Intervenciones de eSalud para la ansiedad y la depresión en niños y adolescentes con enfermedades físicas crónicas
Appendices
Appendix 1. CCMDCT core MEDLINE search
OVID MEDLINE search strategy, used to inform the Cochrane Common Mental Disorders Group's Specialised Register
A weekly search alert based on condition + RCT filter only
1. [MeSH Headings]:
eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]:
(eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]:
(controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Review search: CCMDCTR references register
The CCMD‐CTR‐references register was searched using a sensitive set of terms for: age group + condition + comorbidity + eHealth platforms/computer programs (06/05/16):
[Age Group]
#1. (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young next (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom):ti,ab
[Condition: anxiety/depression]
#2. ((emotion* or psycholog* or mental) next (health or stress* or problem* or disturb* or aspect* or state* or ill*)):ti,ab,kw,ky,emt,mh,mc
#3. (depress* or mood or anxiety or *phobi* or PTSD or post‐trauma* or posttrauma or “post trauma*” or panic* or OCD or obsess* or compulsi* or GAD or "stress disorder*" or “stress reaction*” or "acute stress" or “psychological stress” or “school refusal” or mutism or neurosis or neuroses or neurotic or psychoneuro*):ti,ab,kw,ky,emt,mh,mc
[Comorbidity: chronic physical illnes s]
#4. (“physical* ill*” or “medical* ill*” or “chronic disease” or (chronic* NEXT (ill* or condition*1 or disease* or disorder* or health)) or (long term NEXT (condition*1 or sick*)) or “medical* morbid*” or (medical* NEXT (comorbid* or co morbid*)) or multimorbid* or (multi* NEXT (morbid* or “co morbid*” or comorbid* or physical))):ti,ab,kw,ky,emt,mh,mc
#5. (AIDS or allerg* or angina or aneurysm or “ankylosing spondylitis” or arthropath* or arthriti* or arthrosis or arthroses or asthma* or “atrial fibrillation” or “autoimmune disease*” or “back pain” or blindness or “brain atroph*” or (bone NEXT (disease* or disorder*)) or ((bronchi* or bowel) NEXT (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac NEXT (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) NEAR2 (disease* or disorder* or event*)) or “cerebral palsy” or (cerebrovascular NEAR2 (disease* or disorder* or event*)) or “chronic obstructive” or COPD or pain or cirrhosis or colitis or “congenital abnormalit*” or (congential NEAR3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or “cystic fibrosis” or cystitis)
#6. (deaf* or deformit* or disabled or (physical NEXT (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or “digestive system*” or duoden* or dystonia or eczema or (endocrine NEXT (disease* or disorder*)) or enuresis or epilep* or “eye disease*” or (“fatigue syndrome” or “chronic fatigue”) or fibromyalgia or fibrosis or “food hypersensitivity” or (gastr* NEXT (disease* or disorder*)) or gastritis or “genetic disorder*” or gout or (glomerul* NEXT (disease* or disorder*)) or headache* or ((h?emic or lymph*) NEXT (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) NEAR2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) NEXT (disease* or disorder* or failure)) or (heart NEXT (disease* or disorder* or failure or surg*)) or HIV or “human immunodeficiency virus” or hypertensi* or hypotensi*)
#7. (“inflammatory disease*” or incontinen* or “irritable bowel” or isch?emi* or (joint NEXT (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) NEXT (disease* or disorder* or failure)) or lordosis or “lung disease*” or “lupus erythemat*” or lymphoma or “macular degeneration” or migraine* or “movement disorder*” or musculoskeletal or necrotizing or nephrotic* or neuromuscular or “multiple sclerosis” or myeloma)
#8. (“nephrotic syndrome” or ((nutritional or metabolic) NEXT (disease* or disorder or syndrome*)) or (organ* NEAR2 (transplant* or recipient*)) or (neurological NEXT (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or “otitis media” or otorhinolaryngology* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or “peripheral vascular” or “pick disease*” or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary NEAR2 (disease* or disorder*)))
#9. ((respiratory NEXT (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or “sickle cell an?emia” or ((skin or “connective tissue”) NEXT (disease* or disorder*)) or (“sleep disorder*” or “sleep apn?ea” or insomnia* or dyssomnia* or hypersomnia*) or “spina bifida” or “spinal muscular atropy” or spondylo* or stenosis* or stoma* or (stroke or strokes or “cerebral infarct*”) or tetraplegi* or ((thyroid NEAR (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic NEAR (disorder* or disease*)) or ulcer* or (urogenital NEXT (disease* or disorder*)) or vasculopath* or (vascular NEAR (disease* or disorder*)) or vestibular or ((virus or viral) NEXT disease))
#10. (#1 and (#2 or #3) and (#4 or #5 or #6 or #7 or #8 or #9))
[eHealth]
#11 (android or app or apps or audio* or blog or CBT or CD‐ROM or “cell phone” or cellphone or chat or computer* or cyber* or DVD or eHealth or e‐health or "electronic health*" or e‐Portal or ePortal or eTherap* or e‐therap* or forum* or gaming or iCBT or “information technolog*” or "instant messag*" or internet* or ipad or i‐pad or iphone or i‐phone or ipod or i‐pod or web* or WWW or "smart phone" or smartphone or "social network* site*" or “mobile phone” or e‐mail* or email* or mHealth or m‐health or mobile or multi‐media or multimedia or online* or on‐line or “personal digital assistant” or PDA or SMS or "social medi*" or software or telecomm* or telehealth* or telemed* or telemonitor* or telephone or telepsych* or teletherap* or "text messag*" or texting or podcast or virtual*):ab,ti,kw,ky,emt,mh,mc
#12 (“Brave for Teen*” or “Brave for Child*” or “Camp Cope‐A‐Lot” or “Cool Teens” or Interapy or Memo or Minded or Mindcheck* or “Mood Gym” or Moodgym or Moodhelper or “Mood Helper” or Sparx or “The Journey” or “Think Feel Do”)
#13 (Bebo or “Club Penguin” or Facebook or Franktown or Friendster or Habbo or Jabbersmack or hi5 or iTwixie or MySpace or Orkut or “Sweety High” or Kidzworld or Tumblr or Twitter or Sina Weibo or Yoursphere or YouTube)
#14 (#11 or #12 or #13)
#15 (#10 and #14)
Key to field codes:
ti: title; ab: abstract; kw: CCMD keywords; ky: additional keywords; emt: EMTREE subject headings; mh:MeSH subject headings; mc: MeSH check words
Appendix 3. Review search: CENTRAL search (via CRSO)
The Cochrane Central Register of Controlled Trials (CENTRAL) was searched (via the Cochrane Register of Studies Online (CRSO)), using a sensitive set of terms for age group, condition, comorbidity and intervention (to Issue 8, 2017):
[Age Group]
#1 (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young next (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom):ti,ab
[Condition: anxiety/depression]
#2 ((emotion* or psycholog* or mental) next (health or stress* or problem* or disturb* or aspect* or state* or ill*))
#3 (depress* or mood or anxiety or *phobi* or PTSD or post‐trauma* or posttrauma or “post trauma*” or panic* or OCD or obsess* or compulsi* or GAD or "stress disorder*" or “stress reaction*” or "acute stress" or “psychological stress” or “school refusal” or mutism or neurosis or neuroses or neurotic or psychoneuro*)
[Comorbidity: chronic physical illness]
#4 (“physical* ill*” or “medical* ill*” or “chronic disease” or (chronic* NEXT (ill* or condition*1 or disease* or disorder* or health)) or (long term NEXT (condition*1 or sick*)) or “medical* morbid*” or (medical* NEXT (comorbid* or co morbid*)) or multimorbid* or (multi* NEXT (morbid* or “co morbid*” or comorbid* or physical)))
#5 (allerg* or angina or aneurysm or “ankylosing spondylitis” or arthropath* or arthriti* or arthrosis or arthroses or asthma* or “atrial fibrillation” or “autoimmune disease*” or “back pain” or blindness or “brain atroph*” or (bone NEXT (disease* or disorder*)) or ((bronchi* or bowel) NEXT (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac NEXT (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) NEAR2 (disease* or disorder* or event*)) or “cerebral palsy” or (cerebrovascular NEAR2 (disease* or disorder* or event*)) or “chronic obstructive” or COPD or pain or cirrhosis or colitis or “congenital abnormalit*” or (congential NEAR3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or “cystic fibrosis” or cystitis)
#6 (deaf* or deformit* or disabled or (physical NEXT (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or “digestive system*” or duoden* or dystonia or eczema or (endocrine NEXT (disease* or disorder*)) or enuresis or epilep* or “eye disease*” or (“fatigue syndrome” or “chronic fatigue”) or fibromyalgia or fibrosis or “food hypersensitivity” or (gastr* NEXT (disease* or disorder*)) or gastritis or “genetic disorder*” or gout or (glomerul* NEXT (disease* or disorder*)) or headache* or ((h?emic or lymph*) NEXT (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) NEAR2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) NEXT (disease* or disorder* or failure)) or (heart NEXT (disease* or disorder* or failure or surg*)) or HIV or “human immunodeficiency virus” or hypertensi* or hypotensi*)
#7 (“inflammatory disease*” or incontinen* or “irritable bowel” or isch?emi* or (joint NEXT (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) NEXT (disease* or disorder* or failure)) or lordosis or “lung disease*” or “lupus erythemat*” or lymphoma or “macular degeneration” or migraine* or “movement disorder*” or musculoskeletal or necrotizing or nephrotic* or neuromuscular or “multiple sclerosis” or myeloma)
#8 (“nephrotic syndrome” or ((nutritional or metabolic) NEXT (disease* or disorder or syndrome*)) or (organ* NEAR2 (transplant* or recipient*)) or (neurological NEXT (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or “otitis media” or otorhinolaryngology* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or “peripheral vascular” or “pick disease*” or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary NEAR2 (disease* or disorder*)))
#9 ((respiratory NEXT (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or “sickle cell an?emia” or ((skin or “connective tissue”) NEXT (disease* or disorder*)) or (“sleep disorder*” or “sleep apn?ea” or insomnia* or dyssomnia* or hypersomnia*) or “spina bifida” or “spinal muscular atropy” or spondylo* or stenosis* or stoma* or (stroke or strokes or “cerebral infarct*”) or tetraplegi* or ((thyroid NEAR (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic NEAR (disorder* or disease*)) or ulcer* or (urogenital NEXT (disease* or disorder*)) or vasculopath* or (vascular NEAR (disease* or disorder*)) or vestibular or ((virus or viral) NEXT disease))
#10 ((#1 and (#2 or #3) and (#4 or #5 or #6 or #7 or #8 or #9))
[Intervention: psychological therapies]
#11 MESH DESCRIPTOR Psychotherapy EXPLODE ALL TREES
#12 ((psychologic* or behavio?r or cognitive) adj3 (intervent* or therap* or treat* or manag*)):ti,ab
#13 (abreaction or “acting out” or (acceptance NEAR2 commitment) or “activity scheduling” or adlerian or “analytical therap*” or “anger control” or “anger management” or “art therap*” or “assertive* training” or “attention bias modification” or “autogenic training” or autosuggestion or “aversion therap*” or “balint group” or “behavio* activation” or “behavio* contracting” or “behavio* modification” or “behavio* therap*” or bibliotherap* or “body therap*” or “brief therapy” or catharsis or “client cent* therapy” or “cognitive behavio*”or “cognitive therap*” or CBT or cCBT or iCBT or “cognitive rehabilitation” or “cognitive restructur*” or “colour therap*” or “color therap*” or “compassion focus*” or “compassionate therap*” or “conjoint therap*” or “contingency management” or “conversion therap*” or “conversational therap*” or countertransference or “coping skill*” or counsel* or “covert sensitization” or “crisis intervention” or “crisis management”)
#14 ((dialectic* NEAR2 therap*) or "diffusion therap*" or "distraction therap*" or (dream* NEAR3 analys*) or "eclectic therap*" or "emotion* focus* therap*" or "emotional freedom technique" or "encounter group therap*" or existential or experiential or "exposure therap*" or "expressive therap*" or "eye movement desensiti#ation" or "family therap*" or "focus oriented" or "free association" or freudian or "functional analysis" or gestalt or griefwork or "group therap*" or "guided image*" or "holistic therap*" or humanistic or hypnosis or hypnotherapy or hypnoti#zability or "implosive therap*" or "insight therap*" or "integrative therap*" or "interpersonal therap*" or Jungian or kleinian)
#15 (logotherap* or "logo therap*" or meditation or "mental healing" or metacognitive or meta‐cognitive or milieu or "mind train*" or mindfulness or morita or "multimodal therap*" or music or "narrative therap*" or "nondirective therap*" or non‐directive therap* or "nondirective therap*" or "non‐specific therap*" or "nonspecific therap*" or "object relations" or "personal construct therap*" or "person cent* therap*" or "persuasion therap*" or "pet therap*" or "animal therap*" or "play therap*" or ((pleasant or pleasing) NEAR2 event*) or "present cent* therap*" or "primal therap*" or "problem focus* therap*" or "problem sol*" or "process experiential" or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or psychotherap*)
#16 ("rational emotive" or "reality therap*" or "reciprocal inhibition" or "relationship therap*" or "relaxation stress management" or "relaxation technique*" or "relaxation therap*" or "relaxation training" or "reminiscence therap*" or rogerian or "role play*" or schema or "self analys*" or "self esteem building" or "sensitivity training" or "sleep phase chronotherap*" or "socioenvironment* therap*" or "social skill*" or sociotherap* or "solution focused therap*" or "stress management" or "support group*" or (support NEAR3 psycho*) or "supportive therap*" or "systematic desensiti*" or "systemic *therap*" or "therapeutic communit*" or "therapeutic technique" or "third wave" or "time limited therap*" or "transference therap*" or "transactional analysis" or transtheoretical or "validation therap*")
[Intervention: eHealth]
#17 (Bebo or “Club Penguin” or Facebook or Franktown or Friendster or Habbo or Jabbersmack or hi5 or iTwixie or MySpace or Orkut or “Sweety High” or Kidzworld or Tumblr or Twitter or Sina Weibo or Yoursphere or YouTube)
#18 (“Brave for Teen*” or “Brave for Child*” or “Camp Cope‐A‐Lot” or “Cool Teens” or Interapy or Memo or Minded or Mindcheck* or “Mood Gym” or Moodgym or Moodhelper or “Mood Helper” or Sparx or “The Journey” or “Think Feel Do”)
#19 (android or app or apps or blog or “cell phone” or cellphone or "chat room" or computer* or cyber* or DVD or eHealth or e‐health or "electronic health*" or e‐Portal or ePortal or eTherap* or e‐therap* or forum* or gaming or cCBT or iCBT or “information technolog*” or "instant messag*" or internet* or ipad or i‐pad or iphone or i‐phone or ipod or i‐pod or web* or WWW or "smart phone" or smartphone or "social network* site*" or “mobile phone” or e‐mail* or email* or mHealth or m‐health or mobile or multi‐media or multimedia or online* or on‐line or “personal digital assistant” or PDA or SMS or "social medi*" or software or telecomm* or telehealth* or telemed* or telemonitor* or telepsych*or teletherap* or "text messag*" or texting or podcast or virtual*)
#20 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
#21 (#10 AND #20)
Appendix 4. Review search update 2017
In compliance with Cochrane MECIR standard C37 (searches to be rerun within 12 months of publication), CCMD's information specialist ran an update search on 18 August 2017, details below.
CENTRAL retrieved 209 records and Ovid XSearch 941. These were de‐duplicated against each other and records retrieved in 2016, leaving 900 new records to screen.
1. CENTRAL
CENTRAL was searched (via the Cochrane Register of Studies Online (CRSO)) from Issue 6, 2016 to Issue 8, 2017. The search terms are listed in Appendix 3.
2. Ovid XSearch (MEDLINE, Embase, PsycINFO)
In August 2017, CCMD's Information Specialist also ran a cross‐search of Ovid databases (as the Group's Specialised Register (CCMD‐CTR) was out of date at this time).
Ovid databases searched: MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) 1946 to 18‐Aug‐2017, Embase 1974 to 2017 Week 33, PsycINFO 1806 to August Week 2 2017.
Date limited: 1 Jan 2016 to 18‐Aug‐2017.
Search Terms:
1. (child* or boy* or girl* or infant* or juvenil* or minors or paediatric* or pediatric* or school* or preschool* or pre‐school* or kindergarten or nursery or adolesc* or preadolesc* or pre‐adolesc* or pubert* or pubescen* or prepube* or pre‐pube* or high‐school or teen* or (young adj3 (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or student* or undergrad* or college or campus or classroom).ti,ab,kf,kw,id,hw.
2. ((emotion* or psycholog* or mental) adj3 (health or stress* or problem* or disturb* or aspect* or state* or ill*)).ti,ab,kf,kw,id,hw.
3. (depress* or mood or anxiety or agoraphobi* or phobi* or PTSD or post‐trauma* or posttrauma or post trauma* or panic* or OCD or obsess* or compulsi* or GAD or stress disorder* or stress reaction* or acute stress or psychological stress or school refusal or mutism or neurosis or neuroses or neurotic or psychoneuro*).ti,ab,kf,kw,id,hw.
4. or/2‐3
5. (physical* ill* or medical* ill* or chronic disease or (chronic* adj3 (ill* or condition*1 or disease* or disorder* or health)) or (long term adj3 (condition*1 or sick*)) or medical* morbid* or (medical* adj3 (comorbid* or co morbid*)) or multimorbid* or (multi* adj (morbid* or co morbid* or comorbid* or physical))).ti,ab,kf,kw,id,hw.
6. (allerg* or angina or aneurysm or ankylosing spondylitis or arthropath* or arthriti* or arthrosis or arthroses or asthma* or atrial fibrillation or autoimmune disease* or back pain or blindness or brain atroph* or (bone adj (disease* or disorder*)) or ((bronchi* or bowel) adj (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac adj (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) adj2 (disease* or disorder* or event*)) or cerebral palsy or (cerebrovascular adj2 (disease* or disorder* or event*)) or chronic obstructive or COPD or pain or cirrhosis or colitis or congenital abnormalit* or (congential adj3 (disease or disorder*)) or coxarthrosis or Crohn* or Cushing* or cystic fibrosis or cystitis).ti,ab,kf,kw,id,hw.
7. (deaf* or deformit* or disabled or (physical adj (deform* or disab* or impair*)) or dermatitis or dermato* or dorsopath* or diabet* or digestive system* or duoden* or dystonia or eczema or (endocrine adj (disease* or disorder*)) or enuresis or epilep* or eye disease* or (fatigue syndrome or chronic fatigue) or fibromyalgia or fibrosis or food hypersensitivity or (gastr* adj (disease* or disorder*)) or gastritis or genetic disorder* or gout or (glomerul* adj (disease* or disorder*)) or headache* or ((h?emic or lymph*) adj (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhag* or ((hearing or visual or vision) adj2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) adj (disease* or disorder* or failure)) or (heart adj (disease* or disorder* or failure or surg*)) or HIV or human immunodeficiency virus or hypertensi* or hypotensi*).ti,ab,kf,kw,id,hw.
8. (inflammatory disease* or incontinen* or irritable bowel or isch?emi* or (joint adj (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) adj (disease* or disorder* or failure)) or lordosis or lung disease* or lupus or lymphoma or macular degeneration or migraine* or movement disorder* or musculoskeletal or necrotizing or nephrotic* or neuromuscular or multiple sclerosis or myeloma).ti,ab,kf,kw,id,hw.
9. (nephrotic syndrome or ((nutritional or metabolic) adj (disease* or disorder or syndrome*)) or ((organ* or kidney or stem cell) adj2 (transplant* or recipient*)) or (neurological adj (disease* or disorder*)) or occlusion* or obesity or obese or orthop?edic* or osteo* or otitis media or otorhinolaryngolog* or otosclerosis or pancrea* or papulosquamous or paraplegi* or parkinson* or (peripheral adj (arterial or vascular)) or pick disease* or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or psoriasis or parapsoriasis or (pulmonary adj2 (disease* or disorder*))).ti,ab,kf,kw,id,hw.
10. ((respiratory adj (disease* or disorder*)) or retinopathy or rheumat* or sclerosis or scoliosis or sickle cell an?emia or ((skin or connective tissue) adj (disease* or disorder*)) or (sleep disorder* or sleep apn?ea or insomnia* or dyssomnia* or hypersomnia*) or spina bifida or spinal muscular atropy or spondylo* or stenosis* or stoma* or (stroke or strokes or cerebral infarct*) or tetraplegi* or ((thyroid adj (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic adj5 (disorder* or disease*)) or ulcer* or (urogenital adj (disease* or disorder*)) or vasculopath* or (vascular adj5 (disease* or disorder*)) or vestibular or ((virus or viral) adj disease)).ti,ab,kf,kw,id,hw.
11. or/5‐10
12. exp Psychotherapy/ or exp Psychotherapeutic Techniques/
13. exp Child Psychotherapy/ or exp Adolescent Psychotherapy/
14. ((psychologic* or behavio?r or cognitive) adj3 (intervent* or therap* or treat* or manag*)).ti,ab,id,kf,kw.
15. (abreaction or acting out or (acceptance adj2 commitment) or activity scheduling or adlerian or analytical therap* or anger control or anger management or art therap* or assertive* training or attention bias modification or autogenic training or autosuggestion or aversion therap* or balint or behavio* activation or behavio* contracting or behavio* modification or behavio* therap* or bibliotherap* or body therap* or brief therapy or catharsis or client cent* therapy or cognitive behavio* or cognitive therap* or CBT or cCBT or iCBT or cognitive rehabilitation or cognitive restructur* or colour therap* or color therap* or compassion focus* or compassionate therap* or conjoint therap* or contingency management or conversion therap* or conversational therap* or countertransference or coping skill* or counsel* or covert sensitization or crisis intervention or crisis management).ti,ab,kf,kw,id,hw.
16. ((dialectic* adj2 therap*) or diffusion therap* or distraction therap* or (dream* adj3 analys*) or eclectic therap* or emotion* focus* therap* or emotional freedom technique or encounter group therap* or existential or experiential or exposure therap* or expressive therap* or eye movement desensiti#ation or family therap* or focus oriented or free association or freudian or functional analysis or gestalt or griefwork or group therap* or guided image* or holistic therap* or humanistic or hypnosis or hypnotherapy or hypnoti#zability or implosive therap* or insight therap* or integrative therap* or interpersonal therap* or Jungian or kleinian).ti,ab,id,kf,kw,hw.
17. (logotherap* or logo therap* or meditation or mental healing or metacognitive or meta‐cognitive or milieu or mind train* or mindfulness or morita or multimodal therap* or music or narrative therap* or nondirective therap* or non‐directive therap* or nondirective therap* or non‐specific therap* or nonspecific therap* or object relations or personal construct therap* or person cent* therap* or persuasion therap* or pet therap* or animal therap* or play therap* or ((pleasant or pleasing) adj2 event*) or present cent* therap* or primal therap* or problem focus* therap* or problem sol* or process experiential or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or psychotherap*).ti,ab,kf,kw,id,hw.
18. (rational emotive or reality therap* or reciprocal inhibition or relationship therap* or relaxation stress management or relaxation technique* or relaxation therap* or relaxation training or reminiscence therap* or rogerian or role play* or schema or self analys* or self esteem building or sensitivity training or sleep phase chronotherap* or socioenvironment* therap* or social skill* or sociotherap* or solution focused therap* or stress management or support group* or (support adj3 psycho*) or supportive therap* or systematic desensiti* or systemic *therap* or therapeutic communit* or therapeutic technique or third wave or time limited therap* or transference therap* or transactional analysis or transtheoretical or validation therap*).ti,ab,kf,kw,id,hw.
19. (Bebo or Club Penguin or Facebook or Franktown or Friendster or Habbo or Jabbersmack or hi5 or iTwixie or MySpace or Orkut or Sweety High or Kidzworld or Tumblr or Twitter or Sina Weibo or Yoursphere or YouTube).ti,ab,kf,kw,id,hw.
20. (Brave for Teen* or Brave for Child* or Camp Cope‐A‐Lot or Cool Teens or Interapy or Memo or Minded or Mindcheck* or Mood Gym or Moodgym or Moodhelper or Mood Helper or Sparx or The Journey or Think Feel Do).ti,ab,kf,kw,id,hw.
21. (android or app or apps or blog or cell phone or cellphone or chat room or computer* or cyber* or DVD or eHealth or e‐health or electronic health* or e‐Portal or ePortal or eTherap* or e‐therap* or forum* or gaming or cCBT or iCBT or information technolog* or instant messag* or internet* or ipad or i‐pad or iphone or i‐phone or ipod or i‐pod or web* or WWW or smart phone or smartphone or social network* site* or mobile phone or e‐mail* or email* or mHealth or m‐health or mobile or multi‐media or multimedia or online* or on‐line or personal digital assistant or PDA or SMS or social medi* or software or telecomm* or telehealth* or telemed* or telemonitor* or telepsych*or teletherap* or text messag* or texting or podcast or virtual*):ti,kf,kw,id,hw.
22. or/12‐21
23. trial.ti.
24. (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf,kw,id.
25. (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,kf,kw,id.
26. placebo.hw,ti,ab,kf,kw,id.
27. ((control* adj2 (trial or study or group)) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,kw,id,hw.
28. Randomized Controlled Trial.sh,pt.
29. Double Blind Procedure/
30. Double Blind Method/
31. (clinical trial or empirical study).md.
32. ((single or double or triple) adj2 (blind* or mask* or dummy)).ti,ab,kf,kw,id.
33. or/23‐32
34. ((animal or nonhuman) not (human and (animal or nonhuman))).hw.
35. (33 not 34)
36. (1 and 4 and 11 and 22 and 35)
37. (2016* or 2017*).yr,em,dd,dc,ed.
38. (36 and 37)
39. (case adj (control* or report?)).ti,kf,kw,id,hw.
40. (review or letter or comment*).ti,hw,pt.
41. (dental or dentist* or an?esthes*).ti,hw,jw.
42. or/39‐41
43. (38 not 42)
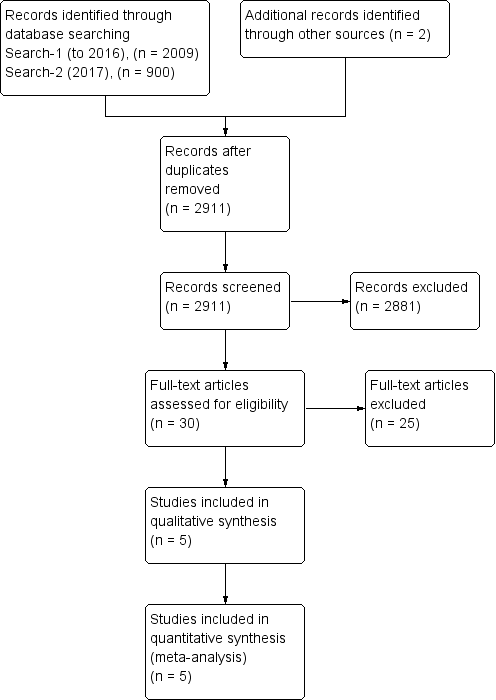
Study flow diagram.
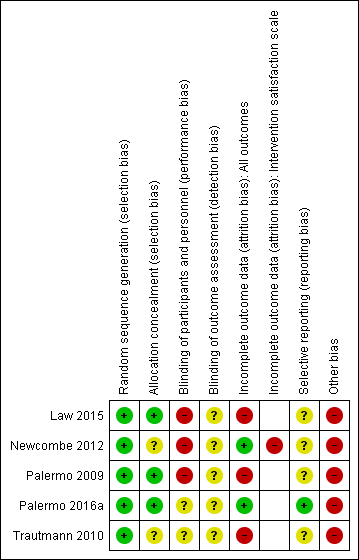
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
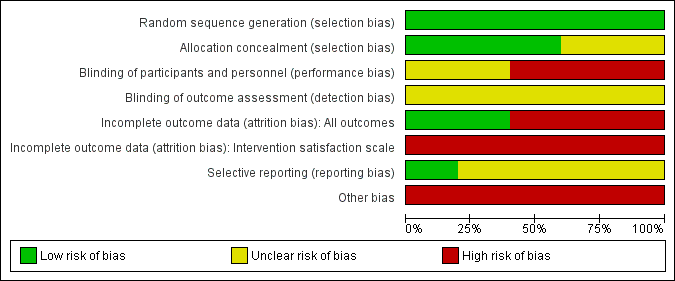
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
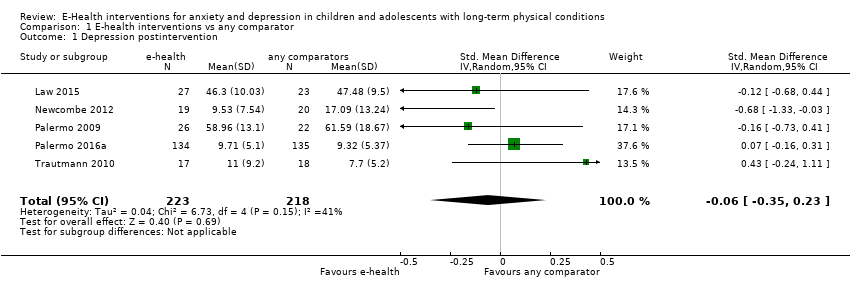
Comparison 1 E‐health interventions vs any comparator, Outcome 1 Depression postintervention.

Comparison 1 E‐health interventions vs any comparator, Outcome 2 Depression 3‐ to 6‐month follow‐up.

Comparison 1 E‐health interventions vs any comparator, Outcome 3 Anxiety postintervention.
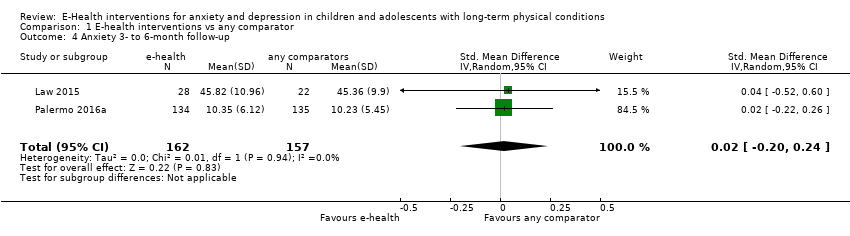
Comparison 1 E‐health interventions vs any comparator, Outcome 4 Anxiety 3‐ to 6‐month follow‐up.

Comparison 1 E‐health interventions vs any comparator, Outcome 5 Treatment acceptability postintervention.

Comparison 1 E‐health interventions vs any comparator, Outcome 6 Quality of life postintervention.
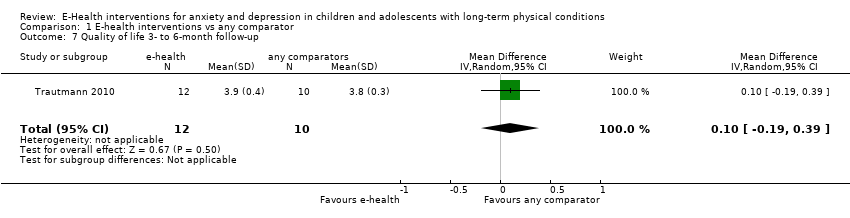
Comparison 1 E‐health interventions vs any comparator, Outcome 7 Quality of life 3‐ to 6‐month follow‐up.

Comparison 1 E‐health interventions vs any comparator, Outcome 8 Functioning postintervention.

Comparison 1 E‐health interventions vs any comparator, Outcome 9 Functioning 3‐ to 6‐month follow‐up.

Comparison 1 E‐health interventions vs any comparator, Outcome 10 Status of long‐term physical condition postintervention.

Comparison 1 E‐health interventions vs any comparator, Outcome 11 Status of long‐term physical condition 3‐ to 6‐month follow‐up.

Comparison 2 E‐health interventions vs attention placebo, Outcome 1 Depression postintervention.
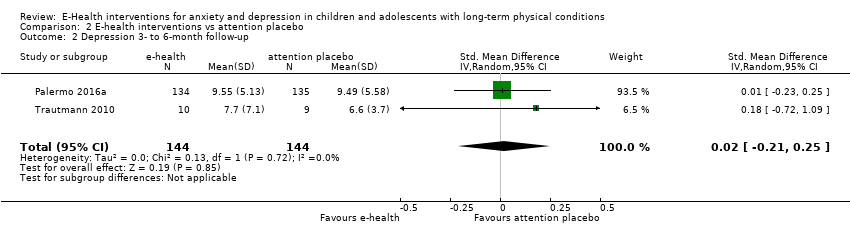
Comparison 2 E‐health interventions vs attention placebo, Outcome 2 Depression 3‐ to 6‐month follow‐up.
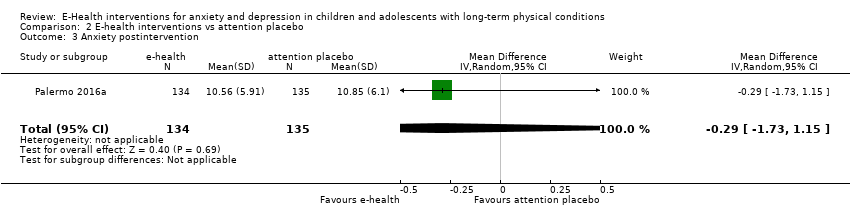
Comparison 2 E‐health interventions vs attention placebo, Outcome 3 Anxiety postintervention.
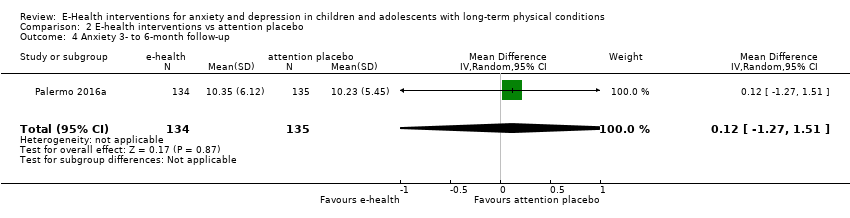
Comparison 2 E‐health interventions vs attention placebo, Outcome 4 Anxiety 3‐ to 6‐month follow‐up.

Comparison 2 E‐health interventions vs attention placebo, Outcome 5 Treatment acceptability postintervention.

Comparison 2 E‐health interventions vs attention placebo, Outcome 6 Quality of life postintervention.

Comparison 2 E‐health interventions vs attention placebo, Outcome 7 Quality of life 6‐month follow‐up.

Comparison 2 E‐health interventions vs attention placebo, Outcome 8 Functioning postintervention.
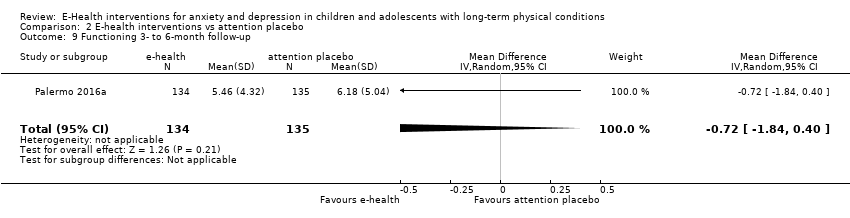
Comparison 2 E‐health interventions vs attention placebo, Outcome 9 Functioning 3‐ to 6‐month follow‐up.

Comparison 2 E‐health interventions vs attention placebo, Outcome 10 Status of long‐term physical condition postintervention.

Comparison 2 E‐health interventions vs attention placebo, Outcome 11 Status of long‐term physical condition 3‐ to 6‐month follow‐up.

Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 1 Depression postintervention.

Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 2 Depression 3‐month follow‐up.
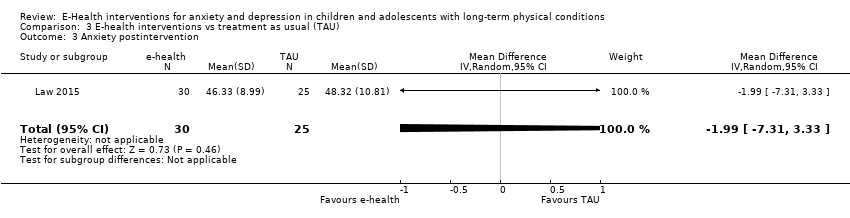
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 3 Anxiety postintervention.
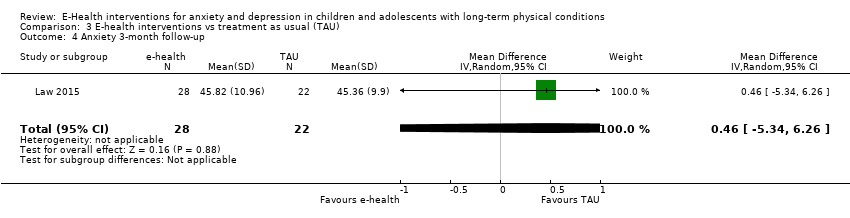
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 4 Anxiety 3‐month follow‐up.
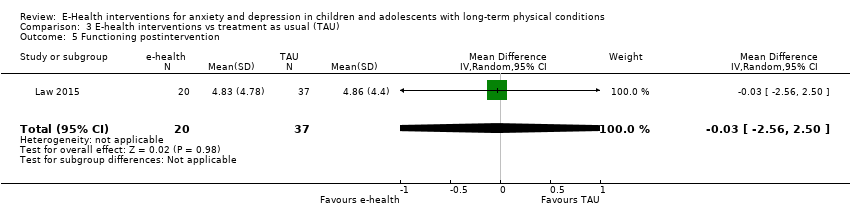
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 5 Functioning postintervention.
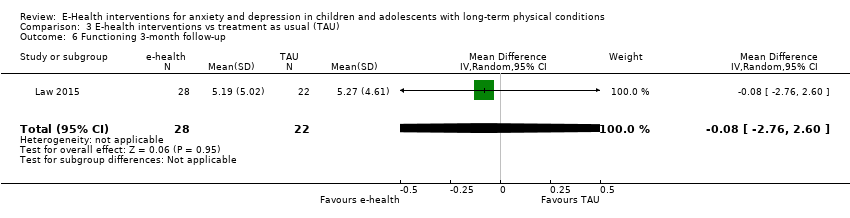
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 6 Functioning 3‐month follow‐up.
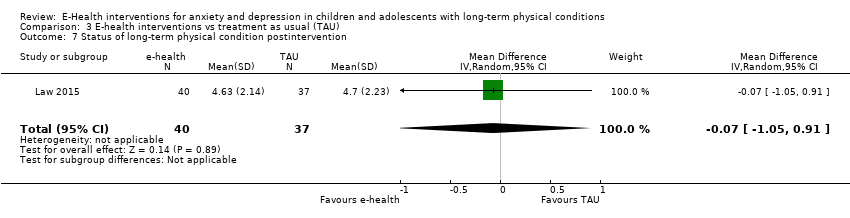
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 7 Status of long‐term physical condition postintervention.
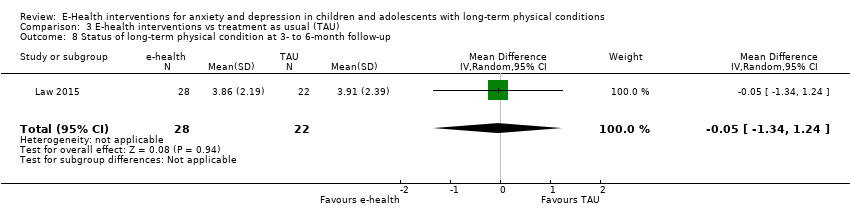
Comparison 3 E‐health interventions vs treatment as usual (TAU), Outcome 8 Status of long‐term physical condition at 3‐ to 6‐month follow‐up.
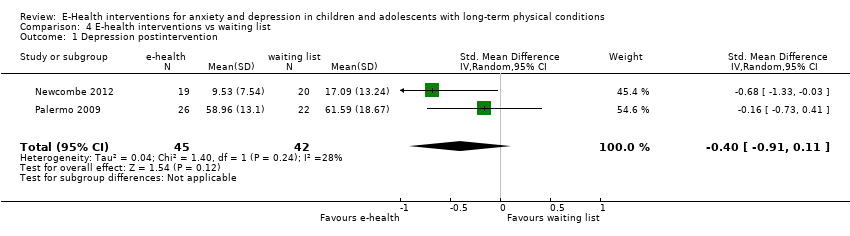
Comparison 4 E‐health interventions vs waiting list, Outcome 1 Depression postintervention.

Comparison 4 E‐health interventions vs waiting list, Outcome 2 Functioning postintervention.

Comparison 4 E‐health interventions vs waiting list, Outcome 3 Status of long‐term physical condition postintervention.
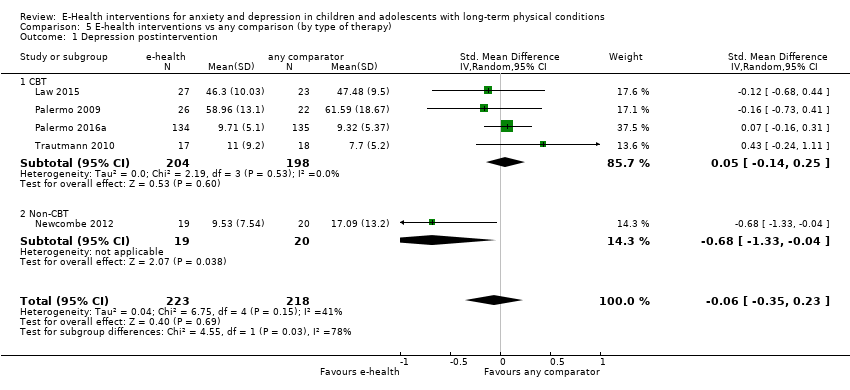
Comparison 5 E‐health interventions vs any comparison (by type of therapy), Outcome 1 Depression postintervention.
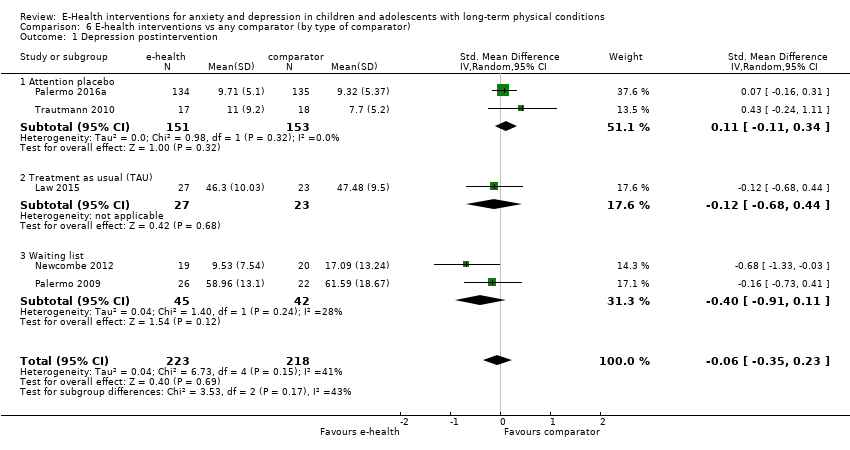
Comparison 6 E‐health interventions vs any comparator (by type of comparator), Outcome 1 Depression postintervention.
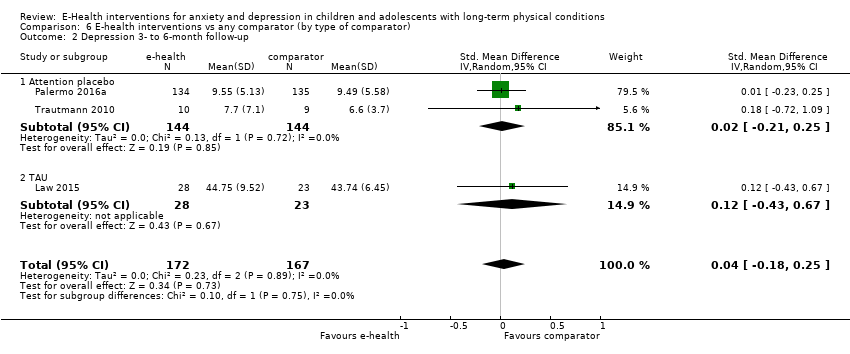
Comparison 6 E‐health interventions vs any comparator (by type of comparator), Outcome 2 Depression 3‐ to 6‐month follow‐up.
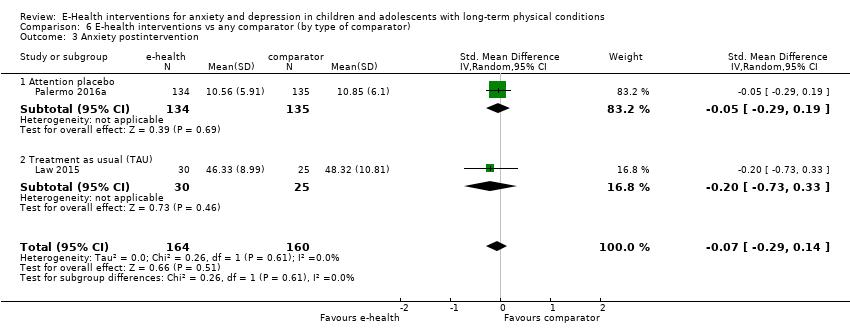
Comparison 6 E‐health interventions vs any comparator (by type of comparator), Outcome 3 Anxiety postintervention.
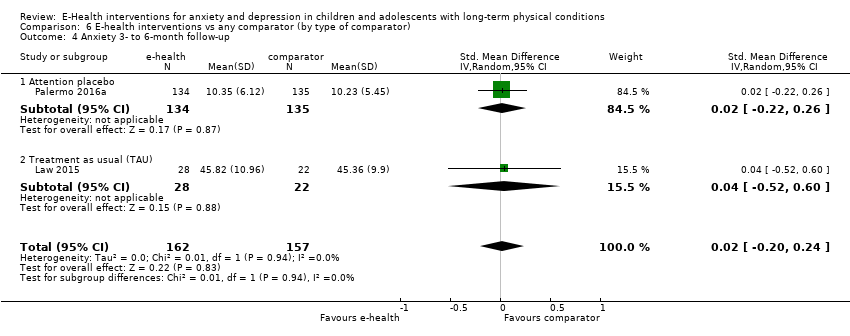
Comparison 6 E‐health interventions vs any comparator (by type of comparator), Outcome 4 Anxiety 3‐ to 6‐month follow‐up.
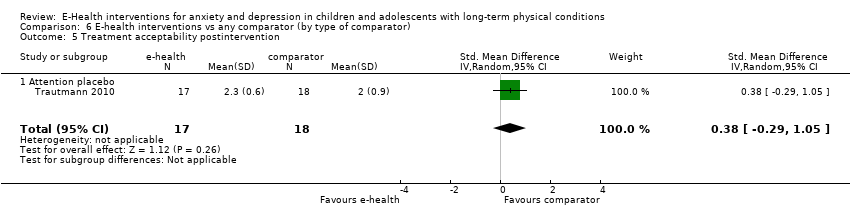
Comparison 6 E‐health interventions vs any comparator (by type of comparator), Outcome 5 Treatment acceptability postintervention.
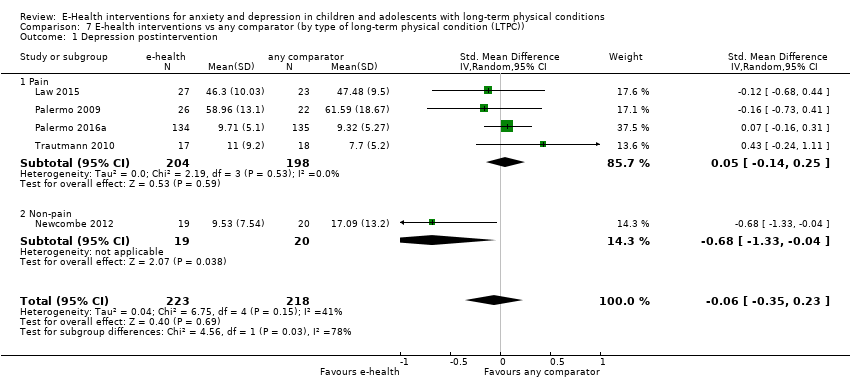
Comparison 7 E‐health interventions vs any comparator (by type of long‐term physical condition (LTPC)), Outcome 1 Depression postintervention.
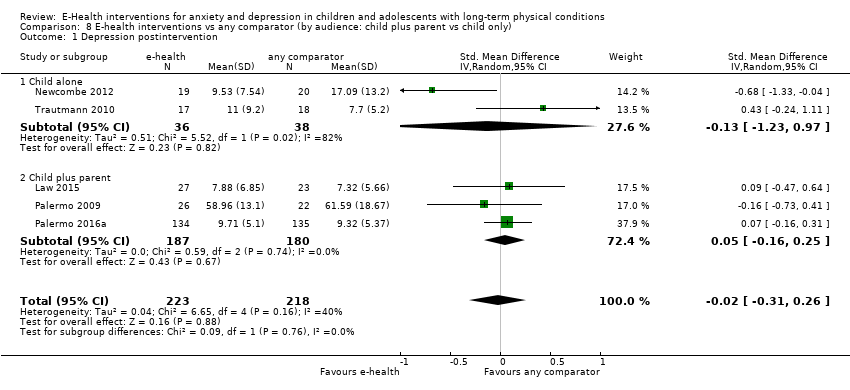
Comparison 8 E‐health interventions vs any comparator (by audience: child plus parent vs child only), Outcome 1 Depression postintervention.
| E‐healthinterventions versus any comparator for anxiety and depression in children and adolescents with long‐term physical conditions | ||||||
| Patient or population: children and adolescents, aged 10 to 18 years, with long‐term physical conditions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with any comparator | Risk with e‐health interventions | |||||
| Depression (postintervention) | The mean self‐reported depression score in the intervention group was 0.06 standard mean deviations lower (0.35 lower to 0.23 higher) | ‐ | 441 | ⊕⊝⊝⊝ | A standard mean deviation of ‐0.06 represents a small difference between groups | |
| Anxiety (postintervention) | The mean self‐reported anxiety score in the intervention group was 0.07 standard mean deviations lower (0.29 lower to 0.14 higher) | ‐ | 324 | ⊕⊝⊝⊝ | A standard mean deviation of ‐0.07 represents a small difference between groups | |
| Treatment acceptability (postintervention) | The mean self‐reported treatment acceptability score in the intervention group was 0.46 standard mean deviations higher (0.23 higher to 0.69 higher) | ‐ | 304 | ⊕⊝⊝⊝ | A standard mean deviation of 0.46 represents a small difference between groups | |
| Quality of life (postintervention) | The mean self‐reported quality of life score in the intervention group was 0.83 standard mean deviations lower | ‐ | 34 | ⊕⊝⊝⊝ | A standard mean deviation of ‐0.83 represents a large difference between groups | |
| Functioning (postintervention) | The mean self‐reported level of functioning in the intervention group was 0.08 standard mean deviations lower | ‐ | 368 | ⊕⊝⊝⊝ | A standard mean deviation of ‐0.08 represents a small difference between groups | |
| Status of long‐term physical condition (postintervention) | The mean self‐reported long‐term physical condition symptom score was 0.06 standard mean deviations higher | ‐ | 463 | ⊕⊕⊝⊝ | A standard mean deviation of 0.06 represents a small difference between groups | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded quality due to a lack of clarity about blinding of participants and outcome assessors, incomplete outcome data, and the fact that all studies were conducted by the developers of the e‐health interventions. b We downgraded for inconsistency due to studies having moderate heterogeneity. c We downgraded for indirectness because most or all of the interventions were not designed to treat anxiety or depression as the primary focus. d We downgraded for imprecision as the upper and lower limits of the confidence intervals include both potential for harm and potential for benefit e We downgraded for imprecision as the total sample size was less than 400 as per guidance from the Consumer and Communication Cochrane Review Group (Ryan 2016) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 5 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 2 Depression 3‐ to 6‐month follow‐up Show forest plot | 3 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.18, 0.25] |
| 3 Anxiety postintervention Show forest plot | 2 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.29, 0.14] |
| 4 Anxiety 3‐ to 6‐month follow‐up Show forest plot | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.20, 0.24] |
| 5 Treatment acceptability postintervention Show forest plot | 2 | 304 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 6 Quality of life postintervention Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.54, ‐0.06] |
| 7 Quality of life 3‐ to 6‐month follow‐up Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.39] |
| 8 Functioning postintervention Show forest plot | 3 | 368 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.33, 0.18] |
| 9 Functioning 3‐ to 6‐month follow‐up Show forest plot | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 10 Status of long‐term physical condition postintervention Show forest plot | 5 | 463 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.12, 0.24] |
| 11 Status of long‐term physical condition 3‐ to 6‐month follow‐up Show forest plot | 3 | 340 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.11, 0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 2 | 304 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.11, 0.34] |
| 2 Depression 3‐ to 6‐month follow‐up Show forest plot | 2 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.21, 0.25] |
| 3 Anxiety postintervention Show forest plot | 1 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.73, 1.15] |
| 4 Anxiety 3‐ to 6‐month follow‐up Show forest plot | 1 | 269 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐1.27, 1.51] |
| 5 Treatment acceptability postintervention Show forest plot | 2 | 304 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 6 Quality of life postintervention Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.54, ‐0.06] |
| 7 Quality of life 6‐month follow‐up Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.39] |
| 8 Functioning postintervention Show forest plot | 1 | 269 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.05, 1.11] |
| 9 Functioning 3‐ to 6‐month follow‐up Show forest plot | 1 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.84, 0.40] |
| 10 Status of long‐term physical condition postintervention Show forest plot | 2 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.34, 0.40] |
| 11 Status of long‐term physical condition 3‐ to 6‐month follow‐up Show forest plot | 2 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.10, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐6.60, 4.24] |
| 2 Depression 3‐month follow‐up Show forest plot | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐3.39, 5.41] |
| 3 Anxiety postintervention Show forest plot | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐7.31, 3.33] |
| 4 Anxiety 3‐month follow‐up Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐5.34, 6.26] |
| 5 Functioning postintervention Show forest plot | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐2.56, 2.50] |
| 6 Functioning 3‐month follow‐up Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐2.76, 2.60] |
| 7 Status of long‐term physical condition postintervention Show forest plot | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.05, 0.91] |
| 8 Status of long‐term physical condition at 3‐ to 6‐month follow‐up Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.34, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.91, 0.11] |
| 2 Functioning postintervention Show forest plot | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐3.31 [‐6.90, 0.28] |
| 3 Status of long‐term physical condition postintervention Show forest plot | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.55, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 5 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 1.1 CBT | 4 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.14, 0.25] |
| 1.2 Non‐CBT | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.33, ‐0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 5 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 1.1 Attention placebo | 2 | 304 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.11, 0.34] |
| 1.2 Treatment as usual (TAU) | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.68, 0.44] |
| 1.3 Waiting list | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.91, 0.11] |
| 2 Depression 3‐ to 6‐month follow‐up Show forest plot | 3 | 339 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.18, 0.25] |
| 2.1 Attention placebo | 2 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.21, 0.25] |
| 2.2 TAU | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.43, 0.67] |
| 3 Anxiety postintervention Show forest plot | 2 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.29, 0.14] |
| 3.1 Attention placebo | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 3.2 Treatment as usual (TAU) | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.73, 0.33] |
| 4 Anxiety 3‐ to 6‐month follow‐up Show forest plot | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.20, 0.24] |
| 4.1 Attention placebo | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.22, 0.26] |
| 4.2 Treatment as usual (TAU) | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.52, 0.60] |
| 5 Treatment acceptability postintervention Show forest plot | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.29, 1.05] |
| 5.1 Attention placebo | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.29, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 5 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 1.1 Pain | 4 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.14, 0.25] |
| 1.2 Non‐pain | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.33, ‐0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression postintervention Show forest plot | 5 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.31, 0.26] |
| 1.1 Child alone | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐1.23, 0.97] |
| 1.2 Child plus parent | 3 | 367 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.16, 0.25] |

