Asistencia respiratoria comparada con ninguna asistencia respiratoria antes del pinzamiento del cordón umbilical en recién nacidos prematuros
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre randomized controlled trial stratified by gestational age and mode of delivery (caesarean section (83%) or vaginal birth (17%)). Sample size of 150 infants estimated to detect a 15% difference in peak haematocrit. A waiver of informed consent was obtained for women presenting in active labour. | |
| Participants | Preterm infants 23 to 31 weeks 6 days' gestation delivered at Sharp Mary Birch Hospital in San Diego. | |
| Interventions | Intervention group: V‐DCC: 75 infants received 60 seconds of CPAP or PPV with room air provided by facemask with a T‐piece resuscitator. Apnoeic infants were briefly stimulated and given PPV followed by CPAP when breathing was established. A colorimetric carbon dioxide detector used to assess adequacy of ventilation. Control group: DCC: 75 infants received 60 seconds of DCC. They were dried and, if apnoeic, received gentle stimulation. | |
| Outcomes | Primary outcome: maximum haematocrit in first 24 hours presented as mean and SD depending on the method of birthing (vaginal or caesarean). Authors provided unpublished data that allowed group comparisons between V‐DCC and DCC to be made. Inhospital mortality rate (1 of the primary outcomes of the current review) was likewise presented according to mode of birth. Condition at birth; measures of cardiovascular status; haematological and respiratory outcomes; and neonatal outcomes including intraventricular haemorrhage, blood transfusion, and duration of phototherapy were also reported by mode of birth. Event rates for categorical variables (e.g. mortality for the V‐DCC and DCC groups as a whole) could be determined from the paper and continuous data (e.g. duration of phototherapy) was calculated from unpublished data. | |
| Notes | Study demonstrated the feasibility of the intervention. 95% of infants had at least 60 seconds of DCC. The majority of infants were delivered by caesarean section. Overall, 91% of participants established breathing before cord clamping. Subgroup analysis of mode of delivery and of infants who breathed during the procedure did not reveal significant differences. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind personnel providing procedure at birth. |
| Blinding of outcome assessment (detection bias) | Low risk | Neonatal team blinded to group allocation at birth. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes presented. |
| Other bias | Low risk | None identified. |
CPAP: continuous positive airway pressure; DCC: delayed cord clamping; PPV: positive pressure ventilation; SD: standard deviation; V‐DCC: delayed cord clamping with ventilation support.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Control group randomized to early cord clamping. | |
| Control group randomized to immediate cord clamping. | |
| Observational study, non‐randomized intervention. | |
| Pilot feasibility study with no control group. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Provision of Breathing Support during Delayed Cord Clamping in Preterm Infants. |
| Methods | Randomized controlled intervention trial with parallel assignment and masking of outcome assessment. |
| Participants | Inclusion criteria: preterm infants, < 31 weeks' gestation undergoing DCC (vaginal and caesarean births) deemed not to have established regular rhythmic breathing (chest wall movement) after 15 seconds of DCC. Exclusion criteria: known congenital abnormality, twin‐to‐twin transfusion syndrome, severe antenatal intrauterine growth restriction (estimated fetal weight < 10th customized centile), placental abruption, delivery of placenta and infant simultaneously (en caul), short umbilical cord, obstetrician refusal, declined antenatal consent. |
| Interventions | Intervention group: V‐DCC. Breathing support in form of PPV or CPAP delivered by facemask and T‐piece resuscitator. Infants will be randomized at 15 seconds of age once their breathing has been assessed. Breathing support will continue for 30 seconds while DCC is occurring (total of 50 seconds of DCC). Infants receiving breathing support will have a carbon dioxide calorimetric detection device inserted into the breathing circuit. Control group: DCC: no respiratory support before the cord is clamped at 50 seconds. |
| Outcomes | Primary outcome: red blood cell transfusion rate during the neonatal admission period. Secondary composite outcomes: chronic lung disease (respiratory support or use of supplemental oxygen at 36 weeks' corrected postnatal age), intraventricular haemorrhage (grade 3 or 4), and death (during the neonatal period). Other secondary outcomes: endotracheal intubation day 1, surfactant usage, admission temperature, echocardiographic assessment of transitional circulation, receipt of phototherapy, neonatal morbidity, length of hospital stay, and neurodevelopmental outcome at 2 years of age. |
| Starting date | February 2016. |
| Contact information | |
| Notes | Australia and New Zealand Clinical Trials Registry: ACTRN12615001026516. Currently recruiting. Expected completion: June 2019. |
| Trial name or title | Stabilisation of Preterm Infants with Intact Umbilical Cord, Aeration, Breathing, then Clamping: a Feasibility Study. |
| Methods | Safety and feasibility study. |
| Participants | Vaginal births 26‐35 weeks' gestation, planned recruitment of 15 infants. |
| Interventions | Intervention group: infants will be placed on the Con‐Cord table at the mother's bedside with monitoring and, with the umbilical cord intact, receive respiratory support according to local resuscitation guidelines. Control group: no respiratory support. |
| Outcomes | Safety and applicability. |
| Starting date | Registered September 2016. |
| Contact information | NTR6095. |
| Notes | Part of a wider ABC project ‐ the feasibility study has recently been completed and is to be followed by an effectiveness study, with the goal of subsequently performing a randomized trial. |
| Trial name or title | VentFirst: a Multicenter RCT of Assisted Ventilation during Delayed Cord Clamping for Extremely Preterm Infants. |
| Methods | Multicentre, randomized controlled intervention trial with parallel assignment and masking of outcomes assessment. Collaborators: Brigham and Women's Hospital, Mayo Clinic, St Louis University, University of Colorado, Denver; Royal Alexandra Hospital Oregon Health and Science University, University of Calgary. |
| Participants | Inclusion criteria: 23 weeks and 0 days to 28 weeks and 6 days' gestation at delivery. Exclusion criteria: life‐threatening condition of fetus (e.g. severe hydrops, lethal chromosomal abnormality, severe congenital malformation); suspected severe fetal anaemia; monochorionic or monoamniotic twins; multiple gestation greater than twins; decision made for comfort care only; medical emergency necessitating emergency delivery (e.g. complete placental abruption); obstetrician or neonatology concern for inappropriateness of the study intervention based on maternal or fetal factors. |
| Interventions | Intervention group: VentFirst (intervention) group will receive assisted ventilation (face mask, CPAP, or PPV) prior to cord clamping at 120 seconds. If the baby is not breathing well, PPV by face mask will be given at 30 seconds, if the baby is breathing well, CPAP by face mask will be given starting at 30 seconds. Control group: standard treatment group will receive DCC 30‐60 seconds after birth, and assisted ventilation after cord clamping. If the infant is not breathing well, cord will be clamped at 30 seconds, and if the baby is breathing well, cord will be clamped at 60 seconds. |
| Outcomes | Primary outcome: intraventricular haemorrhage on head ultrasound 7‐10 days after birth. Secondary outcomes: adverse events in delivery room (assessed at 1 hour after birth), adverse haematological and cardiovascular events (assessed within 24 hours of birth), adverse haematological and respiratory events (assessed within the first 10 days of birth), adverse prematurity‐related events up to 36 weeks' postmenstrual age; and adverse finding on late head ultrasound (birth to 36 weeks' postmenstrual age). |
| Starting date | June 2016. |
| Contact information | Amy K Camblos [email protected]. |
| Notes | Planned enrolment of 940 infants (ClinicalTrials.gov: NCT02742454). Currently recruiting at 6 of 8 sites. Expected study completion June 2023. |
CPAP: continuous positive airway pressure; DCC: delayed cord clamping; PPV: positive pressure ventilation; V‐DCC: delayed cord clamping with ventilation support.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
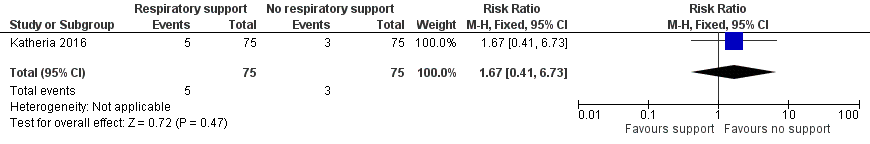
| 1 Mortality during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| Analysis 1.1 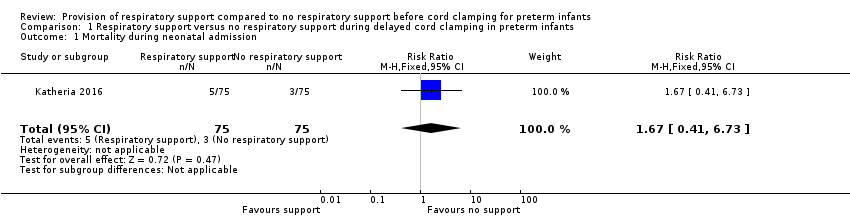 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 1 Mortality during neonatal admission. | ||||
| 2 Need for intubation in delivery room Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.21] |
| Analysis 1.2  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 2 Need for intubation in delivery room. | ||||
| 3 Inotropic support for hypotension Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.63, 2.49] |
| Analysis 1.3  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 3 Inotropic support for hypotension. | ||||
| 4 Peak haematocrit in first 24 hours Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.85, 2.25] |
| Analysis 1.4  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 4 Peak haematocrit in first 24 hours. | ||||
| 5 Blood transfusion during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.54] |
| Analysis 1.5  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 5 Blood transfusion during neonatal admission. | ||||
| 6 Phototherapy for hyperbilirubinaemia Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.31, 0.71] |
| Analysis 1.6 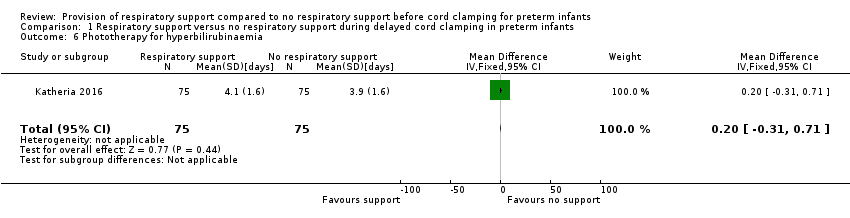 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 6 Phototherapy for hyperbilirubinaemia. | ||||
| 7 Use of surfactant in the first 48 hours of life Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.28] |
| Analysis 1.7  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 7 Use of surfactant in the first 48 hours of life. | ||||
| 8 Bronchopulmonary dysplasia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.50, 2.43] |
| Analysis 1.8  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 8 Bronchopulmonary dysplasia. | ||||
| 9 Intraventricular haemorrhage (of any grade) Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.65, 3.46] |
| Analysis 1.9  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 9 Intraventricular haemorrhage (of any grade). | ||||
| 10 Severe intraventricular haemorrhage grade 3 or 4 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.75] |
| Analysis 1.10  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 10 Severe intraventricular haemorrhage grade 3 or 4. | ||||
| 11 Periventricular leukomalacia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.49] |
| Analysis 1.11 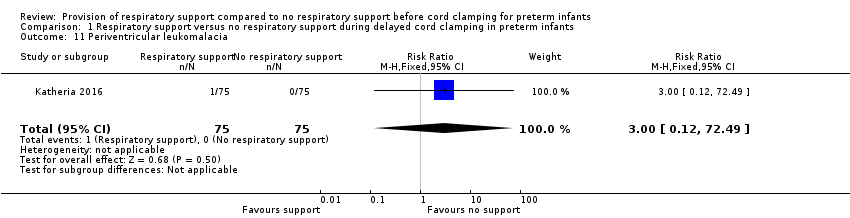 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 11 Periventricular leukomalacia. | ||||
| 12 Necrotizing enterocolitis ≥ Bell's stage 2 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.59] |
| Analysis 1.12  Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 12 Necrotizing enterocolitis ≥ Bell's stage 2. | ||||
| 13 Retinopathy of prematurity requiring treatment Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.59] |
| Analysis 1.13 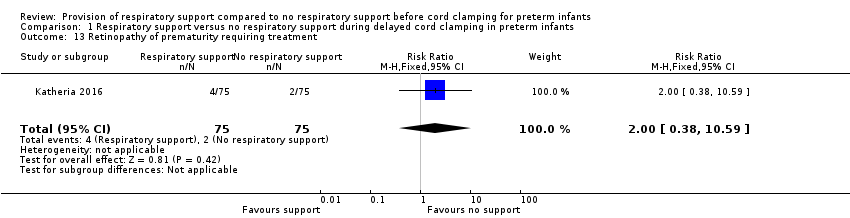 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 13 Retinopathy of prematurity requiring treatment. | ||||
| 14 Sepsis Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| Analysis 1.14 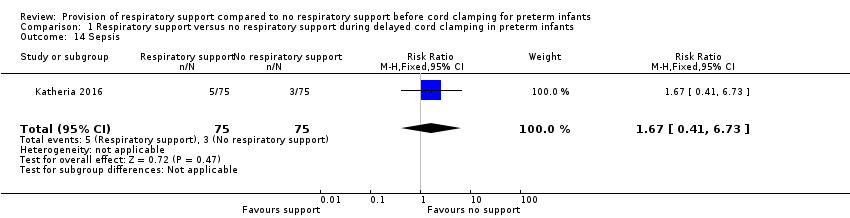 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 14 Sepsis. | ||||
| 15 Pharmacological treatment for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.36] |
| Analysis 1.15 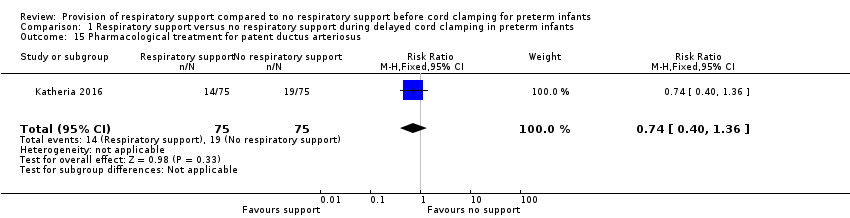 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 15 Pharmacological treatment for patent ductus arteriosus. | ||||
| 16 Surgical ligation for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.85] |
| Analysis 1.16 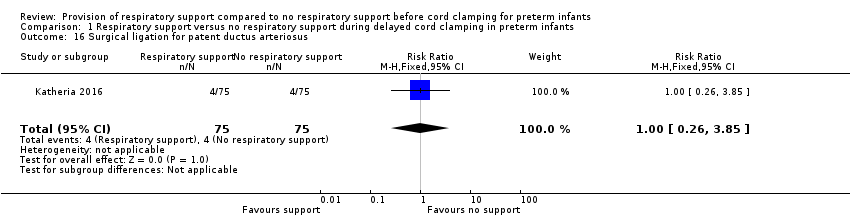 Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 16 Surgical ligation for patent ductus arteriosus. | ||||

Study flow diagram.
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.1 Mortality during neonatal admission.
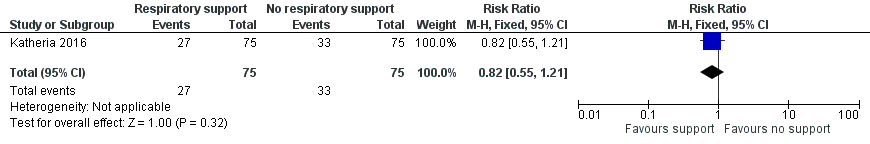
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.2 Need for intubation in delivery room.
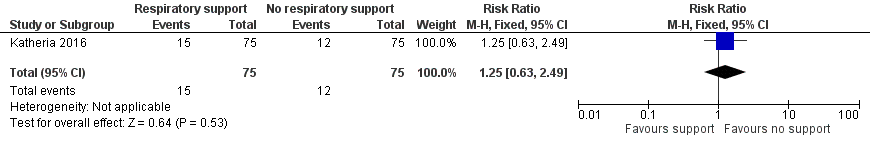
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.3 Inotropic support for hypotension.
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].](/es/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG05.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.5 Blood transfusion during neonatal admission.
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].](/es/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG07.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].
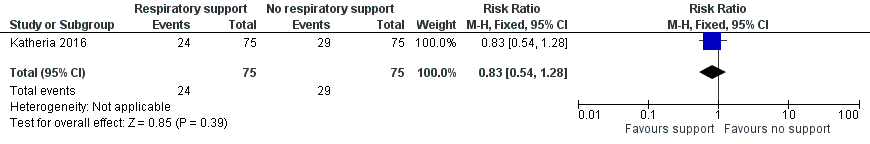
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.7 Use of surfactant in the first 48 hours of life.
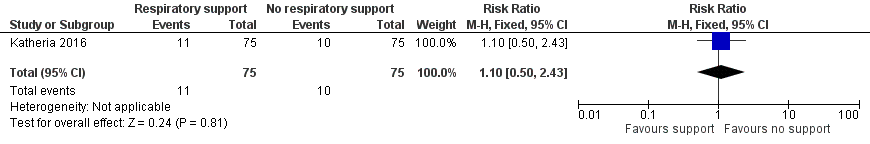
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.8 Bronchopulmonary dysplasia.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.9 Intraventricular haemorrhage (of any grade).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.10 Severe intraventricular haemorrhage grade 3 or 4.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 1 Mortality during neonatal admission.
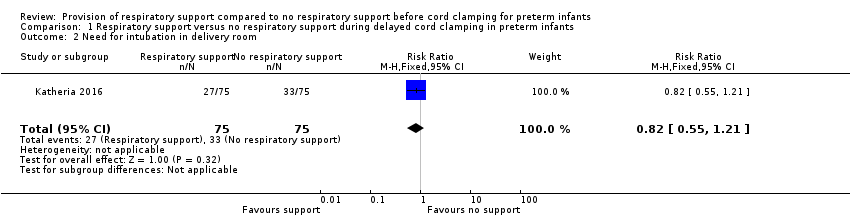
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 2 Need for intubation in delivery room.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 3 Inotropic support for hypotension.
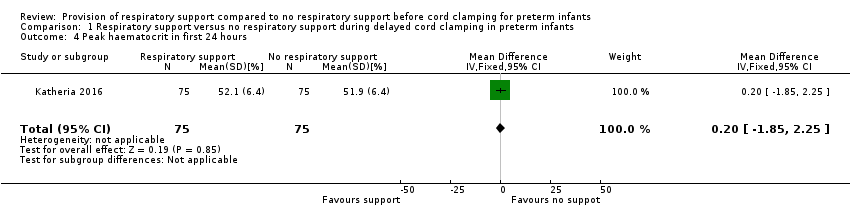
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 4 Peak haematocrit in first 24 hours.
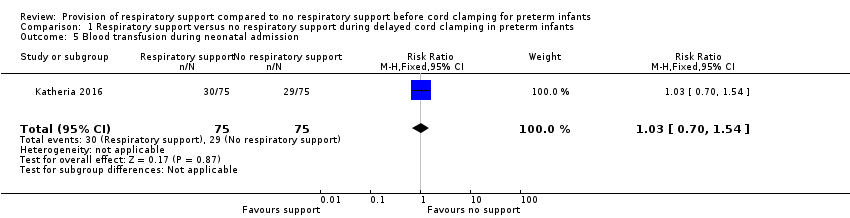
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 5 Blood transfusion during neonatal admission.
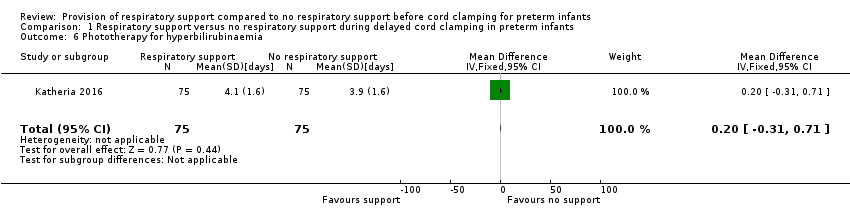
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 6 Phototherapy for hyperbilirubinaemia.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 7 Use of surfactant in the first 48 hours of life.
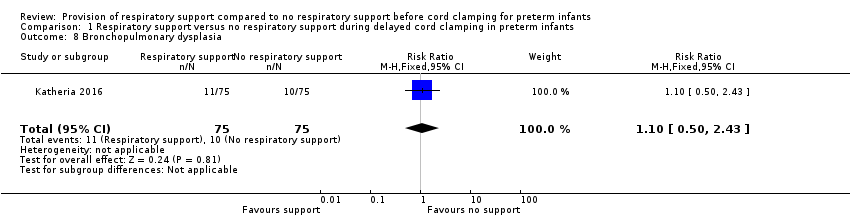
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 8 Bronchopulmonary dysplasia.
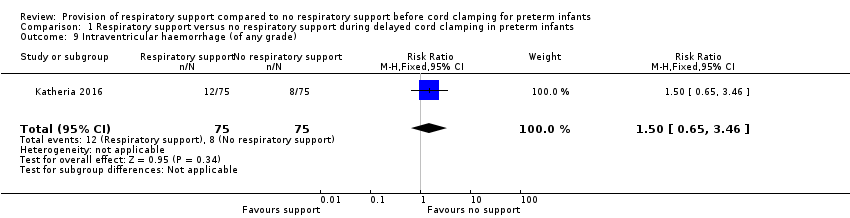
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 9 Intraventricular haemorrhage (of any grade).

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 10 Severe intraventricular haemorrhage grade 3 or 4.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 11 Periventricular leukomalacia.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 12 Necrotizing enterocolitis ≥ Bell's stage 2.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 13 Retinopathy of prematurity requiring treatment.
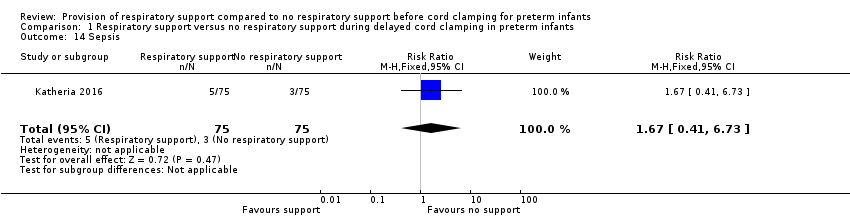
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 14 Sepsis.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 15 Pharmacological treatment for patent ductus arteriosus.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 16 Surgical ligation for patent ductus arteriosus.
| Respiratory support compared with no respiratory support before cord clamping in preterm infants | ||||
| Patient or population: preterm infants Settings: undergoing delayed cord clamping Intervention: respiratory support Comparison: no respiratory support | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Mortality ≤2 years after hospital discharge | RR 1.67 (0.41 to 6.73) | 150 | ⊕⊕⊝⊝ | Secondary study outcome. |
| Inotropic support for hypotension | RR 1.25 (0.63 to 2.49) | 150 | ⊕⊕⊝⊝ | ‐ |
| Peak haematocrit | MD 0.20 (‐1.85 to 2.25) | 150 | ⊕⊕⊕⊝ | Primary outcome. |
| Blood transfusion during neonatal admission | RR 1.03 (0.70 to 1.54) | 150 | ⊕⊕⊕⊝ | 40% with this outcome |
| Phototherapy for hyperbilirubinaemia | MD 0.20 (‐0.31 to 0.71) | 150 | ⊕⊕⊕⊝ | From unpublished data |
| Intraventricular haemorrhage (of any grade) | RR 1.50 (0.65 to 3.46) | 150 | ⊕⊕⊝⊝ | Secondary outcome. |
| Severe intraventricular haemorrhage grade 3 or 4 | RR 1.33 (0.31 to 5.75) | 150 | ⊕⊝⊝⊝ | Uncommon secondary outcome. |
| CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence | ||||
| In the published report, results were presented according to method of delivery. For categorical variables (e.g. mortality), the RR was calculated for the delayed cord clamping with ventilation support (intervention) and delayed cord clamping (control) groups as a whole. For continuous variables (e.g. haematocrit), the authors provided unpublished data that enabled whole group statistics to be determined. 1Downgraded one level due to lack of precision with wide confidence intervals. 2Downgraded two levels due to lack of precision (confidence intervals included both important benefit and harm). The optimal information size for a 30% risk reduction with 80% power and 95% confidence intervals was 3280 infants per group. 3Unpublished data. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| 2 Need for intubation in delivery room Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.21] |
| 3 Inotropic support for hypotension Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.63, 2.49] |
| 4 Peak haematocrit in first 24 hours Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.85, 2.25] |
| 5 Blood transfusion during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.54] |
| 6 Phototherapy for hyperbilirubinaemia Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.31, 0.71] |
| 7 Use of surfactant in the first 48 hours of life Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.28] |
| 8 Bronchopulmonary dysplasia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.50, 2.43] |
| 9 Intraventricular haemorrhage (of any grade) Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.65, 3.46] |
| 10 Severe intraventricular haemorrhage grade 3 or 4 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.75] |
| 11 Periventricular leukomalacia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.49] |
| 12 Necrotizing enterocolitis ≥ Bell's stage 2 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.59] |
| 13 Retinopathy of prematurity requiring treatment Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.59] |
| 14 Sepsis Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| 15 Pharmacological treatment for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.36] |
| 16 Surgical ligation for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.85] |

