Les inhibiteurs de la cholinestérase pour le traitement du délire hors unités de soins intensifs (USI)
Résumé scientifique
Contexte
Le délire est un syndrome clinique commun défini comme une altération de l'attention, accompagnée d'une perturbation supplémentaire de la cognition ou de la perception, qui se développe sur une courte période de temps et tend à fluctuer au cours de l'épisode. Le délire est couramment traité dans les hôpitaux ou en milieu communautaire et est souvent associé à de multiples effets indésirables tels que l'augmentation des coûts, la morbidité et même la mortalité. L'intervention de première ligne implique une approche non pharmacologique à composantes multiples qui comprend l'assurance d'une communication et d'une réorientation efficaces en plus de rassurer ou d'offrir un environnement de soins approprié. Il n'existe actuellement aucun médicament approuvé spécifiquement pour le traitement du trouble délirant. Sur le plan clinique, cependant, divers médicaments sont utilisés pour en soulager les symptômes, comme les antipsychotiques et les inhibiteurs de la cholinestérase, entre autres.
Objectifs
Évaluer l'efficacité et l'innocuité des inhibiteurs de la cholinestérase dans le traitement des personnes atteintes de délire établi dans une unité de soins non intensifs.
Stratégie de recherche documentaire
Nous avons effectué une recherche sur ALOIS, qui est le registre spécialisé du groupe Cochrane sur la démence et les autres troubles cognitifs, le 26 octobre 2017. Nous avons également recoupés les listes de référence des études incluses afin d'identifier tout essai potentiellement admissible.
Critères de sélection
Nous avons inclus des essais contrôlés randomisés, publiés ou non, écrits en anglais ou en chinois, qui comparaient les inhibiteurs de la cholinestérase à un placebo ou à d'autres médicaments destinés à traiter des personnes souffrant de délire établi dans un contexte autre que celui de l'USI.
Recueil et analyse des données
Nous avons utilisé les procédures méthodologiques standard attendues par Cochrane. Les principaux critères de jugement étaient la durée du délire, la gravité du délire et les effets indésirables. Les critères secondaires ont été : l'utilisation de médicaments de secours, une déficience cognitive persistante, la durée de l'hospitalisation, l'institutionnalisation, la mortalité, le coût de l'intervention, l'abandon précoce de l'étude et la qualité de vie. Pour les résultats dichotomiques, nous avons calculé le risque relatif (RR) avec des intervalles de confiance (IC) de 95 % et pour les résultats continus, nous avons calculé la différence moyenne (DM) avec des IC de 95 %. Nous avons évalué la qualité des éléments probants à l'aide de GRADE pour créer un tableau " Résumé des résultats ".
Résultats principaux
Nous avons inclus une étude impliquant 15 participants du Royaume‐Uni. Les participants inclus ont reçu un diagnostic de délire fondé sur les critères de la méthode d'évaluation de la confusion (CAM). Huit hommes et sept femmes ont été inclus, l'âge moyen étant de 82,5 ans. Des 15 participants, sept souffraient de démence comorbide au départ. Le risque de biais était faible dans tous les domaines.
L'étude a comparé la rivastigmine au placebo. Nous n'avons trouvé aucune différence nette entre le deux groupes en ce qui concerne la durée du délire (DM ‐3,6, IC à 95 % ‐15,6 à 8,4), les effets indésirables (nausées, RR 0,30, IC à 95 % 0,01 à 6,29), la prise de médicaments de secours (RR 0,13, IC à 95 % 0,01 à 2,1) la mortalité (RR 0,10, IC à 95 % 0,01 à 1,56), et le fait de quitter l'étude tôt (RR 0,88, IC à 95 % 0,07 à 11,54). On ne disposait pas de données probantes sur la gravité du délire, la déficience cognitive persistante, la durée de l'hospitalisation, le coût de l'intervention ou d'autres critères secondaires prédéfinis.
La qualité des données probantes est faible en raison de la très petite taille de l'échantillon.
Conclusions des auteurs
Les données probantes sont insuffisantes pour appuyer ou réfuter l'utilisation des inhibiteurs de la cholinestérase pour le traitement du délire dans les établissements autres que les USI. On n'a observé aucun effet bénéfique ou nocif clair associé aux inhibiteurs de la cholinestérase comparativement au placebo en raison de l'absence de données. D'autres essais sont nécessaires.
PICO
Résumé simplifié
Les inhibiteurs de la cholinestérase (ICh) chez les patients atteints du trouble délirant qui ne sont pas admis aux soins intensifs
Contexte
Pendant la maladie, une confusion mentale et une altération de la conscience peuvent se manifester chez les personnes. Ces symptômes sont communément associés au trouble délirant. La particularité des patients atteints du trouble délirant est qu’elles passent plus de temps à l'hôpital et ont moins de chances de vaincre leur maladie. Le traitement du trouble délirant devrait être axé sur une réponse à la maladie sous‐jacente et sur des stratégies de réorientation du patient. Cependant, les traitements médicamenteux sont encore utilisés fréquemment. Les médicaments utilisés pour traiter les symptômes de la démence (inhibiteurs de la cholinestérase) peuvent jouer un rôle dans le traitement du délire.
Problématique de la revue
Évaluer si le traitement par inhibiteurs de la cholinestérase permet de réduire la gravité ou la durée du trouble délirant. Les effets secondaires des inhibiteurs de la cholinestérase ont également été étudiés. Le trouble délirant relève souvent de la maladie grave nécessitant le recours aux soins médicaux et infirmiers importants, notamment à l'unité des soins intensifs (USI). Dans cette revue, nous nous sommes concentrés sur des recherches portant sur des patients qui n'étaient pas dans un milieu de soins intensifs.
Caractéristiques des études
Nous avons trouvé un essai mené au Royaume‐Uni, auquel 15 personnes atteintes de délire ont participé. L'âge moyen des participants était de 82,5 ans et l’échantillon de patients était de huit hommes et de sept femmes. Sept participants avaient également des antécédents de démence. Cet essai comparait la rivastigmine (un type d'inhibiteur de la cholinestérase utilisé pour traiter la démence) avec un traitement inactif (placebo).
Résultats principaux
Les résultats n’ont démontré aucune différence d'effet entre l’administration de la rivastigmine et celle du placebo. L'étude a été menée et ses résultats rapportés de manière appropriée, mais le faible nombre de participants limite les conclusions qui pourraient être tirées de cet essai sur l’utilisation de la rivastigmine dans le traitement du délire.
Authors' conclusions
Summary of findings
| Cholinesterase inhibitors (rivastigmine) compared to placebo for the treatment of delirium in non‐ICU settings | ||||||
| Patient or population: people with delirium | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Cholinesterase inhibitors (rivastigmine) | |||||
| Duration of delirium | The mean duration was 9.9 days | The mean duration was 3.6 days lower (15.6 lower to 8.4 higher) | Not estimable | 15 | ⊕⊕⊝⊝ | The data were reported by Overshott 2010. The study was grossly underpowered, and the data were skewed. |
| Severity of delirium | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Adverse events | 143 per 1000 | 43 per 1000 | RR 0.3 | 15 | ⊕⊕⊝⊝ | |
| Persistent cognitive impairment | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Length of hospitalisation | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Mortality | 571 per 1000 | 57 per 1000 | RR 0.1 | 15 | ⊕⊕⊝⊝ | |
| Cost of intervention | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| *The dose of intervention: 1.5 mg once a day increasing to 1.5 mg twice a day after 7 days. **The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision due to very small sample size. | ||||||
Background
This review supersedes a previous Cochrane Review, 'Cholinesterase inhibitors for delirium', which was first published in Issue 1, 2008 (Other published versions of this review).
Description of the condition
Delirium is a common clinical syndrome characterised by alterations in attention and additional disturbances in cognitive function or perception, which has an acute onset and a fluctuating course (APA 2013).
Delirium is a neuropsychiatric disturbance with multiple aetiologies and can be the consequence of a medical condition, substance intoxication or withdrawal, or exposure to a toxin (APA 2013). The causes of delirium are multifactorial and include patient vulnerability factors (such as dementia or cognitive impairment, ageing, medical comorbidity, malnutrition, history of alcohol abuse and prescription opioid or benzodiazepine use, among others) and potentially modifiable factors (such as infections, dehydration, electrolyte abnormalities, polypharmacy, seizures, and surgery) (Inouye 2014; Vasilevskis 2012). The core symptoms of delirium include altered levels of attention and awareness that typically develop over a short period of time and represent a change from the patient's baseline level of attention and awareness. These alterations may fluctuate in severity throughout the course of the episode, at times worsening in the evening and overnight (APA 2013; Schwartz 2016). People with delirium experience increased mortality, postoperative complications (Raats 2015), readmissions (McKhann 2002), poorer functional outcomes (Inouye 1998), risk of dementia (Davis 2012), length of hospital stay (McCusker 2003), and higher healthcare expenditures (Leslie 2008).
Delirium can manifest as hyperactivity, hypoactivity, or mixed (when both hypoactive and hyperactive features are present) (NICE 2014). The fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM‐V) and the 10th Revision of the International Classification of Diseases (ICD‐10) provide the current standard reference diagnostic criteria (APA 2013; WHO 1993). Over the years, various diagnostic and screening instruments have been developed for making the diagnosis of delirium based on the DSM criteria, and these have been used or adapted in various research and clinical applications (Grover 2012; Oh 2017). The Confusion Assessment Method (CAM), which provides a simple diagnostic algorithm, is widely used for identification of delirium worldwide (Hshieh 2018). The 4AT, a new widely used instrument for rapid delirium screening, is also easy and brief to administer and has high sensitivity and specificity (Bellelli 2014; De 2017).
The highest incidence rates of delirium are noted in intensive care unit (ICU) settings, reaching up to 80% (Marcantonio 2017). The incidence of postoperative delirium varies depending on the type of surgical procedure. For example, rates of 12% to 50% have been reported after non‐cardiac surgery (Brouquet 2010; Olin 2005; Shah 2012), up to 51% after cardiac surgery (Smulter 2013), and 12% to 61% after orthopaedic operations (Holmes 2000). The incidence of delirium in palliative care settings ranges from 3% to 45% (Perrar 2013). In general medical and geriatric medicine wards, incidence rates range from 11% to 29% (Inouye 2014). The prevalence of delirium on admission to these wards is also high (18% to 35% in general medical wards) (Inouye 2014). When combined with the incidence rates of newly occurring delirium after admission, the overall occurrence of delirium in these settings is relatively high. The epidemiology of delirium in emergency departments is not as well established (Vasilevskis 2012). Furthermore, delirium is not exclusive to hospital settings. One study found an incidence of delirium of 20% in nursing home residents who experience an acute illness (Flaherty 2013).
The ICU is an organised system that provides intensive and specialised medical and nursing care. Patients in ICU settings appear to have different characteristics when compared with patients in other settings. For example, patients in the ICU are more critically ill than patients in other settings. Treatment priorities also tend to be different. Medical treatment in the ICU focuses on multiple modalities of physiologic organ support to sustain life during a period of acute organ system insufficiency (Marshall 2017). Potential clinical heterogeneity can therefore be expected between ICU and non‐ICU settings. This review complements a review on delirium in ICU settings that is being performed by the Cochrane Anaesthesia Group (Greve 2012).
Description of the intervention
The treatment of delirium aims to enhance recovery, maximise functional status, and improve clinical outcomes. In addition to general symptomatic management, a key element of management is the investigation and treatment of any reversible underlying causes (Schwartz 2016; Young 2010). According to National Institute for Health and Care Excellence (NICE) guidance, multicomponent non‐pharmacological approaches are used as a first‐line intervention for the treatment of delirium in adults. This strategy includes ensuring effective communication and reorientation (e.g. explaining where the person is, who they are, and what your role is), providing reassurance to people diagnosed with delirium, involving family, friends, and caregivers to help in this process, and providing a suitable care environment (NICE 2014). There are currently no drugs specifically approved by the US Food and Drug Administration (FDA) or other medicine licensing bodies to treat delirium. In practice, however, clinicians currently employ various medications for symptomatic relief (Breitbart 2012; NICE 2014).
Antipsychotic medications are often used for the treatment of delirium. This is especially true for second‐generation antipsychotic drugs, which, when compared with first‐generation antipsychotic drugs (such as haloperidol), require a shorter time to take effect and produce fewer extrapyramidal symptoms (Kishi 2016). According to the NICE guidelines, if a person is distressed or presents a substantial risk to themselves or others, antipsychotic drugs (olanzapine or haloperidol) are not recommended for the treatment of delirium unless non‐pharmacological measures have been ineffective or inappropriate (NICE 2014). Benzodiazepines play a role in the treatment of delirium caused by withdrawal from sedatives or alcohol. However, they are not useful in the treatment of delirium from other causes because they can cause confusion and drowsiness, particularly in the elderly (Catic 2011). Some research has demonstrated that dexmedetomidine, an α2‐adrenoceptor agonist, is useful in the treatment of delirium associated with cancer pain, surgery, or alcohol withdrawal (Ayeko 2015; Nguyen 2016). Though cholinesterase inhibitors such as rivastigmine, donepezil, and galantamine have been used to treat delirium, evidence regarding their effectiveness is inconsistent. One randomised controlled trial found that rivastigmine, when added to standard treatment with haloperidol, potentially increased the severity of delirium as well as mortality in people in the ICU (van Eijk 2010). However, other primary prospective studies (non‐randomised) suggested that rivastigmine was useful for delirium associated with Alzheimer's disease, vascular dementia, or stroke in non‐ICU settings (Litvineneko 2010; Oldenbeuving 2008). Other cholinesterase inhibitors such as donepezil have also been studied for the prevention of delirium (Liptzin 2005; Marcantonio 2011; Sampson 2007).
How the intervention might work
The mechanisms underlying the development of delirium are complex and poorly elucidated, though several theories have been proposed (Maldonado 2008). One of the leading hypotheses is that delirium results from an impairment of central cholinergic transmission, considered by some investigators to be 'a common denominator' in this disorder (Blass 1981). Acetylcholine is the main neurotransmitter that mediates learning and attention, functions that are profoundly disturbed during delirium. Impaired cholinergic function also correlates with the cognitive and behavioural changes observed in people with delirium (Trzepacz 1996). Furthermore, drugs with anticholinergic effects may induce delirium, while cholinergic drugs can improve delirium induced by lithium and anticholinergic medications (Oldenbeuving 2008). By inhibiting the activity of the enzymes that metabolise acetylcholine, cholinesterase inhibitors cause increased cholinergic activity at synapses (Masuda 2015). They have also been shown to play a role in improving cognitive function in people with dementia (Chen 2016; Li 2015; Rolinski 2012). Both delirium and dementia share cholinergic deficiency as a mechanistic hypothesis (Hshieh 2008; Wang 2009). The three cholinesterase inhibitors, rivastigmine, donepezil, and galantamine, are currently approved as first‐line drugs for the treatment of dementia associated with Alzheimer's disease (Li 2015; Qaseem 2008), and are also recommended by NICE for the treatment of Lewy body diseases (i.e. dementia with Lewy bodies and Parkinson's disease dementia) (NICE 2011). By treating the presumed cholinergic deficiency in people with delirium, acetylcholinesterase inhibitors may therefore have beneficial effects.
Why it is important to do this review
This review supersedes the previous review 'Cholinesterase inhibitors for delirium', which was published in 2008 (Other published versions of this review). That review included only one trial, Liptzin 2005, which compared donepezil with placebo for the prevention and treatment of postoperative delirium in people over the age of 50 without dementia who were undergoing elective total joint replacement. More studies have since been conducted with various cholinesterase inhibitors in different settings. Compared with patients in non‐ICU settings, patients in the ICU have a higher risk of delirium. In addition, different validation delirium assessment instruments and treatment strategies are employed in the management of ICU patients (Hayhurst 2016; Oh 2017). Since delirium in ICU settings as a sole scope has been examined in previous reviews (Hayhurst 2016; Trogrlić 2015), this review focused on cholinesterase inhibitors for the treatment of delirium in non‐ICU settings.
Objectives
To evaluate the effectiveness and safety of cholinesterase inhibitors for treating people with established delirium in a non‐ICU setting.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials, published or unpublished, which were reported in English or Chinese.
Types of participants
We planned to include participants over 16 years of age, of either sex, diagnosed with delirium by standardised diagnostic criteria (e.g. DSM‐IV, DSM‐V, ICD‐10). If studies stated that people had delirium but did not use standardised diagnostic criteria, we planned to include these studies in the meta‐analysis and conduct sensitivity analyses to test whether the inclusion criteria influenced the results. We also planned to include participants who experienced delirium from any cause (such as medical illnesses and adverse effects from medications) with the exception of alcohol/drug withdrawal. We planned to include studies conducted in either hospital or community settings. We excluded those studies that explicitly mentioned that people were recruited in the ICU, regardless of the type of ICU (such as general ICUs and other special ICUs including coronary care units, trauma ICUs, etc.). However, if the study described the setting as a high dependency unit where patients were cared for more extensively than in a normal ward, but not to the point of intensive care, we planned to include the study.
Types of interventions
We planned to include trials assessing the effect of any of the currently marketed cholinesterase inhibitors (e.g. donepezil, rivastigmine, galantamine), administered at any dose and at any frequency, compared with placebo. We also planned to include head‐to‐head comparisons of a cholinesterase inhibitor with another drug intended to treat delirium (e.g. antipsychotic drugs, α2‐adrenoceptor agonists, benzodiazepines, and melatonin).
We also planned to include trials involving non‐pharmacological management strategies if we could extract data from the groups.
Types of outcome measures
Primary outcomes
-
Response to treatment:
-
duration of delirium;
-
severity of delirium measured by validated scales (e.g. Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997), Delirium Rating Scale (DRS) (Trzepacz 1988), or DRS‐R‐98 (Trzepacz 2001).
-
-
Adverse events
Secondary outcomes
-
Use of rescue medications (e.g. one‐off doses of antipsychotic drug)
-
Persistent cognitive impairment (defined by original studies)
-
Length of hospitalisation
-
Institutionalisation
-
Mortality
-
Cost of intervention (such as direct monetary cost of intervention to participants or healthcare services)
-
Leaving the study early
-
Quality of life
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialised Register, on 26 October 2017.
ALOIS is maintained by the review group's Information Specialist and contained dementia and cognitive improvement studies identified from:
-
quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
monthly searches of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Sciences Literature);
-
monthly searches of trial registers: metaRegister of Controlled Trials (www.isrctn.com/page/mrct); UMIN Clinical Trials Registry (www.umin.ac.jp/ctr/); World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (which covers ClinicalTrials.gov, ISRCTN, Chinese Clinical Trials Register, German Clinical Trials Register, Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
-
monthly searches of a grey literature source: ISI Web of Science Core Collection.
To view a list of all sources searched for ALOIS please see About ALOIS.
We ran additional searches to ensure we had retrieved the most up‐to‐date results. The search strategies used for the retrieval of reports of trials from bibliographic databases and trial registries can be seen in Appendix 1.
Searching other resources
We cross‐checked the reference lists of included studies to identify any potentially eligible trials.
Data collection and analysis
Selection of studies
Two review authors (AY and SW) independently assessed each abstract and title for relevance. We obtained the full texts of citations that described a potentially relevant randomised controlled trial for further assessment. Two review authors independently determined eligibility of these trials for inclusion. Any disagreements at any stage of the study selection process were resolved by discussion or by the involvement of a third review author (ZZ).
Data extraction and management
Two review authors (AY and SW) independently extracted data using prespecified data extraction forms. A pilot data extraction was performed before the formal data extraction. Any discrepancies were resolved by discussion. We collected the following information where possible.
Participant characteristics
-
Age
-
Sex
-
Education
-
Diagnostic criteria for delirium
-
Severity of delirium
-
Underlying aetiology of delirium
-
Baseline comorbid dementia
-
Setting (refers to the environment where the clinical trial was conducted, e.g. palliative care settings, general or geriatric wards, emergency departments)
-
Inclusion or exclusion criteria of the original studies
Intervention characteristics
-
Types of cholinesterase inhibitors
-
Description of the comparator
-
Dose, route, frequency, and duration of cholinesterase inhibitor and comparator
-
Duration of treatment
-
Any concomitant treatments
Outcomes
-
Outcomes as outlined in Types of outcome measures
-
Definition, instruments, and measured time points of outcomes
Methodological characteristics
-
Sample size
-
Duration of follow‐up
-
Information needed for 'Risk of bias' assessment
For continuous data, we extracted the mean, standard deviation, and number of participants for each treatment group at each time point, if available. For dichotomous data, we retrieved the number in each treatment group and numbers experiencing the outcome of interest where possible. If only treatment effects and their standard errors were reported, these would be extracted.
Assessment of risk of bias in included studies
Two review authors (AY and SW) independently assessed the risk of bias of each included study using the Cochrane 'Risk of bias' tool (Higgins 2011), which evaluates the following risk domains: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other potential sources of bias (including source of financial support). We used the criteria reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied the following judgements to each domain: low risk, high risk, or unclear risk (either lack of information or uncertainty over the potential for bias). Any disagreements were resolved by consensus or by consulting a third review author (ZZ) when necessary.
Measures of treatment effect
If trials used the same rating scale to assess the outcome, we planned to calculate the mean difference (MD) with a 95% confidence interval (CI); if different rating scales were used to measure the same outcome, we planned to employ the standardised mean difference (SMD) for continuous data. The treatment effect for dichotomous outcomes was expressed as a risk ratio (RR) with a 95% CI.
Unit of analysis issues
For studies with multiple eligible treatment groups, we planned to use one of the approaches described in Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions to overcome the unit of analysis error (Higgins 2011). Our preferred approach was to merge all relevant experimental intervention groups of the study into a single group and to merge all relevant control intervention groups into a single control group. If this approach was not suitable, we planned to include all relevant experimental groups and split the shared control group.
Dealing with missing data
We planned to report missing outcome data and consider and discuss the potential impact of the missing data on the results for all outcomes. When attrition for a continuous outcome was between 0% and 50%, and only data from people who had completed the study to that point were reported, we planned to reproduce these.
We anticipated that some studies would have used the method of last observation carried forward (LOCF) or other imputation methods. If less than 50% of the data had been imputed, we would present and use these data and report the imputation method used. For studies with more than 50% of imputed data, we would use the data, but would conduct a sensitivity analysis by excluding these studies to test the robustness of the result.
If standard deviations were not reported, we would first attempt to obtain the missing values from the study authors. If this was not possible, we would attempt to calculate standard deviations from the available statistics in the study report according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
Two review authors (AY and SW) independently assessed clinical and methodology heterogeneity, and only planned on conducting meta‐analyses when the study quality, participants, interventions, and outcomes were sufficiently similar. We planned on assessing statistical heterogeneity using the I2 statistic (I2 greater than 50% may represent substantial heterogeneity) combined with the P value from the Chi2 test (P < 0.1), if a meta‐analysis was to be performed (Higgins 2011).
Assessment of reporting biases
If at least 10 studies were available for meta‐analysis, we planned on assessing the effect of publication bias using a funnel plot to identify small‐study effects.
Data synthesis
We planned on conducting meta‐analyses using the Mantel‐Haenszel method for dichotomous outcomes, and the inverse variance method for continuous outcomes. We planned to use a random‐effects model for all analyses. For cases in which the statistical heterogeneity was significant (P value from Chi2 test < 0.1 and I2 greater than 50%), we planned to explore and address the source of heterogeneity as described in the Subgroup analysis and investigation of heterogeneity section.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis
When data allowed, we planned to conduct a subgroup analysis according to:
-
participant age (older than 65 years versus 65 years or younger);
-
different causes of delirium (e.g. postoperative delirium, adverse events to medication, or delirium due to hepatic encephalopathy);
-
presence or absence of pre‐existing dementia or neurocognitive impairment.
Investigation of heterogeneity
Where there was evidence of statistical heterogeneity (P value from Chi2 < 0.1 and I2 greater than 50%) of the treatment effect between trials, if we could identify possible sources of variation, we planned to explore the source of the heterogeneity and conduct subgroup analyses. Otherwise, we would use a random‐effects model to pool the data. Where statistical heterogeneity was significant in a meta‐analysis, we would consider downgrading the quality of evidence using the GRADE approach (GRADEpro GDT 2015; Schünemann 2011a; Schünemann 2011b).
Sensitivity analysis
Where possible, we planned to conduct a sensitivity analysis to explore the influence of the quality of trials by excluding data from low‐quality trials. We would define low‐quality trials as those in which more than 50% of the data in one arm of the study was lost or studies with a high risk of selection bias and a high risk of detection bias due to non‐blind outcome assessment. We also planned to conduct a sensitivity analysis to investigate the difference between results from completers‐only and intention‐to‐treat analysis (for primary outcomes only). We planned on presenting results from both approaches separately and discussing the results at the full review stage.
'Summary of findings' table
For each comparison, we used the GRADE approach to assess the quality of the body of evidence for all outcomes (GRADEpro GDT 2015; Schünemann 2011a; Schünemann 2011b). We presented the following results in the 'Summary of findings' tables.
-
Duration of delirium
-
Severity of delirium
-
Adverse events
-
Persistent cognitive impairment
-
Length of hospitalisation
-
Mortality
-
Cost of intervention
Evidence was given one of four possible ratings: high, moderate, low, or very low quality. A rating of high quality indicated that we were confident in our estimate of the effect and that further research was very unlikely to change this, whereas a rating of very low quality implied that we were very uncertain about the estimate of the effect. The GRADE approach rates evidence from randomised controlled trials as high quality initially, however several factors could lead to the downgrading of the evidence, namely: study limitations (risk of bias); inconsistency; indirectness of evidence; imprecision; and publication bias (Schünemann 2011a; Schünemann 2011b).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 194 records from databases (164 records) and other sources (30 records). The total number of records was unchanged after de‐duplication. We excluded 164 records based on the title and abstract. Of the remaining 30 records assessed in full, we excluded 27 records with reasons (see Excluded studies). One record is an ongoing study (NCT01487317). We eventually included only one study (Overshott 2010, with two records) in this review (see Figure 1 for more details).
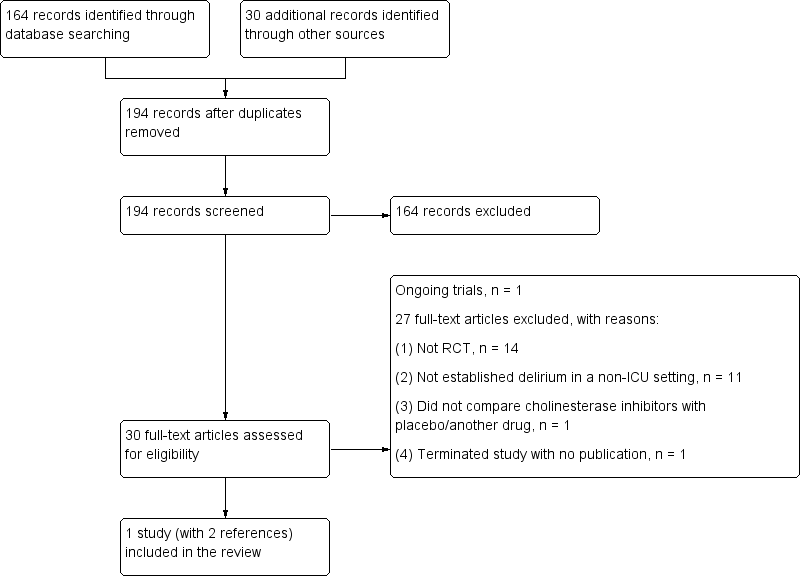
Study flow diagram.
Included studies
Only one study with 15 participants that compared rivastigmine with placebo met the inclusion criteria for this review (see Characteristics of included studies) (Overshott 2010).
The 15 participants were recruited from the UK and were diagnosed with delirium based on the Confusion Assessment Method (CAM) criteria. Seven of the 15 participants had comorbid dementia at baseline. The mean age of the participants was 82.5 years; eight participants were male and seven were female.
The study reported the following outcomes: duration of delirium as assessed by CAM criteria, adverse events, use of rescue medications (additional psychotropic medications received), mortality, and leaving the study early. Other predefined outcomes of this review (severity of delirium, persistent cognitive impairment, length of hospitalisation, institutionalisation, cost of intervention, and quality of life) were not reported.
Excluded studies
See Characteristics of excluded studies.
We excluded a total of 27 studies from this review for the following reasons.
-
Twelve studies were not randomised controlled trials (Chapin 1977; Dautzenberg 2004; Fischer 2001; Gleason 2003; Granacher 1976; Heiser 1974; Hori 2003; Kaufer 1998; Lankarani‐Fard 2006; Listed 2011; Newman 1980; Scicutella 2015; Sheldon 2010; Wengel 1999).
-
Participants of nine studies did not have a diagnosis of delirium in a non‐ICU setting (EUCTR2007‐000262‐20‐GB; Crowell 1967; Doraiswamy 2007; Liptzin 2005; Marcantonio 2011; Moretti 2004; Silver 2006; Tenovuo 2009; Van Eijk 2010; Youn 2017; Zaslavsky 2012).
-
We excluded one study due to the interventions being evaluated (Pitkala 2006). This study compared an intensified, multicomponent geriatric treatment group with a control group, and we were unable to extract data from the groups that differed only in terms of exposure to cholinesterase inhibitor versus placebo.
-
One clinical trial was terminated with no published results (NTR 537). We contacted the primary investigator, who informed us that no data had been published from this trial.
Ongoing studies
See Characteristics of ongoing studies.
We identified one ongoing study begun in France in 2011 (NCT01487317). Though recruitment was complete, no results have been reported or published. We planned to contact the primary investigator for more details but contact information was not available.
Risk of bias in included studies
The summary of risk of bias in the included study is presented in Figure 2 and Figure 3 (Overshott 2010). Please refer to the 'Risk of bias' table in Characteristics of included studies for further details.
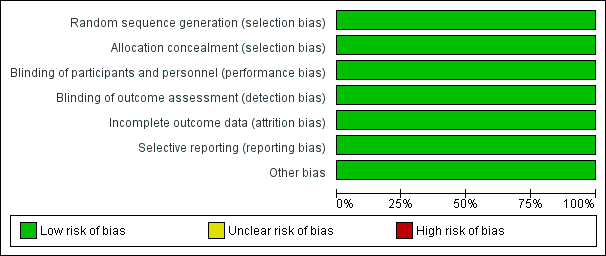
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
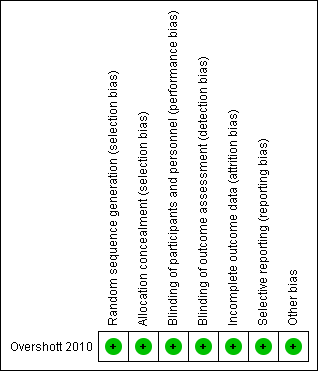
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study stated that participants were randomised by numbered treatment packets developed by the special statistics department, which was independent of the research team. The random sequence was concealed before allocation. We therefore rated this study as at low risk of selection bias.
Blinding
The included study used a convincingly double‐blinded design that ensured blinding of both the participants and researchers. We rated this study as at low risk of performance and detection bias.
Incomplete outcome data
Two participants left the study early due to protocol violation. One person in the rivastigmine group withdrew their consent when CAM was negative for two consecutive days. One person in the placebo group lost the trial medication after being transferred to another ward. The number of dropouts was small and balanced between the two groups. Given that the reasons for the dropouts were not related to the intervention, we rated this study as having a low risk of attrition bias.
Selective reporting
We did not obtain the protocol for this study. The study reported all outcomes that were stated in the methods section, and the primary outcome in this review was reported as well. We rated the study as at low risk of reporting bias.
Other potential sources of bias
We found no other obvious bias in the included study and rated it as at low risk for this domain.
Effects of interventions
We included one study that involved 15 participants and compared rivastigmine with placebo (Overshott 2010). The quality of evidence for the reported outcomes was low due to the very small sample size (see summary of findings Table for the main comparison).
Primary outcomes
Response to treatment: duration of delirium
The study reported that the mean (standard deviation, range) duration of delirium for participants in the rivastigmine and placebo groups was 6.3 (5.7, 1 to 19) days and 9.9 (14.6, 1 to 42) days, respectively. The mean and range of duration of delirium were shorter for the rivastigmine group compared with the placebo group, although the authors did not find a clear difference (mean difference (MD) ‐3.6, 95% confidence interval (CI) ‐15.6 to 8.4; Analysis 1.1) due to the very small sample size (lack of statistical power).
The study used unpaired t‐test for this outcome to measure MD. However, we found that the data were skewed, and therefore a parameter test was not applicable. Hence, we just presented the results as other data in this review.
Response to treatment: severity of delirium
The study did not report this outcome.
Adverse events
Only one participant in the placebo group had nausea. The study found no clear difference in the incidence of nausea between the two groups (risk ratio (RR) 0.30, 95% CI 0.01 to 6.29; Analysis 1.2).
Secondary outcomes
Use of rescue medications
Three participants in the placebo group and no participants in the rivastigmine group received additional psychotropic medication due to behavioural disturbances. The study found no clear difference between the two groups (RR 0.13, 95% CI 0.01 to 2.1; Analysis 1.3).
Persistent cognitive impairment
The study did not report this outcome.
Length of hospitalisation
The study did not report this outcome.
Institutionalisation
The study did not report this outcome.
Mortality
Four participants in the placebo group and no participants in the rivastigmine group died. The study found no clear difference in mortality between the groups (RR 0.10, 95% CI 0.01 to 1.56; Analysis 1.4).
Cost of intervention
The study did not report this outcome.
Leaving the study early
One participant in the rivastigmine group and one participant in the placebo group left the study early. The study found no clear difference in withdrawals between the two groups (RR 0.88, 95% CI 0.07 to 11.54; Analysis 1.5).
Quality of life
The study did not report this outcome.
Subgroup analysis
We did not perform any subgroup analysis due to insufficient data.
Sensitivity analysis
We did not perform any sensitivity analysis due to insufficient data.
Assessment of reporting biases
We did not produce a funnel plot to assess reporting biases because no meta‐analysis included at least 10 studies.
Discussion
Summary of main results
We identified only one study that compared a cholinesterase inhibitor with placebo for the treatment of delirium in non‐ICU patients. Based on the absolute difference, the duration of delirium was shorter in the rivastigmine group (3.6 days on average) compared with the placebo group, and no deaths occurred in the rivastigmine group. However, this study had limited data (15 participants) with low‐quality evidence. Any comparative analysis would be unlikely to show an effect because the study was grossly underpowered. Hence, in actuality we did not find any clear differences in the duration of delirium, adverse events, use of rescue medications, mortality, or the number of participants leaving the study early. No evidence was available to evaluate severity of delirium or the remaining secondary outcomes.
Overall completeness and applicability of evidence
The overall completeness and applicability of the evidence in terms of the participants, interventions, and outcomes were poor and very limited in this one included study. For one, the included participants were diagnosed with delirium using CAM rather than the standardised diagnostic criteria (e.g. DSM‐IV, DSM‐V, ICD‐10). Patient demographics were also limited in terms of country of origin (all participants were from the UK) and age distribution (the average age was over 80 years). Rivastigmine and placebo were the only interventions, and no other cholinesterase inhibitor drugs (e.g. donepezil, galantamine) were evaluated. Furthermore, there were no comparisons of a cholinesterase inhibitor with other drugs intended to treat delirium such as antipsychotic drugs, α2‐adrenoceptor agonists, benzodiazepines, or melatonin. A very small amount of data was reported on outcomes such as duration of delirium, adverse events (nausea), use of rescue medications, mortality, and leaving the study early. Most predefined outcomes in this review were not reported, including the severity of delirium, persistent cognitive impairment, length of hospitalisation, institutionalisation, cost of intervention, and quality of life. The applicability of this current evidence is therefore limited.
Quality of the evidence
The included study had a low risk of bias across all domains. We downgraded the overall quality of evidence to low due to the very small sample size.
Potential biases in the review process
We minimised the potential biases in the review process by performing a thorough and complete search of the databases and other sources. Two review authors (AY and SW) independently screened and extracted the data using prespecified data extraction forms, a process that lessens the likelihood of introducing bias in the review process.
Agreements and disagreements with other studies or reviews
This review supersedes a previous Cochrane Review that was originally published in 2008 (Other published versions of this review). This latter review included one study that evaluated the possible benefit of donepezil versus placebo in the prevention and treatment of postoperative delirium in an elderly population without dementia undergoing elective total joint replacement surgery (Liptzin 2005). In this previous review, the incidence of postsurgical delirium was measured, and there was no clear difference in the duration of postsurgical delirium between the two groups. In this current updated review, we focused on the treatment of delirium. We excluded the previous study, Liptzin 2005, because 90 included participants did not have delirium at the time of randomisation, and only 15 participants developed delirium after treatment. This current review only included participants with established delirium pre‐randomisation. Similar to the previous review, we found no clear difference in the duration of delirium between the rivastigmine and placebo groups in a non‐ICU setting, and no clear difference between groups for other outcomes such as adverse events, use of rescue medications, mortality, and leaving the study early. However, both reviews lacked sufficient evidence to enable any firm conclusions. Though other meta‐analyses have evaluated the prevention of delirium or treatment efficacy of certain interventions (Siddiqi 2016; Tampi 2016), participants did not meet the inclusion criteria of the present review (i.e. they did not have an established diagnosis of delirium).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Study | Interventions | N | MD | 95% CI | P value |
| Overshott 2010 | Cholinesterase inhibitors (rivastigmine) versus placebo | 15 | ‐3.6 | ‐15.6 to 8.4 | 0.5 |
Comparison 1 Cholinesterase inhibitors (rivastigmine) versus placebo, Outcome 1 Duration of delirium (days).

Comparison 1 Cholinesterase inhibitors (rivastigmine) versus placebo, Outcome 2 Adverse events (nausea).

Comparison 1 Cholinesterase inhibitors (rivastigmine) versus placebo, Outcome 3 Use of rescue medications.

Comparison 1 Cholinesterase inhibitors (rivastigmine) versus placebo, Outcome 4 Mortality.

Comparison 1 Cholinesterase inhibitors (rivastigmine) versus placebo, Outcome 5 Leaving the study early.
| Cholinesterase inhibitors (rivastigmine) compared to placebo for the treatment of delirium in non‐ICU settings | ||||||
| Patient or population: people with delirium | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Cholinesterase inhibitors (rivastigmine) | |||||
| Duration of delirium | The mean duration was 9.9 days | The mean duration was 3.6 days lower (15.6 lower to 8.4 higher) | Not estimable | 15 | ⊕⊕⊝⊝ | The data were reported by Overshott 2010. The study was grossly underpowered, and the data were skewed. |
| Severity of delirium | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Adverse events | 143 per 1000 | 43 per 1000 | RR 0.3 | 15 | ⊕⊕⊝⊝ | |
| Persistent cognitive impairment | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Length of hospitalisation | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| Mortality | 571 per 1000 | 57 per 1000 | RR 0.1 | 15 | ⊕⊕⊝⊝ | |
| Cost of intervention | See comment | See comment | See comment | See comment | See comment | No study reported this outcome. |
| *The dose of intervention: 1.5 mg once a day increasing to 1.5 mg twice a day after 7 days. **The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision due to very small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of delirium (days) Show forest plot | Other data | No numeric data | ||
| 2 Adverse events (nausea) Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.29] |
| 3 Use of rescue medications Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.10] |
| 4 Mortality Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.56] |
| 5 Leaving the study early Show forest plot | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.07, 11.54] |

