no statistically significant improvements after the intervention (e‐mental health; early palliative care); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊝⊝⊝ |
| Caregiver physical functioning – not measured | None of the included studies assessed caregiver physical functioning. | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CQOLC: Caregiver QoL Index – Cancer; CES‐D: Center for Epidemiological Studies Depression Scale; CI: confidence interval; DASS‐21: Depression, Anxiety and Stress Scales; HADS: Hospital Anxiety and Depression Scale; LASA: Linear Analogue Self‐Assessment; MBCB: Montgomery‐Borgatta Caregiver Burden; POMS: Profile of Mood States; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; STAI: State‐Trait Anxiety Inventory. | |||
| GRADE Working Group grades of evidence | |||
| aDifferent populations (e.g. mixed cancer caregiver samples, paediatric or adult (or both) caregiver samples). | |||
Background
Description of the condition
The diagnosis and treatment of a brain or spinal cord tumour can have a huge impact on the lives of patients and their families. Approximately 28 per 100,000 adults aged 20 years and over are affected by central nervous system tumours, with the majority of tumours (approximately 66%) being non‐malignant (Ostrom 2014). In children and young adults under 19 years of age, central nervous system tumours are the most common tumour, with an annual age‐adjusted incidence rate of 5.4 per 100,000 (Ostrom 2014).
The treatment and expected outcome depend heavily on the tumour type, molecular markers, tumour grade, and location. Treatment generally consists of surgical intervention, radiotherapy, chemotherapy, or a combination of treatment methods. In making treatment decisions, benefit from treatment is weighed against the expected quality of life (QoL) and symptom burden of patients.
Depending upon the tumour location and treatment adverse effects, patients can experience neurological symptoms such as weakness, sensory loss, and motor dysfunction, or visual‐perceptual deficits and problems with speech and language (Mukand 2001). Cognitive deficits such as problems with memory and concentration occur in most patients, and epilepsy is also common (Armstrong 2016a; Durand 2015; van Loon 2015). Moreover, fatigue, depression, and changes in personality and behaviour are frequently reported throughout the course of the disease (Armstrong 2016b; Cavers 2012; Rooney 2011). These symptoms can influence the degree to which patients can participate in vocational and social activities and can prevent independence and affect QoL (Aaronson 2011; Klein 2001; Macartney 2014).
Patients commonly come to rely on their family caregivers (e.g. spouses, family members, or close friends) for both physical and emotional support. Consequently, many family caregivers experience considerable burden and distress, and consistently report feeling ill‐prepared for their caregiving role (Choi 2012; Sterckx 2013). Therefore, interventions to support caregivers are expected to help the caregiver, the patient, and the family unit.
Various studies have explored the needs of family caregivers in neuro‐oncology, and showed a desire for clear information and communication with healthcare professionals, concerning managing patients' symptoms, treatment, and available resources; health service needs and care co‐ordination; and the need for psychological and social supportive care options (Moore 2012; Sterckx 2013).
Description of the intervention
Individual caregivers' needs can vary greatly depending on the patient's time point in treatment, the caregiver's social support system, expectations and experienced burden (i.e. the stress experienced as a result of the home care situation) (Ownsworth 2015a). Therefore, any intervention programme aimed at improving the well‐being of family caregivers in neuro‐oncology was considered for this review. Here, the term 'well‐being' encompassed all aspects of QoL, psychological distress, coping, and mastery (i.e. the feeling of being in control of the caregiving situation).
The interventions under investigation included, but were not limited to, programmes aimed at supporting family caregivers through:
-
improving information provision (e.g. what to expect from their role as a family caregiver; teaching caregivers what the treatment options are; and educating them on supportive care options);
-
caregiver skills training (e.g. how to recognise (changes in) patients' symptoms; how to manage symptoms or improve patients' everyday functioning); and
-
psychosocial support (e.g. psychosocial interventions to help caregivers cope better; therapeutic interventions to promote a healthy relationship between the patient and caregiver; bereavement support after the patient has died).
Following the National Institute for Health and Care Excellence (NICE) recommended model of psychological assessment and support (NICE 2004), interventions could be any from level 1 (information and general support given by any health and social care professional – or self‐help) to level 4 (specialist psychological or psychiatric interventions delivered by mental health professionals). We did not expect effectiveness of interventions to vary within different subgroups of caregivers (e.g. grade of tumour and age of patient). The interventions were not expected to pose a risk to caregivers; however, length or complexity of intervention programmes may have increased caregiver burden and could have caused caregivers to feel overwhelmed instead of supported.
How the intervention might work
Supportive interventions for family caregivers in neuro‐oncology may help in various ways.
Improving information provision and caregiver skills training can help prepare family members and friends for their caregiving role and activities. When caregivers learn more about the disease and its symptoms, they feel more confident in distinguishing between which (changes in) symptoms could be normal or expected and which may require medical follow‐up. Through this mechanism, patient outcomes may be improved as better symptom management is initiated sooner and new tumour activity may be detected earlier in the disease trajectory, allowing treatment to commence. Moreover, symptoms may be recognised and treated before becoming more serious and requiring inpatient treatment, thus potentially reducing healthcare costs. Finally, increasing caregivers' confidence in dealing with the care situation can substantially improve their feelings of mastery. This may have a positive effect on their overall well‐being, their QoL, and the quality of care they deliver in the home situation.
Psychosocial support can provide caregivers with the tools to improve coping strategies to deal with the psychological burden of being a caregiver to a person who has been diagnosed with a brain or spinal cord tumour. Many patients and caregivers struggle with maintaining a healthy relationship, particularly after changes in the patient's personality and behaviour, and psychological support to caregivers or patient–caregiver dyads can help couples work through these issues together. It is known that patients who go through divorce or separation are more likely to be hospitalised and less likely to complete treatment, become involved in clinical trials, or die at home (Glantz 2009). Therefore, promoting healthy patient–caregiver relationships may have a positive effect on long‐term patient outcomes. This can help decrease caregivers' levels of distress and burden. As many caregivers will provide care for a longer period of time, up to many years on end, decreasing distress and burden may prove beneficial as the physical consequences of long‐term high levels of stress may be prevented. Finally, maintaining good physical as well as emotional health in caregivers will allow them to continue their caregiving tasks, which will benefit patients as well.
Why it is important to do this review
Meeting the needs of family caregivers in neuro‐oncology, by decreasing their distress and burden and improving their sense of mastery, is imperative in order to maintain their emotional and physical health. Protecting caregivers' QoL can enable them to continue their caregiving activities to maintain the best possible level of patients' well‐being. Indeed, caregiver support is listed as a top research priority in neuro‐oncology in the UK through the James Lind Alliance Priority Setting Partnership (Grant 2015). Furthermore, the UK National Health Service (NHS) has made several commitments to caregivers, including supporting caregivers' mental health and well‐being alongside physical needs (NHS England 2014).
Information and support for caregivers of patients with brain and spinal cord tumours is becoming more widely available and caregiver programmes are becoming more common in clinical practice in some centres. Specialised nurses who may also provide caregiver support corresponding with level 1 of the NICE model of psychological support (NICE 2004), are in many countries part of the treatment team. However, large‐scale implementation of caregiver support may be hindered by the lack of high‐quality evidence for the effects of caregiver interventions in populations of brain and spinal tumour patients. Indeed, one report from Macmillan Cancer Support revealed that more than half of family caregivers in oncology did not receive support at present (Macmillan/You Gov 2016). This systematic review will provide an overview of caregiver interventions for those taking care of patients with a brain or spinal tumour, assessed in randomised controlled trials (RCTs). It will also provide a brief economic summary of the health economic benefits where these have been measured. It is expected that this will be useful to make recommendations for policy and practice.
Objectives
To assess the effectiveness of supportive interventions at improving the well‐being of caregivers of people with a brain or spinal cord tumour. To assess the effects of supportive interventions for caregivers in improving the physical and emotional well‐being of patients with a brain or spinal cord tumour and to evaluate the health economic benefits of supportive interventions for caregivers.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs. We included trials that used quasi‐randomised methods if there was sufficient evidence that the treatment and control groups were similar at baseline. If this was unclear, we contacted trial authors to request clarification.
Types of participants
Studies with adult caregivers (aged 18 years or older) for people with a brain or spinal cord tumour. The people for which they provide care could have been of any age, with any type of malignant or non‐malignant, primary or secondary brain or spinal cord tumour, at any time during the disease trajectory.
Types of interventions
Any type of intervention whose primary aim was to improve caregiver well‐being. We included trials that evaluated the effectiveness of individual‐ and group‐based interventions for caregivers or for patient–caregiver dyads as long as they reported caregiver outcomes. We placed no restrictions on: the setting (e.g. hospital, clinic, psychologist office, at home, or elsewhere); the facilitator of the intervention (e.g. a healthcare professional (including nurse specialists), social worker, or (guided) self‐help); or the method of delivery of the intervention (e.g. delivered face‐to‐face, online, written, or by telephone). Thus, interventions could reflect any level of psychological support from the NICE model (NICE 2004). Any control condition was acceptable (e.g. wait list control groups, attention‐only control groups, and information‐only control groups). We contacted trial authors if it was unclear whether a trial met our inclusion criteria.
Types of outcome measures
For all primary outcomes, we accepted recognised caregiver questionnaires or instruments measuring mood, caregiver burden, mastery, marital adjustment, QoL, and physical functioning. Where measured, we assessed the effect on patient emotional and physical well‐being patient questionnaires under Secondary outcomes. Acceptable outcomes are listed below.
Primary outcomes
Outcomes related to caregiver emotional or physical well‐being
-
Psychological distress (depression and anxiety) (e.g. Hospital Anxiety and Depression Scale (HADS; Crawford 2001), Center for Epidemiological Studies Depression Scale (CES‐D; Radloff 1977)).
-
Caregiver burden (e.g. Caregiver Reaction Assessment (CRA; Given 1992)).
-
Caregiver mastery (e.g. Mastery Scale (Pearlin 1978)).
-
Quality of patient–caregiver relationship (e.g. Locke‐Wallace Short Marital Adjustment Test for spousal relationships (Jiang 2013)).
-
Quality of life (QoL), either caregiver specific (e.g. Caregiver QoL index‐cancer (CQOLC; Weitzner 1999), Caregiver Oncology QoL Questionnaire (CarGOQoL; Minaya 2012), or generic, e.g. Short Form Health Survey (SF‐36; McHorney 1993), EuroQol (EQ‐5D Brooks 1996)).
-
Physical functioning (e.g. number of chronic conditions present, physical measures of stress levels (e.g. cytokines), physical subscales of QoL questionnaires).
Secondary outcomes
Outcomes related to patient emotional or physical well‐being
-
Psychological distress (depression and anxiety) (e.g. HADS (Crawford 2001), CES‐D (Radloff 1977)).
-
QoL (e.g. European Organization for Research and Treatment of Cancer QLQ‐C30 (EORTC QLQ‐C30; Aaronson 1993); Functional Assessment of Cancer Therapy (FACT; Weitzner 1995), 36‐item Short Form Health Survey (SF‐36; McHorney 1993)).
-
Symptom management, number or severity (or both) of symptoms (e.g. measured with MD Anderson Symptom Inventory‐Brain Tumor Module (MDASI‐BT; Armstrong 2006), EORTC Brain Cancer Module (EORTC QLQ‐BN20; Taphoorn 2010)).
-
Number of visits to the emergency department (e.g. as detailed in medical records).
-
Number and length of hospitalisations (e.g. as detailed in medical records).
Outcomes related to the health economic effects
-
Caregiver or patient (or both) employment status (e.g. self‐reported).
-
Productivity loss at work of caregiver or patient (or both) (e.g. self‐reported).
-
Caregiver healthcare utilisation for acute or chronic (or both) conditions (e.g. self‐reported or as detailed in caregiver's medical records).
We included trials with different outcomes to those mentioned above, when they measured the same construct.
Search methods for identification of studies
There were no restrictions based on type of publication, year of publication, or language. We considered both published and unpublished RCTs.
Electronic searches
We searched the following databases on 24 August 2018:
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7), in the Cochrane Library, using the search strategy in Appendix 1;
-
MEDLINE via Ovid (1946 to August week 3 2018), using the search strategy in Appendix 2;
-
Embase via Ovid (1980 to 2018 week 34), using the search strategy in Appendix 3.
We also searched ClinicalTrials.gov (clinicaltrials.gov/).
Searching other resources
We handsearched the references of identified studies for studies that were not identified through the electronic search.
We searched conference abstracts and proceedings from 2013 to 2018 through the American Society of Clinical Oncology (ASCO; www.asco.org/ASCO/Meetings), the Society for Neuro‐Oncology (SNO; supplements of Neuro‐Oncology; academic.oup.com/neuro‐oncology), and the International Psycho‐Oncology Society (IPOS; special issues of Psycho‐oncology).
We handsearched the two main journals in the field of neuro‐oncology, Neuro‐oncology and Journal of Neuro‐oncology, for publications from 2017 that were not identified through the electronic search.
We contacted the authors of publications known to focus on improving the well‐being of caregivers of patients with a brain or spinal cord tumour, to enquire about unpublished or ongoing trials. These additional searches were completed on 20 July 2017 (for the initial search) and 23 January 2019 (for the updated search).
Data collection and analysis
Full details on planned data collection and analysis are available in the published protocol (Boele 2017a).
Selection of studies
Two review authors (FB and AR) selected studies for inclusion. After removing duplicates, the two review authors independently screened all titles and abstracts. We excluded studies that did not meet the inclusion criteria while storing these discarded studies in a file as potentially relevant. We retrieved full‐text reports and subjected the eligible studies to further assessment. We documented reasons for exclusion and resolved disagreements between review authors by discussion. If the published report contained too little information to assess the trial, one review author (FB) contacted the study authors to request further details.
Data extraction and management
Two review authors (FB and AR) examined each selected report and extracted data using a data collection form based on Cochrane Consumers and Communication's Group data extraction template (Cochrane CCG 2016). This data collection form included participant characteristics (e.g. age, sex, group size, patients' tumour type, grade, disease stage, etc.) and information about the supportive intervention (e.g. method, duration, delivery, provider); the time points at which the outcomes were assessed; whether outcomes were self‐reported or other; whether the tool was validated; how missing data were handled; statistical methods used; and whether these were appropriate. The form also included details on the results (continuous outcomes: mean difference (MD) and standard error (SE), number of participants; dichotomous outcome data: e.g. number of caregivers who showed an improvement in terms of emotional or physical well‐being as a proportion of the total number treated; and other results e.g. MD, odds ratio, risk difference, confidence intervals (CI), P values), and information on adherence and attrition (Chandler 2013).
Assessment of risk of bias in included studies
The two review authors responsible for the selection of studies and data extraction assessed the risk of bias in accordance with the Cochrane tool for assessing risk of bias (Higgins 2011a). This included several domains: random sequence generation; allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. The risk of bias was categorised as high, low, or unclear. 'High risk' was selected if there was a non‐random component (random sequence generation); if participants or investigators enrolling participants could possibly foresee assignment which could introduce selection bias (allocation concealment); if participants and personnel were not completely blinded and the outcome was likely to be affected by lack of blinding (blinding of participants and personnel); if there was no blinding of outcome assessment and outcome measurement was likely to be influenced (blinding of outcome assessment); if the reason for missing data was likely related to true outcome with either imbalance in numbers or reasons for missing data across groups, if the results for missing outcomes likely induced clinically relevant bias, if participants in 'as treated' analysis did not receive the intervention as planned, or if simple imputation was inappropriately applied (incomplete outcome data); if not all prespecified outcomes were reported or outcomes were reported using measurements, analysis methods or subsets of the data that were not prespecified (selective reporting). The risk of bias in the included studies was discussed between review authors.
Measures of treatment effect
For dichotomous outcome data, we aimed to abstract the number of caregivers who showed an improvement in terms of emotional or physical well‐being as a proportion of the total number treated. We aimed to calculate and present risk ratios (RRs) with 95% CIs.
For continuous outcome data from studies using the same instrument, we aimed to estimate MDs between treatment groups. Where different instruments were used, we aimed to calculate the standardised mean difference (SMD) by dividing the MD in postintervention scores between the intervention and control groups by the standard deviation (SD) of the outcome among participants. We aimed to present both the MD and SMD with 95% CIs for individual outcomes in individual studies. If these data were unavailable, we contacted study authors to request additional information, and if still unavailable, we presented the reported significance levels instead.
Unit of analysis issues
Different levels of randomisation (e.g. at the level of participants or groups) were taken into account. When there were long‐term follow‐up assessments available within trials, we aimed to analyse outcomes for two different follow‐up categories: short term (i.e. zero to three months); or medium to long term (i.e. four months and more). If studies with multiple intervention groups were identified, we aimed to make pair‐wise comparisons between all possible pairs of intervention groups. We aimed to make efforts not to double‐count participants in the analysis.
Dealing with missing data
We contacted the corresponding authors of the trials in writing to request missing data. We evaluated the reporting of important numerical data such as the number of screened and randomised participants, and whether intention‐to‐treat or per‐protocol analyses were done. Missing data were not imputed (Higgins 2011a).
Assessment of heterogeneity
We planned to assess the impact of the heterogeneity of included intervention studies with the I2 statistic for each outcome (Higgins 2011b). Substantial heterogeneity would be defined as I2 greater than 50% and forest plots were to be visually inspected for heterogeneity. In the case of meta‐analysis being possible, we planned to use a random‐effects model as a certain degree of heterogeneity was expected.
Assessment of reporting biases
We planned to draw funnel plots of treatment effect versus precision with the data from all studies (Higgins 2011b), if at least 10 studies were identified. The funnel plots were to be visually inspected to assess whether there was selective reporting of outcomes.
Data synthesis
If trials included different outcomes, we aimed to pool outcomes that measured the same construct, or systematically report on outcomes that did not measure the same construct.
We aimed to perform a meta‐analysis if we found two or more RCTs with a low risk of bias in which study population, intervention, and outcome measures were comparable. With meta‐analysis not possible, two review authors (FB and HB) synthesised the findings of the included studies in summary of findings Table for the main comparison, and rated the overall certainty of the evidence according to the GRADE levels of evidence (Higgins 2011a; Ryan 2016).
Subgroup analysis and investigation of heterogeneity
If sufficient studies were identified (i.e. at least two for each subgroup), we had planned to perform subgroup analyses for the study design (RCT or quasi‐RCT), the type of intervention, the type of control group, timing (e.g. shortly after the patient's diagnosis, during initial antitumour treatment, following initial treatment, in the palliative phase or during the bereavement phase), and patient tumour type.
Sensitivity analysis
If sufficient data were available, we planned to perform a sensitivity analysis to assess the robustness of results (e.g. excluding studies with high risk of bias).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
A flowchart of our search is shown in Figure 1. The initial search yielded 1670 records, supplemented by 413 records found in electronic database searches and handsearching conference abstracts and proceedings from 2013 to 2018, as well as publications in Neuro‐oncology and Journal of Neuro‐oncology from 2017. After removing duplicates, 1666 records remained. Upon screening of titles and abstracts, results were narrowed down to 122 records. Of these, 117 were excluded: 50 records were not (quasi) RCTs; 27 studies did not aim to improve caregiver well‐being; 13 were not focused on caregivers or (or both) neuro‐oncology; 15 were duplicates; four were published protocols only. Thirteen studies were potentially eligible and we requested additional information from the authors. Four publications included less than 20% of neuro‐oncology caregivers in their samples (Cernvall 2015; Hudson 2015; Kissane 2016; Mooney 2015), making it debatable whether the outcomes would be generalisable to the neuro‐oncology caregiver population. There were four ongoing trials (Halkett 2015; Langbecker 2016; Ownsworth 2015b; Roberge 2016).

Study flow diagram. RCT: randomised controlled trial.
The search update yielded a further 413 references. Publications and conference abstracts were also screened at this time. In total, the search update produced 436 records. Of these, 22 were reviewed in detail, and 19 were excluded: 13 records were not (quasi) RCT; two did not aim to improve caregiver well‐being, and three included less than 20% neuro‐oncology caregivers, or an unclear percentage (Epstein 2017; Holm 2016; Lawsin 2017). There was one ongoing trial (NCT03454295). Three additional trials were thus included after the search update.
Eight publications were included in the review.
Included studies
See Characteristics of included studies tables.
Design and setting
Seven studies were RCTs (Andela 2017; Boele 2013; Dionne‐Odom 2015; Klosky 2007a; Reblin 2018; Safarabadi‐Farahani 2016; Wakefield 2016), another was quasi randomised as the last three participants were allocated to the intervention group automatically (Locke 2008). In four publications, randomisation took place at the level of the family or patient–caregiver dyad (Andela 2017; Dionne‐Odom 2015; Klosky 2007a; Locke 2008). In one study, both parents could participate and were then assigned to the same condition, but in different groups (Wakefield 2016). In three studies, caregivers were randomised at the individual level by themselves (Boele 2013; Reblin 2018; Safarabadi‐Farahani 2016). Four trials took place in the US (Dionne‐Odom 2015; Klosky 2007a; Locke 2008; Reblin 2018), one in Australia (Wakefield 2016), two in the Netherlands (Andela 2017; Boele 2013), and one in Iran (Safarabadi‐Farahani 2016).
Participant demographics
The total sample sizes for the included studies ranged from 19 (Locke 2008) to 122 (Dionne‐Odom 2015) randomised participants. However, not all eight studies exclusively focused on neuro‐oncology caregiver populations. We included studies only if more than 20% of caregivers in the sample were taking care of a patient with a primary or secondary brain or spinal cord tumour. As a result, the sample size for the subset of participants of interest to this review were smaller, ranging from 13 (29% of the sample; Wakefield 2016) to 56 (100% of the sample; Boele 2013) neuro‐oncology family caregivers. Participant demographics were only available for the total samples of the included studies. Two studies did not report on caregiver demographics (Klosky 2007a; Locke 2008). Participants' mean age ranged from 42 years (Wakefield 2016) to 61 years (Dionne‐Odom 2015). In all studies except one (Andela 2017 (44%)), the majority of caregivers were female (ranging from 64% of the total sample in Boele 2013 to 95% in Safarabadi‐Farahani 2016). Of note, three studies focused on family caregivers of children diagnosed with cancer (Klosky 2007a; Safarabadi‐Farahani 2016; Wakefield 2016); the other five studies only included those taking care of an adult (Andela 2017; Boele 2013; Dionne‐Odom 2015; Locke 2008; Reblin 2018). Only one study included a significant proportion of caregivers taking care of a patient with secondary brain tumours (brain metastases; Dionne‐Odom 2015).
Intervention characteristics
Four of the interventions focused on patient–caregiver dyads (Andela 2017; Dionne‐Odom 2015; Klosky 2007a; Locke 2008), four aimed to improve specifically caregiver well‐being and did not involve the patient directly (Boele 2013; Reblin 2018; Safarabadi‐Farahani 2016; Wakefield 2016).
Caregiver‐focused interventions
The intervention tested by Boele 2013 was based on the principles of cognitive behavioural therapy (CBT) and psychoeducation. Once every two weeks, for a total of six sessions, a psychologist would meet with the caregiver. During the first session, the caregiver and psychologist reviewed the issues and needs of the caregiver. During the second session, an introduction of the intervention and rationale behind CBT was given. A selection of topics was available for the other four sessions: 1. contact with the patient; 2. the direct environment (contact with family, friends, and other); 3. epilepsy; 4. changes in behaviour, character, and cognition; 5. time for yourself; 6. children (what and how to tell them); and 7. practical and emotional care in the end of life phase. The participants in the control group received care as usual.
Reblin 2018 tested a web‐based intervention called eSNAP, which aimed to help caregivers visualise their existing social network resources. Users were able to list people and groups who could help within six categories of support: 1. hands on; 2. informational; 3. communication; 4. financial; 5. emotional; and 6. self‐care. A visualisation of the support network was created based on caregivers' input and printed in PDF. The intervention was completed within one session (taking approximately 10 to 15 minutes), while waiting for the patient's hospital appointment, and the visualisation report could be revisited by caregivers if they wished. Participants in the control group received care as usual.
The Brief Psychological Intervention (BPI) tested by Safarabadi‐Farahani 2016 employed problem‐solving skills training and psychoeducation. In five sessions lasting between 60 and 90 minutes, a trained social worker covered the following aims: 1. engage and motivate caregivers to participate and develop open communication with the social worker; 2. develop an optimistic attitude, maintaining hope and focus on achievable short‐term goals; 3. provide information about treatments and medication, and living with uncertainty; 4. help caregivers cope with stress and teach stress‐reducing techniques, coping strategies, and promote healthy lifestyle behaviours; and 5. education of self‐care strategies. Sessions were followed up with a telephone call (five in total). The control group participants received care as usual, including counselling and financial support.
The Cascade programme tested by Wakefield 2016 was based on the Uncertainty in Illness model and Family‐Systems‐Illness model. The three‐week programme consisted of weekly 120‐minute online sessions delivered by a psychologist through WebEx. After each session, caregivers would get homework assignments to practice. The programme intended to target intra‐ and interpersonal psychological processes that are important to adaptation in the context of illness (e.g. acceptance of uncertainty). CBT strategies were used to target these core mechanisms of change. The topic areas were not specified in the publication. Control group participants could participate in the Cascade programme after a six‐month waiting list period.
Patient–caregiver dyadic interventions
Andela 2017 tested the 'Patient and Partner Education Programme for Pituitary disease' (PEPP‐pituitary). This was based on a standardised self‐management programme originally developed for Parkinson's disease, and supplemented with information for fatigue, cognitive complaints, and problems with sexuality. It addressed psychological and social issues related to the disease and used techniques from CBT such as cognitive restructuring, situational behavioural analysis, social skills training, and relaxation training. The eight‐week programme consisted of 90‐minute sessions guided by a psychologist or medical social worker. Patients and caregivers participated in separate groups of five to seven participants. The sessions were titled: 1. information; 2. self‐monitoring; 3. health promotion; 4. stress management; 5. management of anxiety and depression/caregivers' challenge; 6. social competence; 7. social support; and 8. evaluation. Control group participants could take part in a single (optional) information meeting in week four or five and were given the option to take part in the PEPP‐pituitary programme after the last assessment.
Dionne‐Odom 2015 tested a dyadic intervention in the ENABLE III (Educate, Nurture, Advise, Before Life Ends III) trial. Based on their earlier ENABLE II palliative care intervention and exploratory interviews with family caregivers, caregivers were more closely involved in the new trial. The COPE framework (Creativity, Optimism, Planning, Expert Information) was applied in developing this coping skills intervention. In three weekly structured educational sessions, two different palliative care nurses supported both caregiver and patient. For caregivers, the first session covered the role of the caregiver, a definition of palliative and supportive care, and an introduction of problem‐solving using the COPE framework. The second session covered caregiver self‐care and effective partnering in patient care. The final session addressed building a support network, decision making and support, and advance care planning. At least once a month, the nurse would follow‐up with telephone calls until patient death or study end. If the patient died during the study, a bereavement call was made. After a waiting list period of three months, participants in the control group could take part in the programme as well.
The programme tested by Klosky 2007a was based on CBT principles. During radiotherapy simulation, families randomised to the intervention group received a CBT‐based programme including exposure to an interactive‐educational ActiMates Barney, an educational video in the clinic room including filmed modelling, and passive distraction via Barney‐narrated stories delivered during the simulation procedure. Families randomised to the control group received a similar intervention with exposure to a non‐interactive children's character, an age‐appropriate cartoon video picked by the child, and stories delivered via cassette tape during simulation.
Locke 2008 tested a dyadic intervention based on cognitive rehabilitation and problem‐solving. In six sessions in a two‐week period, a psychologist or behavioural therapist taught dyads to use a calendar that had a specific format as an external aid to compensate for cognitive symptoms. A model of stress was taught as well as specific positive problem‐solving techniques for its management. Potential disease‐related problems were covered (e.g. adverse effects, psychological distress, family issues, and sexual issues).
Primary and secondary outcomes
Caregiver‐focused interventions
Boele 2013 assessed the primary outcomes of caregiver mastery (Caregiver Mastery Scale) and QoL (SF‐36 Mental Component Summary) at baseline, two, four, six, and eight months' follow‐up. Caregivers also completed questionnaires on patient functioning (QoL (SF‐36); Medical Outcome Study (MOS) Subjective Cognitive Functioning scale; EORTC brain cancer module). Health economic effects were not assessed.
In Reblin 2018, the primary outcome was feasibility. They also assessed caregiver burden (Zarit Caregiver Burden Scale) and distress (HADS) at baseline, three weeks, and six weeks' follow‐up. Caregiver completion of the intervention and satisfaction was also assessed. No patient well‐being or health economic outcomes were included.
Safarabadi‐Farahani 2016 assessed caregiver QoL (Caregiver QoL Index – Cancer, Persian version) at baseline, postintervention, and 30 days' follow‐up. This questionnaire measured four scale scores: mental/emotional burden, lifestyle disruption, positive adaptation, and financial concerns, as well as an overall QoL score. Outcomes related to patient well‐being or health economic effects were not included.
Wakefield 2016 were primarily interested in assessing feasibility (response and attrition rates, participant preference for intervention and questionnaire length; therapist's clinical impressions and technical difficulties) and acceptability (California Psychotherapy Alliance Scale‐Group short version; Youth Satisfaction Questionnaire). Caregiver QoL (QoL – Family Caregiver Tool), psychological functioning (Depression Anxiety Stress Scale (DASS) Short Form), and family functioning (McMaster Family Assessment Device) were also assessed. Caregivers completed questions at baseline, two weeks, and six months. Outcomes related to patient well‐being or health economic effects were not included.
Patient–caregiver dyadic interventions
Andela 2017 did not specify their primary/secondary outcomes. Caregiver measures included mood (Visual Analogue Scale – Mood), self‐efficacy (General Self‐efficacy Scale), illness perceptions (Brief Illness Perception Questionnaire), coping strategies (Utrecht Coping List), QoL (SF‐36), fatigue (Multidimentional Fatigue Inventory), and anxiety and depression (HADS). Assessments took place at baseline, eight weeks, and six months. Patient outcomes included all of the above, plus bother and need for support (Leiden Bother and Needs Questionnaire), participation and autonomy (Impact on Participation and Autonomy), QoL (EQ‐5D in addition to the SF‐36), disease‐specific QoL (AcroQol and CushingQol). Health economic effects were not measured.
Dionne‐Odom 2015 did not specify which were their primary/secondary outcomes. Caregiver QoL (Caregiver QOL Index – Cancer), depression (CES‐D), and caregiver burden (Montgomery‐Borgatta Caregiver Burden) were reported in the main outcomes publication. Complicated grief (Prigerson Inventory of Complicated Grief – Short Form; Dionne‐Odom 2016) and personality (Neo‐3 Personality Inventory) were also assessed (confirmed via correspondence). Measurements took place at baseline and every six weeks until week 24, then every three months until patient death or student completion. Patient outcomes were reported in another publication (Bakitas 2015), and included: QoL (Functional Assessment of Chronic Illness Therapy – Palliative Care and Treatment Outcome Index), symptom impact (QoL at End of Life symptom impact sub scale), mood (CES‐D), one year and overall survival, resource use and location of death (patient‐reported hospital and intensive care unit (ICU) days, emergency department visits; from medical record review or proxy reports: decedents' data for period between last assessment and death, chemotherapy use in the last 14 days, location of death).
In Klosky 2007a, caregivers rated their anxiety (State‐Trait Anxiety Inventory (STAI)) and completed a study‐specific efficacy questionnaire. Assessments took place before and after the intervention, which was on the same day. Child outcomes were reported elsewhere (Klosky 2004; Klosky 2007b; Tyc 2002): a behavioural observational checklist (modified from Observation Scale of Behavioral Distress) as completed by trained clinical observers; heart rate; sedation; state and trait anxiety (STAI) as completed by parents; and radiotherapy questionnaire to rate parents' expectations of their child's distress during radiotherapy. Health economic effects were not assessed.
The Locke 2008 study's primary outcomes were patients' compensation techniques (Compensation Techniques Questionnaire), patient and caregiver feedback (study‐ specific poststudy feedback questionnaire), and patient QoL and functional capacity (Functional Assessment of Cancer Therapy – Brain, Mayo‐Portland Adaptability Inventory – 4). Secondary outcomes were patient cognitive functioning (Repeatable Battery for the Assessment of Neuropsychological Status), overall patient and caregiver QoL (Linear Analogue Self‐Assessment scale), caregiver QoL (Caregiver QoL Index – Cancer), patient and caregiver mood (Profiles of Mood States (POMS)), and patient fatigue (Brief Fatigue Inventory). Health economic effects were not assessed. Assessments were done at baseline, postintervention, and three months' follow‐up.
Statistical analysis
Caregiver‐focused interventions
Boele 2013 analysed only the long‐term effects (eight months' follow‐up). Missing data (42.9%) were handled with the last observation carried forward method, missing data from within completed questionnaires were not imputed. All participants were included in analysis following the intention‐to‐treat principle. Delta scores for change in caregiver mastery and mental functioning were calculated and entered into a multivariate linear regression model together with patient's QoL, cognitive functioning, and neurological functioning.
Reblin 2018 used mixed models to analyse differences in distress, burden, and social support between the intervention group and control group at three and six weeks' follow‐up, corrected for baseline scores. Attrition at three weeks was 7.5% (three participants dropped out in the intervention group; confirmed via correspondence), and was 20% at six weeks' follow‐up. Missing data from within completed questionnaires were not imputed (confirmed via correspondence).
Safarabadi‐Farahani 2016 checked for baseline differences in sociodemographic variables and QoL scores using Chi2 and t‐tests. Repeated measures analysis of variance was done to compare QoL scores at postintervention and 30 days' follow‐up between the intervention and control group. This was not corrected for baseline scores. Questionnaires were checked by the research team directly after completion, resulting in no missing data (confirmed via correspondence).
Wakefield 2016 was a pilot study and not powered to evaluate the efficacy of the intervention. Preliminary analyses using a two (group: intervention versus waiting list) by three (time point: baseline versus postintervention versus follow‐up) mixed analysis of variance were performed following the intention‐to‐treat principle. Caregivers who did not complete all three assessments were excluded from the psychosocial outcomes analysis. It is not clear how the authors handled missing data.
Patient–caregiver dyadic interventions
Andela 2017 compared mood ratings at pre‐ and postintervention with paired sample t‐tests. Linear mixed models with random participant effect and fixed time and group effects were used to assess the effects of the intervention at eight weeks' and six months' follow‐up, taking into account missing data (35% in caregivers). Post hoc analyses (linear mixed models) were performed with data from participants who attended at least six out of eight sessions (52% of caregivers). P values of 0.05 were considered statistically significant, but Bonferroni corrections were also applied (P < 0.005).
Dionne‐Odom 2015 performed two longitudinal, intention‐to‐treat analyses. First, between‐group differences in change from baseline to three months were examined for caregiver QoL, depressed mood, and burden. In the second analysis, data from caregivers of whom the patient had died were examined in a terminal‐decline model with all data from the last 36 weeks of the patient's life. The exact statistical methods used are not specified further. The report stated that patterns of missing data were analysed and the authors referred us to another publication for further information (Bakitas 2015). Here, it is stated that maximum likelihood estimates were used to handle missing outcome data; however, it was unclear if the statistical methods applied would have been the same.
Klosky 2007a did not include a statistical methods paragraph; however, the main outcomes seem to be analysed using t‐tests with a one‐sided P < 0.05 as level of statistical significance. In email correspondence, the authors stated that the statistical methods should be included in the other publications on the same trial (Klosky 2004; Klosky 2007b; Tyc 2002).
The pilot study by Locke 2008 was primarily aimed at assessing the feasibility of the intervention. Recruitment rates, use of taught strategies, and patient satisfaction were analysed using descriptive statistics. Patient QoL and functional capacity were analysed using Wilcoxon signed rank tests (scores from the same group at different time points) and Wilcoxon rank sum tests (scores from different groups at the same time point). All other outcomes including caregiver outcomes were only displayed with descriptive statistics.
Excluded studies
See Characteristics of excluded studies table. Three studies were identified that met the inclusion criteria, but did not include a large enough proportion of family caregivers of patients diagnosed with a primary or secondary brain tumour. For the purpose of this review, the cut‐off was set at 20% of the sample. Four further studies would potentially qualify but authors did not provide us with the number of family caregivers of brain tumour patients.
A 10‐week online guided self‐help programme based on CBT was tested in a Swedish population of parents of children on cancer treatment (Cernvall 2015; Cernvall 2017). The study showed promise in reducing post‐traumatic stress symptoms in parents. Parents of children with brain tumours made up 15% of the sample.
The VOICE (Values and Options in Cancer Care) trial aimed to improve communication between people with advanced cancer, caregivers, and oncologists (Epstein 2017). Significant improvements in doctor–patient communication were found. The authors explained the study is likely to have included caregivers of patients with primary or secondary brain tumours. However, it was unfeasible for the local team to retrieve exact numbers.
Holm 2016 tested a psychoeducational group intervention in family caregivers of patients in specialised palliative home care in Sweden. Preparedness for caregiving improved in 55% of participants randomised to the intervention (Holm 2017). We contacted the authors to enquire about the numbers of caregivers of patients with a brain or spinal cord tumour, but received no reply.
In an effort to prepare caregivers for the role of supporting patients with advanced cancer receiving home‐based palliative care, a three‐arm RCT was initiated comparing a one‐to‐one psychoeducational intervention with one or two visits, and a care as usual control group (Hudson 2013; Hudson 2015). There were no reductions in unmet needs or improvements in positive aspects of caregiving, but the intervention improved caregivers' level of preparedness and competence. Caregivers of patients with brain tumours were included, but only made up 1% of the sample (confirmed via correspondence).
A family therapy programme was trialled in people with advanced cancer and their family caregivers, which was continued into bereavement (Kissane 2016). Compared with standard care, the programme reduced the severity of complicated grief and the development of prolonged grief disorder. The authors confirmed via correspondence that only few, if any, caregivers of patients with a brain or spinal cord tumour were included (no exact percentage provided).
In Australia, the 'Rekindle' programme was tested in a phase II feasibility study (Lawsin 2017). This online programme aimed to provide psychosexual support to cancer patients and their partners. The study was found feasible, with varying levels of participant engagement with the programme. We contacted the authors to enquire about the numbers of caregivers of patients with a brain or spinal cord tumour, but we received no reply.
Finally, an abstract presented at the 2015 IPOS conference focused on an RCT of an automated remote symptom monitoring intervention versus care as usual for family caregivers providing home hospice care (Mooney 2015). Family caregivers reported their own issues as well as patients' symptoms and received automated tailored coaching. Moderate/high symptoms would generate an alert to a hospice nurse. Symptom severity decreased and anxiety and mood improved after the intervention. Family caregivers of patients with primary or secondary brain tumours made up 9% of the sample (confirmed via correspondence).
Ongoing studies
We identified five potentially relevant ongoing studies (Halkett 2015; Langbecker 2016; NCT03454295; Ownsworth 2015b; Roberge 2016; Characteristics of ongoing studies table).
Risk of bias in included studies
The Cochrane risk of bias score was determined for each trial and summarised in Figure 2 and Figure 3.
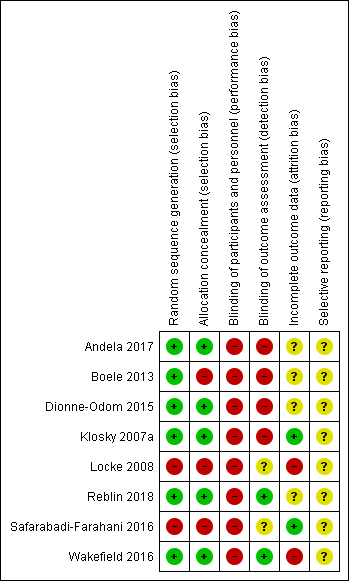
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
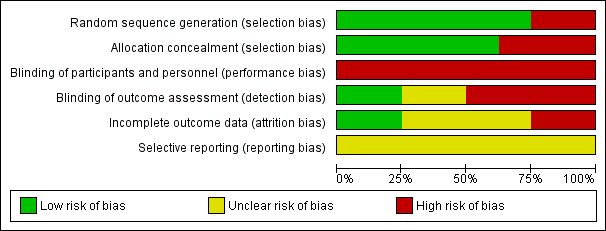
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Most studies described a random component in the sequence generation process. The Locke 2008 publication did not specify the randomisation technique used and was quasi‐randomised, which introduced bias (high risk of bias). In the Safarabadi‐Farahani 2016 study, a team member would number participants (0 to 65) and alternate allocation to the intervention or control group (confirmed via correspondence), leading to an increased risk of bias (high risk of bias). Selection bias may have also been introduced in Boele 2013, as tickets drawn from a concealed box were not numbered (high risk of bias).
Blinding
Blinding of participants and research personnel was generally not possible due to the nature of the interventions (high risk of bias). The person performing the statistical analysis was only blinded in the Wakefield 2016 and Reblin 2018 studies (low risk of bias). It was unclear whether the statisticians were blinded in the Locke 2008 and Safarabadi‐Farahani 2016 studies (unclear risk of bias). This may have introduced performance and detection bias.
Incomplete outcome data
The Klosky 2007a study did not have any attrition, with the pre‐ and postintervention assessments taking place on the same day (confirmed via correspondence) (low risk of bias). In Safarabadi‐Farahani 2016, only 4.6% of the sample dropped out in total, all due to patient death. For all other respondents, data were complete (confirmed via correspondence) (low risk of bias). The other studies report attrition ranging between 17% and 52%. The Boele 2013 study had the highest levels of attrition which were handled using the last observation carried forward method. They reported reasons for dropout (unclear risk of bias). In Andela 2017, 48% of caregivers did not complete all intervention sessions and there were 35% missing data in the caregiver group (confirmed via correspondence), which were handled through linear mixed modelling (unclear risk of bias). The Dionne‐Odom 2015 reported about 32% of caregivers did not complete all follow‐up assessments. There were no significant associations between attrition and caregiver characteristics or outcome, with maximum likelihood methods used to estimate missing outcome data (unclear risk of bias). In Locke 2008, 33% of participants did not complete the intervention, with a 26% dropout at postintervention increasing to 32% at three months. It was not reported how missing data were handled (high risk of bias). In Reblin 2018, 20% of participants did not complete the six‐week follow‐up assessment, with no information provided on reasons for dropout. No imputation was done within completed questionnaires (less than 10% of data was missing), hence missingness was only per person (confirmed via correspondence) (unclear risk of bias). In Wakefield 2016, there was 17% attrition at six months in the intervention group and 27% attrition in the control group. They did not report reasons for dropout and included only complete cases in their evaluation of psychosocial outcomes (high risk of bias).
Selective reporting
Wakefield 2016 was linked to a published protocol (Wakefield 2015). However, this protocol referred to the follow‐up study, not the pilot. None of the other studies had published protocols available, leading to an unclear risk of reporting bias. In Boele 2013, caregiver burden data were collected but not used as many participants failed to complete the questionnaire in the intended way (unclear risk of bias). In the Dionne‐Odom 2015 study, personality was assessed but not reported on (unclear risk of bias). The remaining studies were also at unclear risk of bias.
Effects of interventions
The heterogeneity of populations and methodologies used in the included studies hindered pooling of data and, therefore, meta‐analysis was not carried out. See summary of findings Table for the main comparison.
Primary outcomes
Caregiver psychological distress
We found low‐certainty evidence that supportive interventions were more effective than any control condition. Across six studies, 163 neuro‐oncology caregivers were included. Psychological distress was measured with six outcomes (DASS‐21, the Fear of Recurrence Questionnaire – Family Member, CES‐D, HADS, POMS, or STAI). Three trials reported improvements after the intervention (Dionne‐Odom 2015: early versus delayed palliative care; CES‐D between‐group difference changed from baseline: mean –3.4 (SE 1.5); P = 0.02; d = –0.32; Klosky 2007a: interactive‐educational programme; STAI State change score mean –19.6 (SD 3.3) intervention versus mean –14.5 (SD 2.7) control; Trait change score mean –13.5 (SD 2.9) intervention versus mean –6.9 (SD 2.6) control; Andela 2017: self‐management programme; HADS Anxiety group by time interaction from baseline to six months (MD score –2.65), HADS depression group by time interaction from baseline to eight weeks and baseline to six months (MD: eight weeks: –2.60; six months: –3.47), HADS total score group by time interaction from baseline to eight weeks and baseline to six months (MD: eight weeks: –4.54; six months: –6.51; P < 0.05). Andela 2017 also compared mood (Visual Analogue Scale – Mood) before and after each session and reported caregivers' mood improved significantly after the last three sessions (mean: session six: from 73.00 (SD 6.95) to 77.17 (SD 6.46); P = 0.005; session seven: from 75.08 (SD 7.32) to 78.15 (SD 7.03); P = 0.025; session eight: from 73.08 (SD 6.09) to 77.54 (SD 7.66); P = 0.030).
Three feasibility/pilot trials also measured distress. In Reblin 2018, (electronic social network intervention) depression decreased after the intervention (mean: HADS depression scale: 9.795 with intervention versus 11.822 with control; F = 3.432; P = 0.072). There were no effects for anxiety. One trial found no significant effects (Wakefield 2016: e‐mental health intervention; DASS‐21; Fear of Recurrence Questionnaire – Family Member). The other trial only reported descriptives (Locke 2008; cognitive rehabilitation and problem solving), with POMS mean scores being the same postintervention in both groups. At three months, scores in control group were higher (mean 74 (SD 23.9) with control versus 73 (SD 16.1) with intervention).
Caregiver burden
There was low‐certainty evidence that supportive interventions were not more effective than control conditions. One study that included a heterogeneous sample of 27 neuro‐oncology caregivers (advanced cancer only; including those taking care of people with brain metastases) reported caregiver burden outcomes (Dionne‐Odom 2015). There was no statistically significant difference between the groups for any of the Montgomery‐Borgatta Caregiver Burden subscales (objective, demand, stress burden). A pilot study of electronic social network mapping found no differences over time in caregiver burden (Zarit Caregiver Burden Scale) between the intervention and control groups (Reblin 2018).
Caregiver mastery
Very low‐certainty evidence found that psychological support was more effective than care as usual. In one study among 56 caregivers, mastery (Caregiver Mastery Scale) improved in the intervention group compared with the control group (ΔR2 = 0.055, P = 0.021), corrected for the confounding factors of changes in patient's communication deficits (EORTC QLQ BN‐20), cognitive functioning (MOS). and physical functioning (SF‐36 Physical Component Summary) (Boele 2013). Self‐efficacy and coping skills, concepts closely linked to mastery, did not improve in a self‐management programme compared to controls (Andela 2017).
Quality of patient–caregiver relationship
Very low‐certainty evidence found that a supportive e‐mental health intervention was not more effective than a waiting list control condition (Wakefield 2016). This feasibility study, which included 13 parents of patients with a childhood brain tumour, showed no statistically significant difference on the McMaster Family Assessment Device family communication, problem‐solving, and general functioning subscales.
Caregiver quality of life
We found very low‐certainty evidence that supportive interventions were more effective than control conditions. Across six studies which included 157 neuro‐oncology caregivers in total, two trials found improvements after intervention (Andela 2017: self‐management programme; SF‐36 Vitality sub scale group by time interaction from baseline to eight weeks and baseline to six months (MD: eight weeks: 14.03; six months: 15.45; P = 0.026); Safarabadi‐Farahani 2016: psychosocial intervention; mean CQOLC total, mental/emotional burden, lifestyle disruption, positive adaptation sub scale scores higher than control group mean scores over time; all P < 0.001). One trial found stable QoL (SF‐36 Mental Component Summary) in the intervention versus the control group (psychological support; Boele 2013), which was no longer statistically significant after controlling for changes in patient functioning. A trial and a pilot trial found no evidence for improvements in QoL after the intervention (Dionne‐Odom 2015 using CQOLC; Wakefield 2016 using QoL – Family Caregiver Tool); one pilot trial only reported descriptive results (Locke 2008), with CQOLC scores appearing to improve slightly in both the intervention group (mean: from 95 (SD 20.1) at baseline to 98 (SD 19.5) postintervention) and control group (mean: from 93 (SD 174) at baseline to 102 (SD 11.7) postintervention).
Caregiver physical functioning
None of the included studies evaluated caregiver physical functioning.
Secondary outcomes
Patient emotional or physical well‐being
Psychological distress (depression and anxiety)
There was no support for the effectiveness of caregiver support on reducing patient psychological distress. Three trials did not show statistically significant differences (Andela 2017: HADS; Dionne‐Odom 2015 (Bakitas 2015): CES‐D; Klosky 2007a: OBD). Andela 2017 did report improved mood (Visual Analogue Scale – Mood) after all intervention sessions except the first (before range: 65.27 to 70.76; after range: 73.11 to 77.93; all changes P < 0.0015). Klosky 2007a collected further data on STAI scores but did not report descriptive statistics or between‐group changes. One trial only reported descriptive results, with POMS scores appearing to improve slightly in the intervention group (mean: baseline: 65 (SD 24.6); two weeks' follow‐up: 72 (SD 7.0); three months' follow‐up: 76 (SD 11.3)) and declining slightly in the control group (mean: baseline: 81 (SD 2.3); two weeks' follow‐up: 76 (SD 9.4); three months' follow‐up: 79 (SD 8.7)) (Locke 2008).
Quality of life
There was little information for the effectiveness of caregiver support on improving patient QoL. Three studies found no statistically significant differences between the intervention and control groups for overall QoL (Andela 2017: EQ‐5D, SF‐36, CushingQol, AcroQol; Dionne‐Odom 2015: FACIT‐Pal; Locke 2008: FACT‐BR), although Locke 2008 reported better scores after the intervention on the physical well‐being sub scale (MD 3.25 95% CI 0.07 to 6.43; P = 0.04). Furthermore, Locke 2008 reported descriptive results on LASA scores, which appear to be comparable between the intervention and control groups over time. One trial collected further data on proxy‐measured patient QoL, but did not report descriptive statistics or between‐group changes (Boele 2013: SF‐36).
Symptom management, number or severity (or both) of symptoms
No support was found for the effectiveness of caregiver support on patient symptom management. Two studies did not find statistically significant differences between the intervention and control groups (Dionne‐Odom 2015: QoL at End of Life (QUAL‐E) Symptom Impact sub scale; Locke 2008: Mayo‐Portland Adaptability Inventory (MPAI‐4)). Two studies assessed patient fatigue. Andela 2017 (Multidimentional Fatigue Inventory‐20) reported no statistically significant differences. Locke 2008 (Brief Fatigue Inventory) reporting descriptive results indicating a slight improvement in the intervention group over time (mean: baseline: 4.4 (SD 2.5); two weeks' follow‐up: 4.2 (SD 2.7); three months' follow‐up: 3.2 (SD 2.8)), whereas scores seemed to improve for the control group at postintervention (mean: baseline: 2.6 (SD 3.0); two weeks' follow‐up: 1.8 (SD 1.7)), and then deteriorate again at three months' follow‐up (mean: 3.0 (SD 3.5)). Locke 2008 also assessed neuropsychological functioning (Repeatable Battery for the Assessment of Neuropsychological Status); however, results were not presented due to a lack of sufficient follow‐up data. One trial further collected data on proxy‐reported patient symptom burden (Boele 2013: EORTC QLQ‐BN20 and MOS Cognitive Functioning scale), but did not report descriptive statistics or between‐group changes.
Number of visits to the emergency department
There was no support for the effectiveness of caregiver support on the number of patient visits to the emergency department. Only one trial assessed this outcome (Dionne‐Odom 2015). There was no significant difference between the early and delayed palliative care groups; however, the rate of resource use appeared to be lower in the early palliative care group (0.14, 95% CI 0.09 to 0.2) than in the delayed group (0.19, 95% CI 0.14 to 0.26).
Number and length of hospitalisations
There was no support for the effectiveness of caregiver support on the number and length of patient hospitalisations. Only one trial assessed this outcome and found no significant difference between the groups (Dionne‐Odom 2015). However, hospital visits and ICU stays were less frequent in the early (rate of hospital days: 0.95, 95% CI 0.61 to 1.46; rate of ICU days: 0.1, 95% CI 0.04 to 0.24) versus delayed palliative care group (rate of hospital days: 1.3, 95% CI 0.91 to 1.86; rate of ICU days: 0.15, 95% CI 0.07 to 0.3).
Other relevant patient well‐being outcomes
The self‐management programme investigated by Andela 2017 found group by time interactions in patient self‐efficacy with improvements in the control group (GSE MD: at eight weeks: 1.35; at six months: 1.74; both P < 0.05). Results for bother and need for support (various scales of the Leiden Bother and Needs Questionnaire for patients with pituitary disease (LBNQ‐Pituitary)) were variable, showing some improvement at eight weeks after intervention, yet heightened scores indicating more bother at six months. Differences from baseline to six months were not statistically significant. There were no differences for illness perceptions (Brief Illness Perception Questionnaire (BIPQ)), coping (Utrecht's Coping List (UCL)), or participation and autonomy (Impact of Participation and Autonomy Questionnaire (IPA)). The early versus delayed palliative care intervention trialled by Dionne‐Odom 2015 (Bakitas 2015) showed a 15% difference at one‐year survival (63% early group versus 48% delayed group; P = 0.038). There was no statistically significant difference in overall median survival between the groups. The use of chemotherapy in the last 14 days of life was not statistically different between the groups. About 54% of people in the early group and 47% of people in the delayed group died at home.
Outcomes related to the health economic effects
None of the included studies reported outcomes related to health economic effects (e.g. caregiver or patient (or both) employment status; productivity loss at work; caregiver healthcare utilisation for acute or chronic (or both) conditions).
Discussion
The aim of this review was to assess the effectiveness of supportive interventions at improving neuro‐oncology caregiver well‐being. The review included eight published studies.
Summary of main results
Overall, evidence was sparse. There was some evidence for positive effects of caregiver support on psychological distress, mastery, and QoL (low‐ to very low‐certainty evidence). No studies reported significant effects on caregiver burden or quality of patient–caregiver relationship (low‐ to very low‐certainty evidence). None of the studies assessed caregiver physical functioning. For secondary outcomes (patient emotional or physical well‐being; health economic effects), we found very little to no evidence for the effectiveness of caregiver support interventions.
Overall completeness and applicability of evidence
None of the studies measured all of the primary outcomes in this review, and even fewer provided data on our secondary outcomes. Of note, none of the studies assessed caregiver physical functioning or health economics outcomes. The scarcity of data available was a major hindrance in assessing the effectiveness of caregiver support.
This review included trials from the US, Australia, the Netherlands, and Iran. The perspectives of non‐Western countries was under‐represented, which may be highly relevant as differences in cultural values including family obligations and social support networks could influence the family caregiving experience (Knight 2010).
We purposefully kept inclusion criteria broad, allowing any type of supportive intervention, any control group, tested in any population which included at least 20% adult family caregivers who took care of a patient (of any age) with a primary or secondary brain or spinal cord tumour. As a result, there was significant heterogeneity in populations and interventions. Populations ranged from parental caregivers of childhood cancer survivors (three studies), to caregivers of patients with advanced cancer including brain metastases (one study), to caregivers of patients with pituitary disease (one study), to caregivers of adult patients with primary brain tumours (three studies); indicating a degree of heterogeneity in the caregiver–patient relationship, as well as patient disease and prognosis. None of the studies included caregivers of patients with spinal cord tumours. Four studies tested dyadic interventions which also involved the patient; four were focused solely on caregivers. A broad range of interventions was included: face‐to‐face support based on the principles of CBT, problem‐solving skills training, psychoeducation or cognitive rehabilitation, or both; early access to palliative care which included coping skills training; and web‐based programmes with or without guidance from a psychologist. Exposure to the intervention ranged from a single session taking 10 to 15 minutes to multiple sessions across three months, with one programme providing monthly follow‐up telephone calls into the bereavement phase. Although beneficial for external validity, this heterogeneity precluded pooling of data and meta‐analysis.
Quality of the evidence
We included seven RCTs and one quasi‐RCT, which limited the overall potential selection bias. Together, these studies included 250 neuro‐oncology caregivers (range within studies: 13 to 56). This highlights the main weakness of the state of the evidence – trials that are likely underpowered to measure the effectiveness of caregiver support in neuro‐oncology introduce potential type II errors and make it difficult to draw conclusions on efficacy. Authors described three out of eight studies as 'pilot' or 'feasibility' studies, and in two cases it remained unclear whether these led on to larger‐scale studies. The Wakefield 2016 study led on to a full RCT (Wakefield 2015). Due to the nature of supportive interventions, participant blinding is often not possible or desirable; however, more efforts could have been made to ensure that the person carrying out statistical analyses is naive to group allocation – which was only done in two out of eight studies. Participant attrition and lack of reported reasons may have introduced further (attrition) bias. Lack of published protocols led to unclear risk of reporting bias across all included studies.
Outcome measures assessed were not consistent between studies, with six out of eight studies measuring psychological distress and QoL, but only two studies assessed caregiver burden and mastery, and only one study assessed quality of patient–caregiver relationship. Where possible to assess this, study results seemed consistent with either no evidence of effect found, or a positive effect on caregiver well‐being found. The GRADE certainty of evidence overall was low to very low, with the main reasons for downgrading being the small sample of neuro‐oncology caregivers; and the heterogeneity of interventions and populations studied.
Potential biases in the review process
We have searched three databases, handsearched relevant conference abstracts since 2013, and articles published since 2017 in the two main journals in the field. We searched for ongoing trials and contacted known experts in the field. Despite this rigorous review process, it may still be possible that we did not identify all eligible trials, in particular when the protocol or results were published after our updated search date (August 2018). Therefore, regular updates of this review are needed.
Agreements and disagreements with other studies or reviews
In general, this Cochrane Review highlighted a scarcity of RCTs investigating support for family caregivers in neuro‐oncology. This lack of literature mirrors the findings of other systematic (Langbecker 2015; Madsen 2011; Piil 2016; Russell 2014; Sterckx 2013), and non‐systematic (Boele 2017b; Sherwood 2016), reviews that include neuro‐oncology caregiver studies, all concluding that more research is required on caregivers' unmet needs and the effectiveness of specific programmes to support neuro‐oncology caregivers.
There is some additional literature on non‐controlled efforts to improve neuro‐oncology caregivers' well‐being. Programmes include nurse‐led interventions, support groups, caregiver workshops, psycho‐education, patient navigation or care co‐ordination, use of a telephone hotline or brain tumour website, and a dyadic yoga programme (Boele 2017b; Langbecker 2015; Milbury 2018; Sherwood 2016). These generally show encouraging results such as good uptake percentages and qualitative evidence of increased family autonomy; however, these still need to be evaluated in a randomised controlled setting.
There has been similar research of other family caregiver populations. In caregivers of people with terminal illness, one Cochrane systematic review concluded that support may help reduce caregivers' psychological distress (Candy 2011). Another Cochrane systematic review on non‐pharmacological interventions for caregivers of people with stroke concluded that too few high‐quality RCTs had been published to determine effectiveness (Legg 2011). In cancer caregivers, one systematic review reporting on 49 intervention studies (not all RCTs) concluded that 65% of the interventions led to positive and significant improvements in caregiver or patient well‐being (Applebaum 2013). A state of the science review supported this and stressed the need for guidelines to advocate changes in clinical practice (Northouse 2012).
The samples of studies included in our Cochrane Review consisted of very few caregivers of patients with brain metastases and none of the studies included caregivers of patients with spinal cord tumours. Two systematic reviews similarly reported that very little is known about the burden and support needs of patients with brain metastases and their family caregivers (Magbool 2017; Saria 2017). We are not aware of any systematic reviews which have included support needs or interventions for caregivers of patients with spinal cord tumours. In adults, most systematic reviews focus on family caregivers of patients with a high‐grade primary malignant brain tumour (Piil 2016; Russell 2014; Sterckx 2013), which seems reflected in the samples of the studies included in this review.
This pattern is generally mirrored in the series of guidelines published by the European Association for Neuro‐Oncology (EANO). For example, the guidelines on meningiomas (Goldbrunner 2016), ependymal tumours (Ruda 2017), and brain metastases (Soffietti 2017) did not mention caregiver support, whereas the guidelines for adults with astrocytic or oligodendrial tumours (Weller 2017) briefly acknowledged the importance of supporting family caregivers. The palliative care guidelines refer to caregiver needs throughout (Pace 2017).
Of note, although it is commonly emphasised that support should not end after the patient has deceased (Petruzzi 2015; Piil 2019), only one of the included studies in this Cochrane Review continued to provide some support after the death of the patient (Dionne‐Odom 2015).

Study flow diagram. RCT: randomised controlled trial.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
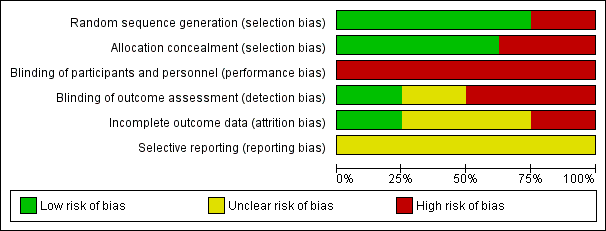
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Interventions to help support caregivers of people with a brain or spinal cord tumour | |||
| Patient or population: caregiver well‐being | |||
| Outcomes | Impact | № of participants | Certainty of the evidence |
| Caregiver psychological distress Follow‐up: range 1 days to 8 months | 4 studies found improvements after the intervention (early palliative care; interactive‐educational programme; electronic social network intervention; self‐management programme); 1 found no significant effects (e‐mental health); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊕⊝⊝ |
| Caregiver burden Follow‐up: range 6 weeks to 3 months | 2 studies found no statistically significant differences in burden scales between the intervention and control groups (early vs delayed palliative care; electronic social network intervention). | (2 RCTs) | ⊕⊕⊝⊝ |
| Caregiver mastery Follow‐up: range 6 months to 8 months | 1 study found improvements in mastery after the intervention (psychoeducation and cognitive behavioural therapy) compared to care‐as‐usual, corrected for changes in patient functioning. 1 study found no improvements in self‐efficacy or coping strategies (self‐management programme). | (2 RCTs) | ⊕⊝⊝⊝ |
| Quality of patient–caregiver relationship | 1 study found no statistically significant differences in family functioning between the intervention and control groups (e‐mental health vs waiting list). | (1 RCT) | ⊕⊝⊝⊝ |
| Caregiver quality of life Follow‐up: range 30 days to 8 months | 2 studies found improvements in QoL over time in the intervention group (psychosocial intervention; self‐management programme) compared to the control group; 1 study found stable QoL in the intervention group vs decline in the control group (no longer statistically significant after controlling for patient functioning); 2 studies found no statistically significant improvements after the intervention (e‐mental health; early palliative care); 1 only reported descriptives (cognitive rehabilitation and problem‐solving). | (6 RCTs) | ⊕⊝⊝⊝ |
| Caregiver physical functioning – not measured | None of the included studies assessed caregiver physical functioning. | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CQOLC: Caregiver QoL Index – Cancer; CES‐D: Center for Epidemiological Studies Depression Scale; CI: confidence interval; DASS‐21: Depression, Anxiety and Stress Scales; HADS: Hospital Anxiety and Depression Scale; LASA: Linear Analogue Self‐Assessment; MBCB: Montgomery‐Borgatta Caregiver Burden; POMS: Profile of Mood States; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; STAI: State‐Trait Anxiety Inventory. | |||
| GRADE Working Group grades of evidence | |||
| aDifferent populations (e.g. mixed cancer caregiver samples, paediatric or adult (or both) caregiver samples). | |||

