Tomografía de emisión de positrones (TEP) y resonancia magnética (RM) para la evaluación de la resecabilidad tumoral en el cáncer peritoneal/de las trompas de Falopio/ovárico epitelial primario avanzado
Appendices
Appendix 1. MEDLINE search strategy
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to 2017 February 23rd
# Searches
1 exp Ovarian Neoplasms/
2 Fallopian Tube Neoplasms/
3 Peritoneal Neoplasms/
4 ((ovar* or fallopian* or peritone*) adj5 (cancer* or neoplasm* or carcin* or cystadenocarcinoma* or malign* or tumo?r*)).ti,ab,kw,kf.
5 1 or 2 or 3 or 4
6 exp MAGNETIC RESONANCE IMAGING/
7 (MRI or MRi or NMRI or NMRi).ti,ab,kw,kf.
8 ((magn*or MR or MTC or MT or NMR or spin or chemical shift or diffus*) adj3 (imag* or scan* or resonance* or tomogra$)).ti,ab,kw,kf.
9 Diffusion‐weighted.ti,ab,kw,kf.
10 exp POSITRON‐EMISSION TOMOGRAPHY/
11 (pet adj3 scan*).ti,ab,kw,kf.
12 (positr* adj4 tomogr*).ti,ab,kw,kf.
13 (pet‐ct or petct or fdg‐pet).ti,ab,kw,kf.
14 (CT adj3 (cine or scan* or x‐ray* or xray*)).ab,ti,kw,kf.
15 (ct or mdct).ti.
16 ((electron beam* or comput* or axial) adj3 tomography).ab,ti,kw,kf.
17 tomodensitometry.ab,ti,kw,kf.
18 exp TOMOGRAPHY, X‐RAY COMPUTED/
19 radiography.fs.
20 radionuclide imaging.fs.
21 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 5 and 21
Appendix 2. Embase search strategy
Embase Classic+Embase 1946 to 2017 February 23rd
1 ((exp ovary tumor/ or uterine tube tumor/ or exp peritoneum tumor/) or (((ovar* or fallopian* or peritone*) adj5 (cancer* or neoplasm* or carcin* or cystadenocarcinoma* or malign* or tumo?r*)).ti,ab,kw.))
2 (exp nuclear magnetic resonance imaging/ or (MRI or MRi or NMRI or NMRi or Diffusion‐weighted or ((magn*or MR or MTC or MT or NMR or spin or chemical shift or diffus*) adj3 (imag* or scan* or resonance* or tomogra$))).ti,ab,kw.)
3 ((positron emission tomography/ or exp computer assisted tomography/ or computer assisted emission tomography/) or ((pet adj3 scan*) or (positr* adj4 tomogr*) or (pet‐ct or petct or fdg‐pet) or (CT adj3 (cine or scan* or x‐ray* or xray*)) or ((electron beam* or comput* or axial) adj3 tomography) or tomodensitometry).ti,ab,kw or (ct or mdct).ti.)
4 1 AND (2 OR 3)
Appendix 3. Clinicaltrials.gov search strategy
ClinicalTrials.gov search strategy
(MRI OR MRi OR NMRI OR NMRi OR Diffusion‐weighted OR magnetic imaging OR chenical‐shift OR pet‐ct or petct or fdg‐pet OR PET‐scan OR CT‐scan) | ((ovarian OR ovary OR fallopian OR peritoneal) AND (cancer OR neoplasm OR carcinoma OR cystadenocarcinoma OR malignant OR malignancy OR tumor OR tumour))
Appendix 4. ICTRP search strategy
ICTRP search strategy
ovarian cancer AND MRI OR "fallopian cancer" AND MRI OR "ovarian tumor" AND MRI OR "ovarian tumour" AND MRI OR "peritoneal cancer" AND MRI OR "peritoneal tumor" AND MRI OR "peritoneal tumour" AND MRI OR "ovarian neoplasm" AND MRI OR "ovarian carcinoma" AND MRI
OR
ovarian cancer AND "magnetic imaging" OR "fallopian cancer" AND "magnetic imaging" OR "ovarian tumor" AND "magnetic imaging" OR "ovarian tumour" AND "magnetic imaging" OR "peritoneal cancer" AND "magnetic imaging" OR "peritoneal tumor" AND "magnetic imaging" OR "peritoneal tumour" AND "magnetic imaging" OR "ovarian neoplasm" AND "magnetic imaging" OR "ovarian carcinoma" AND "magnetic imaging"
OR
ovarian cancer AND "Diffusion‐weighted" OR "fallopian cancer" AND "Diffusion‐weighted" OR "ovarian tumor" AND "Diffusion‐weighted" OR "ovarian tumour" AND "Diffusion‐weighted" OR "peritoneal cancer" AND "Diffusion‐weighted" OR "peritoneal tumor" AND "Diffusion‐weighted" OR "peritoneal tumour" AND "Diffusion‐weighted" OR "ovarian neoplasm" AND "Diffusion‐weighted" OR "ovarian carcinoma" AND "Diffusion‐weighted"
OR
ovarian cancer AND "chemical‐shift" OR "fallopian cancer" AND "chemical‐shift" OR "ovarian tumor" AND "chemical‐shift" OR "ovarian tumour" AND "chemical‐shift" OR "peritoneal cancer" AND "chemical‐shift" OR "peritoneal tumor" AND "chemical‐shift" OR "peritoneal tumour" AND "chemical‐shift" OR "ovarian neoplasm" AND "chemical‐shift" OR "ovarian carcinoma" AND "chemical‐shift"
OR
ovarian cancer AND "CT‐scan" OR "fallopian cancer" AND "CT‐scan" OR "ovarian tumor" AND "CT‐scan" OR "ovarian tumour" AND "CT‐scan" OR "peritoneal cancer" AND "CT‐scan" OR "peritoneal tumor" AND "CT‐scan" OR "peritoneal tumour" AND "CT‐scan" OR "ovarian neoplasm" AND "CT‐scan" OR "ovarian carcinoma" AND "CT‐scan"
OR
ovarian cancer AND "pet‐scan" OR "fallopian cancer" AND "pet‐scan" OR "ovarian tumor" AND "pet‐scan" OR "ovarian tumour" AND "pet‐scan" OR "peritoneal cancer" AND "pet‐scan" OR "peritoneal tumor" AND "pet‐scan" OR "peritoneal tumour" AND "pet‐scan" OR "ovarian neoplasm" AND "pet‐scan" OR "ovarian carcinoma" AND "pet‐scan"
OR
ovarian cancer AND "fdg‐pet" OR "fallopian cancer" AND "fdg‐pet" OR "ovarian tumor" AND "fdg‐pet" OR "ovarian tumour" AND "fdg‐pet" OR "peritoneal cancer" AND "fdg‐pet" OR "peritoneal tumor" AND "fdg‐pet" OR "peritoneal tumour" AND "fdg‐pet" OR "ovarian neoplasm" AND "fdg‐pet" OR "ovarian carcinoma" AND "fdg‐pet"
OR
ovarian cancer AND "pet‐ct" OR "fallopian cancer" AND "pet‐ct" OR "ovarian tumor" AND "pet‐ct" OR "ovarian tumour" AND "pet‐ct" OR "peritoneal cancer" AND "pet‐ct" OR "peritoneal tumor" AND "pet‐ct" OR "peritoneal tumour" AND "pet‐ct" OR "ovarian neoplasm" AND "pet‐ct" OR "ovarian carcinoma" AND "pet‐ct"
Appendix 5. Operational definitions of QUADAS‐2 items
| Risk of bias | Applicability | ||||||
| Quality indicator | Notes | Quality indicator | Notes | ||||
| Domain 1 Patient Selection | Could the selection of patients have introduced bias? (High/low/unclear) | Are there concerns that the included patients and settings do not match the review question? (High/low/unclear) | |||||
| 1. Was a consecutive or random sample of patients enrolled? | 'Yes' if a consecutive or random sample of patients was enrolled. 'No' if a selected group of patients was enrolled. 'Unclear' if there was insufficient information on enrolment. | 1. Were the patients diagnosed by conventional diagnostic work‐up for advanced stage ovarian cancer? | 'Yes' if patients were diagnosed by conventional diagnostic work‐up with advanced stage‐ovarian cancer. 'No' if patients included in the trial were diagnosed with low stage‐disease (FIGO I or II) only. No high stage‐disease patients in the trial. 'Unclear' if there was insufficient information on recruitment method, criteria for diagnosis of ovarian cancer. | ||||
| 2. Did the study avoid inappropriate exclusions? | 'Yes' if there were no inappropriate exclusions. 'No' if there were inappropriate exclusions. 'Unclear' if there was insufficient information on exclusions. | 2. Were the patients scheduled for primary debulking surgery after conventional diagnostic work‐up? | 'Yes' if the patients were scheduled for primary debulking surgery after conventional diagnostic work‐up. 'No' if none of the patients were scheduled for primary debulking surgery. 'Unclear' if there was insufficient information. | ||||
| Domain 2 Index Test | Could the interpretation of the Index test have introduced bias? (High/low/unclear) | Were there concerns that the index test, its conduct, or the interpretation differed from the review question? (High/low/unclear) | |||||
| 1. Were the index test results interpreted without the knowledge of the results of the reference standard? | This will always be rated as 'yes', because the index test is performed before the reference standard. | 1. Were the same clinical data available when test results were interpreted as would be available when the test is used in clinical practice? | 'Yes' if all usual clinical data (except laparotomy results) were available when the index test was interpreted, including details of physical examination, serum tumour markers, ultrasound, and CT/MRI imaging. Also answer 'yes' if one of the items was missing. 'No' if clinical information (as mentioned by 'yes') was not available to the gynaecologist. 'Unclear' if insufficient information was reported. | ||||
| 2.Was the threshold used prespecified? | 'Yes' if a clear description of the threshold was given which was specified before start of the study. 'No' if no clear description was given beforehand. 'Unclear' if there was insufficient information within the paper to determine whether or not a prespecified threshold was used. | 2. Did the study provide a clear definition of what was considered to be a ’positive’ result for the index test? | 'Yes' if a clear description was given about when the index test was positive or negative (e.g. what the cut‐off for too extensive abdominal disease was). 'No' if there was no clear description given about what was classified as too extensive disease or not. 'Unclear' if there was insufficient information within the paper to determine whether or not a defined threshold was used for a positive test result. | ||||
| 3. Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis? | 'Yes' if all patients underwent the reference standard (laparotomy). 'No' if not all patients underwent reference standard. 'Unclear' if insufficient information was provided. | ||||||
| 4. Did patients receive the same reference standard regardless of the index test result? | 'Yes' if patients who underwent the reference standard had laparotomy. 'No' if patients did not undergo laparotomy. 'Unclear' if insufficient information was provided. | ||||||
| Domain 3 Reference Standard | Could the interpretation of the reference standard have introduced bias? (High/low/unclear) | Were there concerns that the target condition as defined by the reference standard did not match the question? (High/low/unclear) | |||||
| 1. Was the reference standard likely to correctly classify the target condition? | 'Yes' if the reference standard was laparotomy. 'No' if the reference standard used was not the one defined in the protocol. 'Unclear' if the information was insufficient. | 1. Did the study provide a clear definition of what was considered to be a ’positive’ result for the reference standard? | 'Yes' if a clear description was given about when the reference standard was positive or negative (e.g. if description was given about the size of the tumour deposits left after surgery). 'No' if there was no clear description of tumour deposit size after surgery. 'Unclear' if there was insufficient information within the paper that described tumour size after surgery. | ||||
| 2. Were the reference standard results interpreted without the knowledge of the results of the index test? | 'Yes' if the report stated that the reference test was performed by individuals who did not perform the index test. 'No' if the reference test was done by the same person performing the index test. 'Unclear' if not reported. | ||||||
| 3. Was the surgeon's expertise adequate to perform the reference standard? | 'Yes' if the reference test was performed by a gynaecological oncologist. 'No' if the reference test was not performed by a gynaecological oncologist. 'Unclear' if not reported. | ||||||
| Domain 4 Flow and Timing | Could the patient flow have introduced bias? (High/low/unclear) | ||||||
| 1. Was the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | 'Yes' if the time period between the index test and reference standard did not extend 6 weeks. 'No' if the time period was more than 6 weeks for an unacceptably high proportion of patients. 'Unclear' if the information on the timing of tests was not provided. | ||||||
| 2. Did all patients receive the same reference standard? | 'Yes' if all patients underwent the reference standard (laparotomy, diagnostic laparoscopy or image‐guided biopsy of distant metastases). 'No' if not all patients underwent the reference standard. 'Unclear' if insufficient information was provided. | ||||||
| 3. Were all patients included in the analysis? | 'Yes' if for all patients entered in the study were included in the analysis. 'No' if not all the patients in the study were included in the analysis. 'Unclear' if it was not clear whether all patients were accounted for. | ||||||
| 4.Were withdrawals from the study reported? | 'Yes' if, for all patients entered in the study,it was reported what happened during the study, also those who withdrew or answered 'Yes' if no withdrawals were reported, and results were reported for all patients who entered in the study. 'No' if not all the patients in the study completed the study and these patients were not accounted for. 'Unclear' if it was not clear whether all patients were accounted for. | ||||||
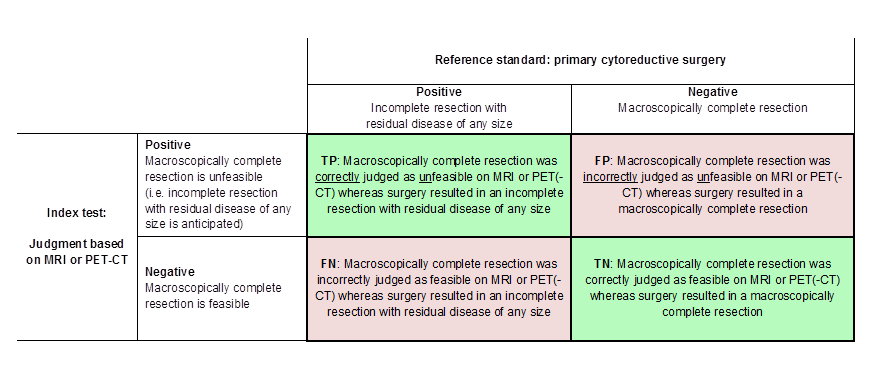
Definitions of the two by two table, wherein the index tests are tabulated against the reference standard outcome, on the analysis: macroscopic debulking versus incomplete debulking with residual disease of any size (i.e. consisting of deposits ≤ 1 cm and > 1 cm in diameter ). TP = true positive, FP = false positive, FN = false negative, TN = true negative.

Definitions of the two by two table, wherein the index tests are tabulated against the reference standard outcome, on the analysis: macroscopic debulking or incomplete debulking with residual disease ≤ 1 cm in diameter versus incomplete resection with residual disease > 1 cm in diameter. TP = true positive, FP = false positive, FN = false negative, TN = true negative.

Visual representation of 2 x 2 table. TP = true positive, FP = false positive, FN = false negative, TN = true negative.

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Forest plot of tests: 1 PET/CT for assessing incomplete debulking with residual disease of any size, 4 MRI for assessing incomplete debulking with residual disease of any size, 2 MRI for assessing incomplete debulking with residual disease > 1 cm, 3 MRI for assessing incomplete debulking with residual disease > 2 cm.

PET/CT for assessing incomplete debulking with residual disease of any size.

MRI for assessing incomplete debulking with residual disease > 1 cm.

MRI for assessing incomplete debulking with residual disease > 2 cm.

MRI for assessing incomplete debulking with residual disease of any size.
| What is the diagnostic accuracy of FDG‐PET/CT or MRI for assessing tumour resectability in advanced epithelial ovarian/fallopian tube/primary peritoneal cancer? Patients Women suspected of ovarian cancer scheduled for surgery Prior testing Conventional diagnostic work‐up (e.g. physical examination, ultrasound) Setting University hospitals or specialised cancer institutes Index test FDG‐PET/CT or MRI. In all studies, the index test was evaluated as a replacement of abdominal CT. No studies were identified that followed an add‐on design. Target condition Residual disease assessed after debulking surgery | ||||||||
| Test | Target condition | No. of women (studies) | Prevalence in study | Sensitivity (95% CI) | Specificity (95% CI) | No. of false negatives* per 1000 tested | No. of false positives** per 1000 tested | Test accuracy certainty (quality) of evidence (sensitivity/specificity)a |
| FDG‐PET/CT | Residual disease > 0 cm | 23/343 (2) | 26%/65% | 1.0 (0.54 to 1.0) and 0.66 (0.60 to 0.73) | 1.0 (0.80 to 1.0) and 0.88 (0.80 to 0.93) | 211 (167 to 248)b | 46 (27 to 76)b | Lowc/moderated |
| DW‐MRI | Residual disease > 0 cm | 94 (1) | 53% | 0.94 (0.83 to 0.99) | 0.98 (0.88 to 1.00) | 37 (6 to 105)b | 8 (0 to 46)b | Lowc/moderated |
| DW‐MRI | Residual disease > 1 cm | 34 (1) | 23.5% | 0.75 (0.35 to 0.97) | 0.96 (0.80 to 1.00) | 59 (7 to 153) | 31 (0 to 153) | Very low/very low e, f |
| Conventional MRI | Residual disease > 2 cm | 50 (1) | 22% | 0.91 (0.59 to 1.00) | 0.97 (0.87 to 1.00) | 20 (0 to 90) | 23 (0 to 101) | Very low/very low e,g |
| CTh | Residual disease > 0 cm | 94 (1) | 53% | 0.66 (95% CI 0.52 to 0.78) | 0.77 (95% CI 0.63 to 0.87) | 211 (136 to 298)b | 87 (49 to 141)b | Low/lowc |
| CI: confidence interval a. According to GRADE for sensitivity (false negatives (FNs)) and specificity (false positives (FPs)), respectively | ||||||||
| Criteria to consider primary debulking unfeasible according to study methods | |||||
| Alessi | Shim | Espada | Forstner | Michielsen | |
| Site of tumour involvement | |||||
| Liver/porta hepatis | Yes | No | No | Yes | Yes |
| Mesentery | Yes | Yes | Yes | Yes | No |
| Colon | Yes, when necessitating > 4 bowel resections | No | No | No | Yes, when necessitating multiple bowel resections |
| Stomach | Yes | No | Yes | No | Yes |
| Pancreas | Yes | No | No | No | Yes |
| Duodenum | Yes | No | No | No | Yes |
| Diaphragm | No | Yes | No | Yes | No |
| Ascites | No | Yes | No | No | No |
| Peritoneal carcinomatosis | Yes | Yes | No | No | No |
| Lesser sac/bursa omentalis | No | No | Yes | Yes | No |
| Spleen/splenic hilum | No | No | Yes | No | No |
| Lymph nodes above level of renal vessels/at coeliac axis | No | No | Yes | Yes | Yes |
| Gastrosplenic ligament | No | No | No | Yes | No |
| Presacral extraperitoneal disease | No | No | No | Yes | No |
| Extra‐abdominal distant metastasis | No | No | No | No | Yes |
| Vessels of coeliac trunk | No | No | No | No | Yes |
| Hepatoduodenal ligament | No | No | No | No | Yes |
| Superior mesenteric artery | No | No | No | No | Yes |
| Yes: site of tumour involvement is selected as one of the criteria to consider primary debulking unfeasible | |||||
| Test | No. of studies | No. of participants |
| 1 PET/CT for assessing incomplete debulking with residual disease of any size Show forest plot | 2 | 366 |
| 2 MRI for assessing incomplete debulking with residual disease > 1 cm Show forest plot | 1 | 34 |
| 3 MRI for assessing incomplete debulking with residual disease > 2 cm Show forest plot | 1 | 50 |
| 4 MRI for assessing incomplete debulking with residual disease of any size Show forest plot | 1 | 94 |

