Erythropoietin plus iron versus control treatment including placebo or iron for preoperative anaemic adults undergoing non‐cardiac surgery
Abstract
Background
Approximately 30% of adults undergoing non‐cardiac surgery suffer from preoperative anaemia. Preoperative anaemia is a risk factor for mortality and adverse outcomes in different surgical specialties and is frequently the reason for blood transfusion. The most common causes are renal, chronic diseases, and iron deficiency. International guidelines recommend that the cause of anaemia guide preoperative anaemia treatment. Recombinant human erythropoietin (rHuEPO) with iron supplementation has frequently been used to increase preoperative haemoglobin concentrations in patients in order to avoid the need for perioperative allogeneic red blood cell (RBC) transfusion.
Objectives
To evaluate the efficacy of preoperative rHuEPO therapy (subcutaneous or parenteral) with iron (enteral or parenteral) in reducing the need for allogeneic RBC transfusions in preoperatively anaemic adults undergoing non‐cardiac surgery.
Search methods
We searched CENTRAL, Ovid MEDLINE(R), Ovid Embase, ISI Web of Science: SCI‐EXPANDED and CPCI‐S, and clinical trial registries WHO ICTRP and ClinicalTrials.gov on 29 August 2019.
Selection criteria
We included all randomized controlled trials (RCTs) that compared preoperative rHuEPO + iron therapy to control treatment (placebo, no treatment, or standard of care with or without iron) for preoperatively anaemic adults undergoing non‐cardiac surgery.
We used the World Health Organization (WHO) definition of anaemia: haemoglobin concentration (g/dL) less than 13 g/dL for males, and 12 g/dL for non‐pregnant females (decision of inclusion based on mean haemoglobin concentration). We defined two subgroups of rHuEPO dosage: 'low' for 150 to 300 international units (IU)/kg body weight, and 'high' for 500 to 600 IU/kg body weight.
Data collection and analysis
Two review authors collected data from the included studies. Our primary outcome was the need for RBC transfusion (no autologous transfusion, fresh frozen plasma or platelets), measured in transfused participants during surgery (intraoperative) and up to five days after surgery. Secondary outcomes of interest were: haemoglobin concentration (directly before surgery), number of RBC units (where one unit contains 250 to 450 mL) transfused per participant (intraoperative and up to five days after surgery), mortality (within 30 days after surgery), length of hospital stay, and adverse events (e.g. renal dysfunction, thromboembolism, hypertension, allergic reaction, headache, fever, constipation).
Main results
Most of the included trials were in orthopaedic, gastrointestinal, and gynaecological surgery and included participants with mild and moderate preoperative anaemia (haemoglobin from 10 to 12 g/dL). The duration of preoperative rHuEPO treatment varied across the trials, ranging from once a week to daily or a 5‐to‐10‐day period, and in one trial preoperative rHuEPO was given on the morning of surgery and for five days postoperatively.
We included 12 trials (participants = 1880) in the quantitative analysis of the need for RBC transfusion following preoperative treatment with rHuEPO + iron to correct preoperative anaemia in non‐cardiac surgery; two studies were multiarmed trials with two different dose regimens.
Preoperative rHuEPO + iron given to anaemic adults reduced the need RBC transfusion (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.38 to 0.80; participants = 1880; studies = 12; I2 = 84%; moderate‐quality evidence due to inconsistency). This analysis suggests that on average, the combined administration of rHuEPO + iron will mean 231 fewer individuals will need transfusion for every 1000 individuals compared to the control group.
Preoperative high‐dose rHuEPO + iron given to anaemic adults increased the haemoglobin concentration (mean difference (MD) 1.87 g/dL, 95% CI 1.26 to 2.49; participants = 852; studies = 3; I2 = 89%; low‐quality evidence due to inconsistency and risk of bias) but not low‐dose rHuEPO + iron (MD 0.11 g/dL, 95% CI −0.46 to 0.69; participants = 334; studies = 4; I2 = 69%; low‐quality evidence due to inconsistency and risk of bias).
There was probably little or no difference in the number of RBC units when rHuEPO + iron was given preoperatively (MD −0.09, 95% CI −0.23 to 0.05; participants = 1420; studies = 6; I2 = 2%; moderate‐quality evidence due to imprecision).
There was probably little or no difference in the risk of mortality within 30 days of surgery (RR 1.19, 95% CI 0.39 to 3.63; participants = 230; studies = 2; I2 = 0%; moderate‐quality evidence due to imprecision) or of adverse events including local rash, fever, constipation, or transient hypertension (RR 0.93, 95% CI 0.68 to 1.28; participants = 1722; studies = 10; I2 = 0%; moderate‐quality evidence due to imprecision).
The administration of rHuEPO + iron before non‐cardiac surgery did not clearly reduce the length of hospital stay of preoperative anaemic adults (MD −1.07, 95% CI −4.12 to 1.98; participants = 293; studies = 3; I2 = 87%; low‐quality evidence due to inconsistency and imprecision).
Authors' conclusions
Moderate‐quality evidence suggests that preoperative rHuEPO + iron therapy for anaemic adults prior to non‐cardiac surgery reduces the need for RBC transfusion and, when given at higher doses, increases the haemoglobin concentration preoperatively. The administration of rHuEPO + iron treatment did not decrease the mean number of units of RBC transfused per patient.
There were no important differences in the risk of adverse events or mortality within 30 days, nor in length of hospital stay. Further, well‐designed, adequately powered RCTs are required to estimate the impact of this combined treatment more precisely.
PICO
Plain language summary
The use of erythropoietin plus iron to correct anaemia before surgery to reduce the risk of blood transfusion
Background
Erythropoietin is a hormone produced by the kidneys. It stimulates the bone marrow to produce more red blood cells (RBCs). Erythropoietin is frequently used to correct anaemia caused by a reduced number of red blood cells or low haemoglobin concentration in cases of chronic renal failure. The haemoglobin in red blood cells carries oxygen around the body.
Adult patients undergoing non‐cardiac surgery, such as bone (orthopaedic) surgery, have blood tests before surgery to check their general health. Many individuals are found to be anaemic, which means they may need RBC transfusions with donor blood before, during, or after surgery to increase their haemoglobin concentrations. Giving donor blood can cause an allergic reaction such as a rash, headache, fever, raised blood pressure, temporary loss of kidney function, and blood clotting. If it is possible to diagnose the cause of the anaemia before the operation, then erythropoietin can be used together with iron to treat most of the causes of anaemia.
Review question
We wanted to know whether erythropoietin therapy in combination with iron before surgery is effective in reducing the need for RBC transfusions in the immediate recovery period in anaemic adults undergoing non‐cardiac surgery, compared to not giving the treatment.
Erythropoietin therapy can be given by injection below the skin (subcutaneously) or into a vein (intravenously). Iron can be given by mouth (delivered into the gut) or intravenously.
Study characteristics
We identified 12 randomized controlled trials involving a total of 1880 adult participants who were anaemic. The participants were randomly1 assigned to a treatment group receiving erythropoietin and iron or a control group. The control group received either placebo (dummy treatment) or no treatment with or without iron. We looked at the following outcomes: haemoglobin concentrations, need for transfusion, the amount of RBC units transfused per participant, adverse events, and any deaths. Erythropoietin was given either in a low dose (150 to 300 international units (IU)/kg body weight) or high dose (500 to 600 IU/kg body weight).
The evidence is current to August 2019.
Key results
We found that giving erythropoietin with iron supplementation reduces the need for RBC transfusions amongst participants (12 studies; 1880 participants). Higher doses of erythropoietin with iron increased haemoglobin concentrations before surgery (7 studies; 1186 participants), but at lower doses any effect was unclear. The number of units of RBCs required was not changed.
Adverse events did not differ between study groups (10 studies; 1722 participants). Only two studies reported on deaths, with no apparent differences between groups.
We are uncertain whether adults suffering from preoperative anaemia who were treated with erythropoietin and iron had shorter hospital stays than adults with preoperative anaemia who did not receive preoperative treatment before surgery (3 studies; 293 participants).
Quality of the evidence
The quality of the evidence was moderate for the outcome need for RBC transfusion, due to some inconsistency between eligible trials; low for haemoglobin concentration before surgery due to inconsistency between eligible trials and risk of bias; moderate for the amount of RBC units transfused, due to differences in how studies measured the number of units; low for length of hospital stay due to inconsistency in findings and imprecision of the studies; and moderate for mortality and adverse events and death, as only a small number of events were reported.
Authors' conclusions
Summary of findings
| rHuEPO with iron compared to control treatment with or without iron in preoperative anaemic adults undergoing non‐cardiac surgery | |||||
| Patient or population: adult participants, irrespective of gender and type of non‐cardiac surgery, with mild or severe preoperative anaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Risk with control treatment | Risk with rHuEPO + iron | ||||
| Transfusion (measured in participants during or up to 5 days after operation) | 444 per 1000 | 231 per 1000 | RR 0.55 | 1880 | ⊕⊕⊕⊝ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | The mean Hb ‐ rHuEPO high 500 to 600 IU/kg BW ranged across control groups from 10.7 to 12.4 g/dL. | MD 1.87 (1.26 higher to 2.49 higher) | ‐ | 852 | ⊕⊕⊝⊝ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | The mean Hb ‐ rHuEPO low 150 to 300 IU/kg BW ranged across control groups from 11.9 to 12.1 g/dL. | MD 0.11 (0.46 lower to 0.69 higher) | ‐ | 334 | ⊕⊕⊝⊝ |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | The mean number of RBC units ranged across control groups from 1.28 to 2.69. | MD 0.09 lower | ‐ | 1420 | ⊕⊕⊕⊝ |
| Mortality (within 30 days after surgery) | 45 per 1000 | 54 per 1000 | RR 1.18 | 230 | ⊕⊕⊕⊝ |
| Adverse events (number of participants with any adverse event) | 88 per 1000 | 81 per 1000 | RR 0.93 | 1722 | ⊕⊕⊕⊝ |
| Length of hospital stay | The mean length of hospital stay ranged across control groups from 7 to 13. | MD 1.07 lower (4.12 lower to 1.98 higher) | ‐ | 293 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to inconsistency. Unexplained inconsistency, with widely different point estimates. | |||||
Background
Description of the condition
Anaemia is defined by the World Health Organization (WHO) as a haemoglobin concentration of less than 12 g/dL in non‐pregnant adult women, and less than 13 g/dL in adult men (WHO 2008). Anaemia before surgery (preoperative anaemia) is common amongst adults undergoing non‐cardiac surgery, with a prevalence from 27%, reported in Baron 2014, to 39%, reported in Musallam 2011. Preoperative anaemia is associated with an increased requirement for red blood cell (RBC) transfusion and postoperative morbidity and mortality (Jans 2014; Munoz 2008; Munoz 2012; Musallam 2011). It is also associated with a prolonged postoperative hospital stay and increased perioperative costs (Shander 2010). The recently published German guidelines on the diagnosis and treatment of preoperative anaemia investigated the risk of anaemia before non‐cardiac surgery, using the GRADE system to assess the published evidence until early 2015 (German S3 Guideline 2018). This meta‐analysis showed that preoperative anaemia increased the risk ratio for mortality by 3.97 (95% confidence interval (CI) 2.54 to 6.19), the risk ratio to be transfused by 3.57 (95% CI 2.59 to 4.92), and prolonged the length of hospital stay after non‐cardiac surgery by a median 4.01 days (95% CI −0.21 to 8.22). Haemoglobin concentrations of 10 g/dL were considered to be standard liberal threshold concentrations for transfusion (Blair 1986; McIntyre 2004). Allogeneic RBC transfusion, per se, is an important risk factor for mortality, infection, pneumonia, and sepsis (Jans 2014; Morton 2010). In addition, RBC resources due to demographic changes are contemporaneously decreasing (Greinacher 2011).
Description of the intervention
Pre‐ and intra‐operative strategies to avoid transfusion are recommended in the guidelines of the Network for the Advancement of Transfusion Alternatives (NATA), Goodnough 2011, and the guidelines of the European Society of Anaesthesiology for the management of severe perioperative bleeding (Kozek‐Langenecker 2013). In the case of preoperative anaemia, guidelines recommend the use of supplemental iron ‐ oral or intravenous ‐ in preoperative iron deficiency. Treatment with erythropoiesis‐stimulating agents (ESA) is recommended when iron deficiency has been either ruled out or corrected (Goodnough 2011).
Erythropoiesis‐stimulating agents are recombinant erythropoietin preparations with an amino acid sequence comparable to endogenous human erythropoietin (Jelkmann 2013). Recombinant human erythropoietin (rHuEPO) is the most frequently used substance of the ESA group. After publications of both guidelines, several studies were conducted addressing the application of rHuEPO in combination with intravenous or oral iron in people with preoperative anaemia in non‐cardiac surgery (Feagan 2000; Lin 2013; Weber 2005). These studies mainly focused on the haemoglobin concentration and the proportion of participants with the need for allogeneic RBC transfusion receiving the intervention of interest.
rHuEPO acts as a growth factor on progenitor cells. It stimulates the proliferation and differentiation of red cell precursors in the bone marrow and can increase erythropoiesis, resulting in increased haemoglobin concentrations and haematocrit in anaemic people. After parenteral application of rHuEPO in clinically relevant single doses of 50 international units (IU)/kg body weight, the drug is eliminated at a first‐order kinetic rate followed by a rapid distribution phase (Jelkmann 2013). After subcutaneous administration, peak plasma concentrations of rHuEPO are achieved after 12 to 18 hours, but the slow absorption allows for about 30% lower drug requirements compared to an intravenous application (Jelkmann 2013).
One of the first investigations examined the safety and efficacy of rHuEPO in reducing the need for allogeneic blood transfusion in non‐cardiac surgery (Canadian OPESG 1993). They proved rHuEPO reduced the need for perioperative allogeneic transfusion without any increased risk of deep venous thrombosis, high blood pressure, or other adverse experiences (Canadian OPESG 1993). When anaemic participants scheduled for primary hip arthroplasty received rHuEPO with oral iron application, starting four weeks before surgery, rHuEPO reduced the overall need for perioperative allogeneic transfusion as well as increased the haemoglobin concentration. The incidence of thromboembolic events did not differ amongst the different regimens. rHuEPO treatment supplemented with oral or intravenous iron in preoperative anaemic participants undergoing major orthopaedic surgery led to increased haemoglobin concentration in the postoperative period and reduced transfusion rates (Weber 2005). Concomitantly, rHuEPO treatment did not affect postoperative recovery (time to ambulation, time to discharge, and infection rate). However, postoperative recovery was impaired when participants were transfused.
Similar to the findings for non‐cardiac surgery, the use of rHuEPO with intravenous iron in anaemic participants before off‐pump coronary bypass surgery confirmed an increase of the haemoglobin concentration and a decrease of allogenic blood transfusion in the rHuEPO‐treated group (Weltert 2010). A Cochrane Review evaluated the efficacy of rHuEPO, pre‐ and perioperatively, in reducing allogeneic blood transfusions and improving haemoglobin, quality of life, recurrence rate, mortality, and survival following colorectal surgery. The authors concluded that there was insufficient evidence to support a recommendation for pre‐ and perioperative rHuEPO for any mentioned outcome apart from haemoglobin concentrations, but also concluded that the occurrence of thrombotic events did not increase (Devon 2009).
Iron deficiency and anaemia mediated by chronic diseases are common causes of preoperative anaemia. Early preoperative oral iron supplementation may be suitable to refill iron stores (Casalduero 2008). Due to gastrointestinal side effects and poor intestinal absorption, parenteral iron application can be indicated. In the case of persisting iron deficiency despite adequate iron application, or in the case of severe preoperative anaemia, the use of rHuEPO should be considered. In most of the investigations and clinical practice, rHuEPO application is primarily supplemented by oral or parenteral iron (Canadian OPESG 1993; Na 2011). It has been suggested that people may develop a functional iron deficiency postoperatively due to a surge in circulating inflammatory cytokines (Lin 2013). In this state of iron‐restricted erythropoiesis, iron stores are normal but unavailable for erythropoiesis, and intravenous iron is required to restore concentrations of accessible transferrin‐bound iron (Lin 2013). The role of iron therapy without rHuEPO in people with preoperative anaemia was addressed in a recent Cochrane Review (Ng 2015). This review identified only three trials of preoperative iron therapy, finding no reduction in the proportion of participants who received an allogeneic blood transfusion compared to no iron therapy.
How the intervention might work
Depending on the degree and cause of preoperative anaemia, a weekly administration over four weeks prior to operation in anaemic participants was typically used in the clinical trials (Weber 2005). rHuEPO has a half‐life of one day, but the effect is evident only five days later when the induced red cell proliferation is mature enough for release into the circulation (Fisher 2003). People should receive iron supplementation throughout the course of rHuEPO therapy to optimize both the response to rHuEPO therapy and RBC production in the presurgical setting (Goodnough 2011).
An internal turnover of 20 mg of iron per day is required for erythropoiesis in the bone marrow (Munoz 2008), which is available by iron recycling from ageing erythrocytes and by daily exchange within iron‐containing enzymes and iron stores (Steinbicker 2013). An amount of 1 mg to 2 mg is compensated by iron absorption from the diet. Oral iron supplementation may be less effective due to insufficient absorption of iron and increased gastrointestinal side effects. Nevertheless, in the context of supportive iron therapy in preoperative anaemic patients undergoing rHuEPO treatment, iron is still administrated orally in a daily dose of 200 mg (Feagan 2000). Recent investigations recommended the parenteral application of iron in the case of rHuEPO treatment in preoperative anaemic patients, to bypass the reduced absorption of oral iron (Ralley 2014). People diagnosed with preoperative anaemia due to iron deficiency or chronic disease may respond better to preoperative treatment with intravenous iron, depending on the timescale before surgery, tolerance of iron, and iron status (Goodnough 2011). In case of an insufficient increase in haemoglobin, additional treatment with rHuEPO may be indicated.
Why it is important to do this review
One of the main therapeutic strategies to avoid allogeneic RBC transfusion in preoperative anaemic people is preoperative treatment with rHuEPO and iron. This strategy aims to normalize the haemoglobin concentration and red cell mass.
The recommendations of rHuEPO and iron in the NATA guidelines (Goodnough 2011), as well as partly in the European Society of Anaesthesiology guidelines (Kozek‐Langenecker 2017), are based on a limited number of randomized controlled trials in participants with preoperative anaemia. The major clinical impact of preoperative anaemia and the associated socioeconomic burden suggest that a contemporary update of the evidence for the use of rHuEPO in combination with iron would be appropriate. This Cochrane Review should add systematic evidence on the use of rHuEPO + iron therapy in people with preoperative anaemia, as well as safety data. It will also assess the influence of gender on the treatment of preoperative anaemia and provide evidence to update and strengthen existing recommendations.
There are currently several studies which are partly summarized in systematic reviews investigating the use of rHuEPO and iron in people with different causes of anaemia, or people scheduled for autologous blood donation. However, there are a limited number of studies investigating rHuEPO with iron supplementation in the case of preoperative anaemia (Alsaleh 2013; Lin 2013). Recommendations for rHuEPO in preoperative anaemia are therefore based mainly on studies of rHuEPO augmented with preoperative autologous blood donation (Goodnough 2011; Kozek‐Langenecker 2017). This existing gap between current recommendations based on a limited body of evidence, and clinical practice, requires a current evaluation of the body of evidence of rHuEPO in conjunction with iron supplementation in preoperative anaemic people.
Objectives
To evaluate the efficacy of preoperative rHuEPO therapy (subcutaneous or parenteral), in combination with iron (enteral or parenteral), in reducing the need for allogeneic RBC transfusions in preoperatively anaemic adults undergoing non‐cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We also planned to include trials published only in abstract form, even if insufficient information was available; however, we did not identify any relevant abstracts.
Types of participants
We included trials enrolling preoperative adult participants (older than 17 years), irrespective of gender and type of non‐cardiac surgery, with mild to severe anaemia (WHO criteria on haemoglobin concentrations: males < 13 g/dL, females < 12 g/dL; WHO 2008; decision of inclusion based on mean haemoglobin concentrations).
We excluded studies that focused on perioperative anaemia in pregnant women undergoing, for example, caesarean section.
Types of interventions
We included all trials where rHuEPO + iron (enteral or parenteral) of any dose was initiated preoperatively at any time from the decision to operate up to preoperatively on the day of surgery. We accepted any dose or duration of rHuEPO + iron. Where rHuEPO was started preoperatively (including directly before incision/surgery), administration of additional intra‐ and postoperative rHuEPO was accepted.
We accepted trials comparing active rHuEPO treatment to control treatment (placebo or no treatment/standard of care, including the administration of iron).
Types of outcome measures
Primary outcomes
-
Need for allogeneic RBC transfusion (no autologous transfusion or other transfusions like haemostatic blood products in the form of fresh frozen plasma or thrombocytes), measured in participants during or up to five days after operation.
Secondary outcomes
-
Haemoglobin concentration (g/dL) preoperatively (directly before surgery).
-
Number of allogeneic RBC units transfused per participant (one unit contains 250 to 450 mL of RBC) during or up to five days after operation.
-
Mortality (within 30 days after surgery)
-
Adverse events (renal dysfunction, delirium, heart failure, cardiac arrest, atrial fibrillation, venous thromboembolism, systemic infection, wound infections and haematomas, hypertension, allergic reaction or local rash likely due to rHuEPO administration, headache, fever, constipation), measured in numbers of participants with adverse events between day of surgery and discharge from hospital.
-
Length of hospital stay.
Search methods for identification of studies
We searched for studies using the systematic and sensitive search strategies described in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Electronic searches
We ran searches in the following databases.
-
Cochrane Central Register of Controlled Trials (CENTRAL) (first search Issue 7, 2018; second search Issue 8, 2019).
-
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (first search 1946 to 17 July 2018, second search to 29 August 2019).
-
Embase Classic + Embase (OvidSP) (first search 1947 to 17 July 2018, second search to 29 August 2019).
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (first search 1970 to 17 July 2018, second search to 29 August 2019).
-
ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (first search 1990 to 17 July 2018, second search to 29 August 2019).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch).
For our detailed search strategies, see Appendices.
We did not apply restrictions to language or publication status. We developed a subject‐specific search strategy in MEDLINE and used that as a basis for the search strategies in the other databases listed. All search strategies are shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6.
Searching other resources
We searched the following conference proceedings electronically and manually for the years not included in CENTRAL.
-
American Society of Anesthesiology (ASA) (2014 to 2019).
-
European Society of Anaesthesiology (2014 to 2019).
-
German Society of Anaesthesiology and Intensive Care (DGAI) (2014 to 2019).
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials. When necessary, we contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (LK, AH) independently scanned the retrieved titles and abstracts of all studies and applied the predefined selection criteria to identify all potentially eligible studies. If a decision on inclusion could not be made based on review of the title and abstract, we obtained the full‐text manuscript to assess eligibility. Any disagreements in the selection process were resolved through consensus with all review authors. We documented the number of studies identified, excluded, and included, with reasons at every stage of searching and screening, in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (CvH, LK) extracted data relevant to each study using a predefined, standardized data extraction form. We listed all necessary information, including general information, study characteristics, participant characteristics, type of intervention or control, and outcome in the Characteristics of included studies tables. Any disagreements were resolved by discussion amongst all review authors. When information was unclear, we attempted to contact the study investigators for further details. We entered data into Review Manager 5 software (Review Manager 2014), and one review author (AH) checked the data entry for accuracy.
Assessment of risk of bias in included studies
Two review authors (LK, CvH) independently assessed the risk of bias (methodological quality) of all eligible studies using the methods suggested in Chapter 8 of version 5.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We considered the following 'Risk of bias' domains.
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants, personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective outcome reporting (reporting bias)
-
Other sources of bias
We assessed each domain as at low, high, or unclear risk of bias (Higgins 2011). We recorded this judgement in 'Risk of bias' tables in Characteristics of included studies and presented a summary 'Risk of bias' figure. To improve the transparency of the judgements, we included quotations from the full‐text article or supplemental information from the trial author in the 'Notes' section of the 'Risk of bias' tables.
Overall 'Risk of bias' summary
We summarized the risk of bias for each study based on our assessment of the following domains: random sequence generation, blinding of participant and personnel, and incomplete outcome data. We classified each study as 'low risk of bias' when we judged all of these items as 'low risk of bias'. If we judged at least one item as 'high risk of bias', or all items as 'unclear risk of bias', then we classified the overall risk of bias for the study as 'high risk of bias'. In the remaining cases, we classified the study as 'unclear risk of bias'. We used the overall study‐level 'Risk of bias' judgements for sensitivity analyses.
Measures of treatment effect
We carried out statistical analysis using Cochrane's statistical software, Review Manager 5 (Review Manager 2014).
For dichotomous data (e.g. RBC transfusion), we used the risk ratio (RR), obtained from the intervention and control event rates.
For continuous outcomes (e.g. preoperative haemoglobin concentration), we used the mean difference (MD) or standardized mean difference (SMD), estimated from means and standard deviations (SDs) of the intervention and control groups.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually randomized trials. However, we did not identify any relevant cluster‐randomized trials in the current review.
Multiarmed studies
We overcame a unit of analysis error for studies that could contribute multiple comparisons to the same outcome by dividing the shared group into two groups with smaller sample size, and including two reasonably independent comparisons in the presented data. We included two studies with two comparisons in this review (Christodoulakis 2005; Feagan 2000).
Dealing with missing data
In the case of missing data, we contacted the relevant trial authors to obtain further information. We planned that if we did not receive the information required (e.g. missing SD), we would calculate, or estimate, missing data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). In the current review, there were no missing outcome data, such as SDs required for meta‐analysis. If there were missing participants in the study groups, we performed available‐case analysis (used data on only those whose results are known). We have not imputed missing participant data.
Assessment of heterogeneity
We classified heterogeneity following the interpretation specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
In the case of trials being sufficiently similar in clinical and methodological design, we included them in a meta‐analysis.
We evaluated the extent of heterogeneity by visually examining the forest plots, and used the I2 statistic to quantify it. We used the I2 statistic to assess substantial heterogeneity. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. We considered there to be substantial statistical heterogeneity if I2 was greater than 50% (Higgins 2019).
If substantial heterogeneity was suggested in the overall analysis, we investigated possible causes of the heterogeneity via subgroup analysis by dose (high versus low). If this did not explain the statistical heterogeneity, we would perform a sensitivity analysis excluding studies at high risk of bias (Table 1). We presented all meta‐analyses using the fixed‐effect model as sensitivity analysis in a tabular format (Table 2)
| Outcome | Statistical method | All studies | Trials with low risk of bias for random sequence generation, blinding, incomplete outcome data | ||
|---|---|---|---|---|---|
| Studies | Effect estimate (95% CI) | Studies | Effect estimate (95% CI) | ||
| Transfusion (measured in participants during or up to 5 days after operation) | Risk ratio | 12 | RR 0.55 (0.38; 0.80) | 5 | RR 0.79 (0.61; 1.01) |
| Hb concentration preoperatively (directly before surgery) | Mean difference | 7 | MD 0.97 (0.07; 1.87) | 2 | MD −0.31 (−0.69; 0.08) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | Mean difference | 3 | MD 1.87 (1.26; 2.49) | 0 | ‐ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | Mean difference | 4 | MD 0.11 (−0.46; 0.69) | 2 | MD 0.15 (−1.28; 1.58) |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | Mean difference | 6 | MD −0.09 (−0.23; 0.05) | 3 | MD −0.09 (−0.27; 0.09) |
| Mortality (within 30 days after surgery) | Risk ratio | 2 | RR 1.18 (0.39; 3.63) | 1 | RR 1.25 (0.35; 4.52) |
| Adverse events (number of participants | Risk ratio | 10 | RR 0.97 (0.70; | 5 | RR 0.92 (0.63; |
| Length of hospital stay | Mean difference | 3 | MD −1.07 (−4.12; 1.98) | 2 | MD 0.65 (0.05; |
BW: body weight; CI: confidence interval; Hb: haemoglobin; IU: international unit; IV: inverse variance; MD: mean difference; MH: Mantel‐Haenszel; RBC: red blood cells; rHuEPO: recombinant human erythropoietin; RR: risk ratio
| Outcome | Statistical heterogeneity (I2) | Fixed‐effect (95% CI) | Random‐effects (95% CI) |
|---|---|---|---|
| Transfusion (measured in participants during or up to 5 days after operation) | 84% | RR 0.52 (0.45, 0.59) | RR 0.55 (0.38, 0.45) |
| Hb concentration preoperatively (directly before surgery) | 97% | MD 1.58 (1.47, 1.70) | MD 0.97 (0.07, 1.87) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | 69% | MD −0.11 (−0.39, 0.17) | MD 0.11 (−0.46, 0.69) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | 89% | MD 1.94 (1.81, 2.07) | MD 1.87 (1.26, 2.49) |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | 0% | MD −0.09 (−0.23, 0.05) | MD −0.09 (−0.23, 0.05) |
| Mortality (within 30 days after surgery) | 0% | RR 1.19 (0.39, 3.63) | RR 1.18 (0.39, 3.63) |
| Adverse events (number of participants with any adverse event) | 0% | RR 0.93 (0.68, 1.28) | RR 0.97 (0.70, 1.34) |
| Length of hospital stay | 87% | MD 0.59 (−0.00, 1.18) | MD −1.07 (−4.12, 1.98) |
BW: body weight; CI: confidence interval; Hb: haemoglobin; IU: international unit; MD: mean difference; RBC: red blood cells; rHuEPO: recombinant human erythropoietin; RR: risk ratio
Assessment of reporting biases
If there were 10 or more studies in a meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If visual assessment suggested asymmetry, we performed exploratory analyses such as Rücker's arcsine test for dichotomous data and trim and fill sensitivity analysis to investigate it. We used the R package meta (version 4.9‐4) for analysis (Schwarzer 2018).
Data synthesis
We estimated summary statistics by fixed‐effect and random‐effects meta‐analysis using inverse‐variance estimators for RR and MD. We presented results as summary RRs and MDs with the z statistic, P value, and 95% confidence interval (CI) in tables and forest plots. When I2 was greater than 50% amongst trials, and the trial estimates were in different directions, we evaluated heterogeneity. We did not present orphan studies (comparisons with only a single included study) in analysis with forest plots.
Subgroup analysis and investigation of heterogeneity
If we identified heterogeneity (I2 > 50%), we investigated it using subgroup analysis and sensitivity analysis. We carried out the following subgroup analyses.
-
Different dosages of rHuEPO:
-
low dose (150 IU to 300 IU subcutaneous/kg body weight);
-
high dose (500 IU to 600 IU subcutaneous/kg body weight).
-
If administration of rHuEPO was reported in bolus dose of international units (IU), we calculated the IU per kg body weight by dividing the bolus unit/70 kg (body weight for a mean adult).
We compared subgroups for qualitative interaction (direction of effect reversed) and quantitative interaction (magnitude of effect differs). We assessed the Review Manager 5 calculated interaction z statistic with P value to declare a difference between subgroups (Review Manager 2014).
Sensitivity analysis
We conducted sensitivity analysis to test the robustness of the effect estimates based on methodological quality as determined by overall study‐level 'Risk of bias' judgements. We conducted the analysis by including only those RCTs with high methodological quality (studies that were classified as having a 'low risk of bias' based on the approach outlined in the Assessment of risk of bias in included studies section. We identified only one trial at low risk of bias for all domains (Higgins 2019).
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables, including a rating of the quality of evidence. The assessment of the quality of evidence was based on the principles of the GRADE Working Group (www.gradeworkinggroup.org) (Guyatt 2008; Guyatt 2011; Schünemann 2009).
We created one primary 'Summary of findings' table using GRADEpro GDT for the following outcomes (GRADEpro GDT).
-
Need for allogeneic RBC transfusion (no autologous transfusion or other transfusions like haemostatic blood products in the form of fresh frozen plasma or thrombocytes), measured in participants during or up to five days after operation.
-
Haemoglobin concentration preoperatively (directly before surgery).
-
Number of allogeneic RBC units transfused per participant (one unit contains approximately 450 mL of blood) during or up to five days after operation.
-
Mortality (within 30 days after surgery).
-
Length of hospital stay.
-
Adverse events (renal dysfunction, delirium, heart failure, cardiac arrest, atrial fibrillation, venous thromboembolism, systemic infection, wound infections and haematomas, hypertension, allergic reaction or local rash likely due to erythropoietin administration, headache, fever, constipation), measured in numbers of participants with adverse events between day of surgery and discharge from hospital.
Two review authors (AH, LK) independently assessed the quality of the body of evidence for each outcome. The assessments reflect five factors: risk of bias (study limitations), publication bias, indirectness, imprecision, and inconsistency. We appraised the quality of evidence only for relative treatment effects and not for treatment rankings.
The GRADE assessment resulted in one of four levels of 'quality':
-
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
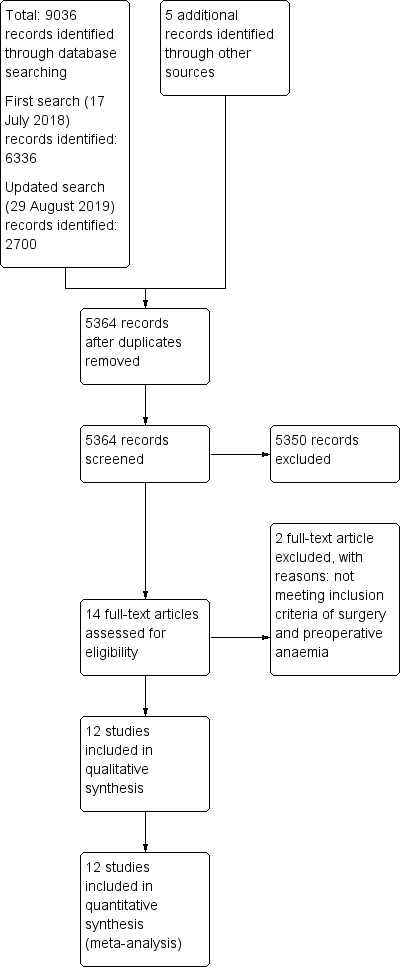
The results of the literature search process are presented in a PRISMA flow chart (Figure 1). We performed the first search on 17 July 2018, and retrieved 6336 records through the database search and 5 additional records through other sources. After removal of duplicate studies, 3427 records remained for assessment. We excluded 3413 of these records by reading the title or abstract. We reviewed the remaining 14 records, excluding two studies that did not meet the inclusion criteria of preoperative anaemia, and including 12 studies in the synthesis of the review. All included studies were published in English.

Study flow diagram.
We performed an updated second search on 29 August 2019 including all records since the first search. We found 2700 additional records in this updated search. After removal of duplicates, 1937 retrieved titles and abstracts were screened for their eligibility for inclusion in the review. No new records were found in this updated search.
Included studies
We included 12 RCTs (1880 participants) that evaluated the use of preoperative rHuEPO + iron to treat preoperative anaemia in adults undergoing non‐cardiac surgery (Bernabeu-Wittel 2016; Christodoulakis 2005; Dousias 2003; Feagan 2000; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Larson 2001; Moonen 2008; Na 2011; Qvist 1999; Weber 2005). A detailed description of the trials is provided in the Characteristics of included studies section. All included studies were published in English, one excluded study were published in russian (Gerasimov 2012).
Details on rHuEPO with iron administration
Two of the included studies reported data from two different dosing regimens of rHuEPO (Christodoulakis 2005; Feagan 2000).
If administration of rHuEPO was reported as a bolus IU, we calculated the IU per kg body weight with division of the bolus through the assumed body weight of a mean adult with 70 kg.
Seven studies reported low rHuEPO dosage (Feagan 2000; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Larson 2001; Na 2011; Qvist 1999), and five studies reported rHuEPO dosage subgroup for the analysis of the outcomes (Bernabeu‐Wittel 2016; Dousias 2003; Feagan 2000; Moonen 2008; Weber 2005).
Duration of the administration of rHuEPO ranged from daily dosages for 10 days, Christodoulakis 2005, to weekly dosages for four weeks (Feagan 2000; Larson 2001; Moonen 2008).
Additional iron was given intravenously in four studies (Bernabeu‐Wittel 2016; Kettelhack 1998; Kosmadakis 2003; Na 2011), and orally in eight studies (Christodoulakis 2005; Dousias 2003; Feagan 2000; Heiss 1996; Larson 2001; Moonen 2008; Qvist 1999; Weber 2005). Participants in two studies received rHuEPO with iron and additional folic acid (Christodoulakis 2005; Heiss 1996).
Comparators
In six trials, participants in the comparator arm received control treatment with saline (Bernabeu‐Wittel 2016; Feagan 2000; Kettelhack 1998; Kosmadakis 2003; Na 2011; Qvist 1999). In six trials, participants in the comparator arm received standard care or no treatment (Christodoulakis 2005; Dousias 2003; Heiss 1996; Larson 2001; Moonen 2008; Weber 2005). Intravenous iron was given to participants of three trials (Bernabeu‐Wittel 2016; Kettelhack 1998; Kosmadakis 2003). Oral iron was given to participants in seven trials (Dousias 2003; Feagan 2000; Heiss 1996; Larson 2001; Moonen 2008; Na 2011; Qvist 1999), and additional folic acid was given in two studies (Christodoulakis 2005; Heiss 1996). In one trial, participants in the control arm received the usual standard of care, including oral or intravenous iron (Weber 2005).
Surgical procedures
Participants undergoing colorectal cancer surgery were analysed in four trials (Christodoulakis 2005; Heiss 1996; Kettelhack 1998; Qvist 1999). Participants of five trials obtained joint replacement or arthroplasty in orthopaedic surgery (Weber 2005), such as hip joint arthroplasty (Bernabeu‐Wittel 2016; Feagan 2000), hip or knee replacement (Moonen 2008), or bilateral total knee replacement (Na 2011). Gastrointestinal surgery was performed in one trial (Kosmadakis 2003), and participants received hysterectomy in two trials (Dousias 2003; Larson 2001).
Setting
Five trials were multicentre trials (Bernabeu‐Wittel 2016; Christodoulakis 2005; Feagan 2000; Kettelhack 1998; Weber 2005).
One study was conducted in a community hospital in Canada (Feagan 2000), 10 studies in Europe (Bernabeu‐Wittel 2016; Christodoulakis 2005; Dousias 2003; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Larson 2001; Moonen 2008; Qvist 1999; Weber 2005), and one study in South Korea (Na 2011). All studies were carried out in hospitals.
Inclusion and exclusion criteria of participants
The criteria for inclusion and exclusion of participants in the included studies varied widely (see Characteristics of included studies). All studies reported preoperative anaemia as an inclusion criterion. The proportion of female and male participants varied in the trials. In three trials, only female participants were investigated (Dousias 2003; Larson 2001; Na 2011).
Study sample size
The overall sample size ranged from 30 participants, Heiss 1996, to 733 participants, Weber 2005. Six studies reported a sample size calculation for their primary outcome (Bernabeu‐Wittel 2016; Feagan 2000; Kettelhack 1998; Larson 2001; Moonen 2008; Na 2011). The remaining six studies did not report any sample size calculation for their primary outcome (Christodoulakis 2005; Dousias 2003; Heiss 1996; Kosmadakis 2003; Qvist 1999; Weber 2005); it is possible that these trials were underpowered. The primary endpoints are listed in the Characteristics of included studies section.
Source of funding
Financial support was provided by government funding in one trial (Bernabeu‐Wittel 2016).
Two trials explicitly stated that there was no funding (Moonen 2008; Na 2011), and three trials reported industry funding (Feagan 2000; Qvist 1999; Weber 2005). The remaining trials did not mention the source of funding in their publications (Characteristics of included studies). The authors of four trials declared their conflicts of interest (Bernabeu‐Wittel 2016; Moonen 2008; Na 2011; Weber 2005); the authors of the remaining trials did not report this information.
Reported outcomes
All 12 included studies reported at least one primary and secondary outcome of interest for this review. The primary outcome 'need for allogeneic RBC transfusion' of this review was reported as transfusion rate (Weber 2005), transfusion incidences (Na 2011), indication of blood transfusions (Kosmadakis 2003; Larson 2001), need for blood transfusion (Christodoulakis 2005; Dousias 2003; Feagan 2000; Heiss 1996; Kettelhack 1998; Moonen 2008; Qvist 1999), and the percentage of participants who received RBC transfusion (Bernabeu‐Wittel 2016).
Excluded studies
We excluded one study because it did not include surgical patients (Gerasimov 2012), and one study because the population did not include individuals with anaemia (Stowell 2009)
For details, see Characteristics of excluded studies.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We estimated the risk of bias of each of the included studies as described in the 'Risk of bias' tables (Characteristics of included studies). The results of the quality assessments are presented graphically in Figure 2. The overall risk of bias concerning selection bias (random sequence generation), performance bias (blinding of participants and personnel), and attrition bias (incomplete outcome data) was low in more than 50% of the included studies (Figure 3). For selection bias (allocation concealment), detection bias, and other bias, we judged risk of bias to be low for only approximately 20% of the included studies. For reporting bias, we assessed the risk of bias as low for only 10% of the included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All 12 included studies reported random sequence generation. However, only three trials sufficiently described the methods to generate the allocation sequence (Bernabeu-Wittel 2016; Moonen 2008; Na 2011). We assessed risk of selection bias as unclear due to insufficient reporting on allocation sequence for more than 75% of the analysed studies (Figure 3).
Blinding
Participants and personnel were blinded in six trials (Bernabeu‐Wittel 2016; Feagan 2000; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Qvist 1999), and in one trial it was unclear whether blinding was used (Moonen 2008). Four studies were open‐label, and blinding was not performed (Dousias 2003; Larson 2001; Na 2011; Weber 2005). In two trials, blinding was unclear due to unblinded providers for the transfusion decision, Christodoulakis 2005, and different preoperative haemoglobin concentrations in the treatment and control groups, Moonen 2008. The risk of performance bias across all studies was less than 50% (Figure 3).
Incomplete outcome data
All 12 included studies assessed data for each primary and secondary outcome, including attritions and exclusions and reasons for them. In addition, the numbers of analysed and randomized participants in the treatment and control groups were reported. We found no attrition bias due to incomplete outcome data (Figure 3).
Selective reporting
Only one study published a study protocol in advance (Bernabeu‐Wittel 2012). The published study report included all expected outcome parameters of the protocol, and the study was reported as prespecified. For all other trials, a study protocol was not pre‐published, and it remains unclear if the published reports included all expected outcomes (Figure 3). For the primary outcome, we visually analysed the funnel plot for publication bias (Figure 4).

Funnel plot of comparison: 1 rHuEPO versus control treatment, outcome: 1.1 Transfusion.
Other potential sources of bias
Other potential sources of bias included non‐reported funding, or reported funding by medical companies that gave input into the design and reporting of the study (Feagan 2000), as well as missing conflict of interest declaration of the investigators. Three studies reported funding as well as conflicts of interest of the investigators, with no important concerns about bias not addressed in the studies (Bernabeu-Wittel 2016; Moonen 2008; Na 2011). It remains unclear whether there were other potential sources of bias in more than 75% of studies (Figure 3).
Effects of interventions
The effects of interventions by primary and secondary outcomes, including the subgroup analysis, are shown in the analyses and forest plots for each outcome, as well as in summary of findings Table 1 for the main comparison of rHuEPO + iron versus control for preoperative anaemia in non‐cardiac surgery. Two multiarmed studies were included, and the control group for analysis was split accordingly (Christodoulakis 2005; Feagan 2000).
Primary outcome
Need for allogeneic RBC transfusion
Twelve studies reported the proportion of participants with preoperative anaemia who received allogeneic RBC transfusions in each group up to five days after operation (Bernabeu-Wittel 2016; Christodoulakis 2005; Dousias 2003; Feagan 2000; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Larson 2001; Moonen 2008; Na 2011; Qvist 1999; Weber 2005). Overall, these trials enrolled 1880 participants (100% of the participants included in the review), of which 1109 (59%) were allocated to the rHuEPO + iron group and 771 (41%) to control. Eight trials investigated allogeneic RBC transfusion as a primary outcome, whilst four trials reported transfusion as a secondary outcome (Dousias 2003; Larson 2001; Na 2011; Qvist 1999).
The administration of rHuEPO + iron reduced the need for allogeneic RBC transfusion in anaemic participants (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.38 to 0.80; participants = 1880; studies = 12; I2 = 84%; Analysis 1.1; Figure 5). This means that when rHuEPO + iron was given, 231 (200 to 262) fewer of 1000 individuals received no RBC transfusion compared to control. We rated the quality of evidence as moderate due to inconsistency (summary of findings Table 1).

Forest plot of comparison: 1 rHuEPO versus control treatment, outcome: 1.1 Transfusion.
The estimated effect for need for allogeneic blood cell transfusion was preserved in a sensitivity analysis including only studies at low risk of overall bias (RR 0.79, 95% CI 0.61 to 1.01; 5 studies; Table 1).
Visual assessment of the funnel plot for transfusion (12 studies, including two substudies), revealed asymmetry by two studies (Figure 4). Rücker's arcsine test did not suggest funnel plot asymmetry (P = 0.11), and trim and fill sensitivity analysis did not change the conclusion.
Secondary outcomes
Haemoglobin concentration (g/dL) preoperatively (directly before surgery)
Four trials with 334 participants using low‐dose administration of rHuEPO + iron, Kettelhack 1998; Larson 2001; Na 2011; Qvist 1999, and three trials with 852 participants using high doses of rHuEPO + iron reported a pre‐surgery haemoglobin concentration (g/dL) (Dousias 2003; Moonen 2008; Weber 2005).
Pre‐surgery haemoglobin concentrations (g/dL) were increased when a high dose of rHuEPO + iron was given (mean difference (MD) 1.87, 95% CI 1.26 to 2.49; participants = 852; studies = 3; I2 = 89%; low‐quality evidence), compared to unchanged preoperative haemoglobin concentration (g/dL) when a low dose of rHuEPO + iron was given (MD 0.11, 95% CI −0.46 to 0.69; participants = 334; studies = 4; I2 = 69%; low‐quality evidence) (Analysis 1.2; Figure 6). There is a difference between both dose regimens (Chi2 = 166.54, df = 1; P < 0.001).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.2 Haemoglobin concentration immediately prior to surgery (g/dL).
Number of allogeneic RBC units
Six studies involving 1420 participants investigated the number of RBC units transfused per participant (where one unit contains approximately 250 to 450 mL of blood; intraoperative and postoperative transfusion, up to five days after operation) in each treatment group (Bernabeu-Wittel 2016; Christodoulakis 2005; Feagan 2000; Heiss 1996; Qvist 1999; Weber 2005). There was no difference in the number of allogeneic RBC units transfused between the rHuEPO + iron and control groups (MD −0.09, 95% CI −0.23 to 0.05; participants = 1420; studies = 6; I2 = 2%; Analysis 1.3; Figure 7). We rated the quality of evidence as moderate due to imprecision.

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.3 Number of RBC units transfused per participant (intraoperative to 5 days postoperative).
Mortality
Two studies involving 230 participants reported mortality (within 30 days after surgery) (Bernabeu-Wittel 2016; Heiss 1996). There was no difference in mortality between groups (RR 1.19, 95% CI 0.39 to 3.63; participants = 230; studies = 2; I2 = 0%; Analysis 1.4; Figure 8). We rated the quality of evidence as moderate due to imprecision.

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.4 Mortality (within 30 days after surgery).
One study reported one‐year survival of 80.6% of participants in the rHuEPO + iron group compared to 59.3% of participants in the control group (Kosmadakis 2003).
Adverse events
Ten studies reported adverse events. No adverse events were reported in two studies (Dousias 2003; Na 2011). There was no difference in adverse events between rHuEPO + iron and control groups (RR 0.93, 95% CI 0.68 to 1.28; participants = 1722; studies = 10; I2 = 0%; Analysis 1.5; Figure 9). We rated the quality of evidence as moderate due to imprecision. One trial reported a local rash that was likely due to rHuEPO administration (Christodoulakis 2005). Fever and constipation may have been associated with rHuEPO administration in one trial (Kettelhack 1998), whilst three participants suffered from transient hypertension after rHuEPO administration (Heiss 1996; Kosmadakis 2003).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.5 Adverse events (number of participants with any adverse event).
Length of hospital stay (days)
Three trials involving 293 participants investigated length of hospital stay (days) (Bernabeu-Wittel 2016; Kosmadakis 2003; Larson 2001). rHuEPO + iron did not clearly reduce the length of hospital stay (MD −1.07, 95% CI −4.12 to 1.98; participants = 293; studies = 3; I2 = 87%; Analysis 1.6; Figure 10). We rated the quality of evidence as low due to imprecision.

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.6 Length of hospital stay.
Discussion
Summary of main results
We included 12 RCTs (1880 participants) that evaluated preoperative rHuEPO with iron to correct preoperative anaemia in non‐cardiac surgery. Preoperative anaemia treatment was mainly investigated in orthopaedic surgery (five studies: Bernabeu-Wittel 2016; Feagan 2000; Moonen 2008; Na 2011; Weber 2005), and gastrointestinal surgery (five studies: Christodoulakis 2005; Heiss 1996; Kettelhack 1998; Kosmadakis 2003; Qvist 1999). Two trials were performed in women scheduled for hysterectomy (Dousias 2003; Larson 2001). rHuEPO with iron was mainly compared to placebo, whilst four studies investigated rHuEPO + iron versus control treatment with oral iron only (Christodoulakis 2005; Dousias 2003; Larson 2001; Weber 2005). Two trials compared rHuEPO with iron to no treatment (Moonen 2008), or to control treatment with iron supplementation (Qvist 1999).
All included studies reported the proportion of participants requiring RBC transfusion. The risk of transfusion is probably reduced up to five days postoperatively when rHuEPO + iron is given to preoperatively anaemic adults (RR 0.55, 95% CI 0.38 to 0.80; participants = 1880; studies = 12; I2 = 84%; moderate‐quality evidence due to inconsistency).
A high dose of rHuEPO + iron may result in increased pre‐surgery haemoglobin concentrations (g/dL) (MD 1.87, 95% CI 1.26 to 2.49; participants = 852; studies = 3; I2 = 89%; low‐quality evidence due to inconsistency and risk of bias). In the low‐dose rHuEPO + iron regimen, pre‐surgery haemoglobin concentration (g/dL) may not differ between groups (MD 0.11, 95% CI −0.46 to 0.69; participants = 334; studies = 4; I2 = 69%; low‐quality evidence due to inconsistency and risk of bias).
There is probably no difference in the number of allogeneic RBC units transfused between rHuEPO + iron and control groups (MD −0.09, 95% CI −0.23 to 0.05; participants = 1420; studies = 6; I2 = 2%; moderate‐quality evidence due to different counting system of the units).
Mortality within 30 days of surgery is probably not affected when rHuEPO + iron is administered to preoperatively anaemic adults before non‐cardiac surgery compared to control (RR 1.19, 95% CI 0.39 to 3.63; participants = 230; studies = 2; I2 = 0%; moderate‐quality evidence due to imprecision).
There is probably no difference in adverse events between rHuEPO + iron and control (RR 0.93, 95% CI 0.68 to 1.28; participants = 1722; studies = 10; I2 = 0%; moderate‐quality evidence due to imprecision).
We are uncertain if the administration of rHuEPO + iron compared to control influences length of hospital stay (days) (MD −1.07, 95% CI −4.12 to 1.98; participants = 293; studies = 3; I2 = 87%; low‐quality evidence due to inconsistency and imprecision).
Overall completeness and applicability of evidence
The evidence that rHuEPO + iron reduces the need for allogeneic RBC transfusion in preoperatively anaemic patients remains limited. The generalizability of the results to all kind of operations in non‐cardiac surgery is limited because most included trials were in orthopaedic, gastrointestinal, and gynaecological surgery. We are uncertain if the results apply to other surgical fields. Potential differences in patient characteristics in terms of age, gender, comorbidity or malignancies in different areas than non‐cardiac surgery may alter the generalizability.
In general, participants in the included studies had mild to moderate preoperative anaemia (haemoglobin from 10 to 12 g/dL). We thus cannot be certain that rHuEPO + iron would be effective in avoiding transfusion in patients with a haemoglobin concentration < 10 g/dL, or whether it would be even more effective in those patients.
It may appear contradictory that rHuEPO reduces the need for allogeneic RBC transfusion, but there is no indication of a difference between groups in the number of RBC units administered. It is possible that despite their higher starting haemoglobin concentration, there may have been some unknown clinical reason for some individuals to receive more units when transfusion was required in the rHuEPO + iron group, but the amount of RBC units per participant in total remained unchanged. Alternatively, the result could be explained by the 'in practice' administration of complete units of RBCs rather than the exact volume of RBCs each individual needed. The available information in these reports on the transfusion protocols used was insufficient to support or refute this supposition.
Two different rHuEPO treatment regimens were investigated in the studies. For low‐dose rHuEPO, no changes in presurgical haemoglobin concentration (g/dL) were observed. Nevertheless, data were insufficient to conclude that low‐dose rHuEPO is less efficient compared to the high‐dose regimen. One reason might be that transfusion, as well as haemoglobin concentration (g/dL), are direct and clinically relevant parameters for the efficiency of rHuEPO, but they did not directly reflect the pharmacological‐kinetic stimulation of erythropoiesis. However, both outcomes are of high clinical relevance and are often used as primary and secondary outcomes in clinical trials, especially in anaemic patients in the perioperative setting.
The duration of preoperative rHuEPO treatment was inconsistent across the trials. In four studies, rHuEPO was given either once a week, Dousias 2003; Moonen 2008; Weber 2005, or twice a week, Larson 2001, whereas in all the other trials, rHuEPO was given daily over a 5‐to‐10‐day period. In one trial, preoperative rHuEPO was given on the morning of surgery and for five days postoperatively (Na 2011). As yet no conclusion can be drawn either in favour of a weekly or daily dose regimen of preoperative rHuEPO or a defined continuous administration over the first postoperative days.
Concomitant iron supplementation was mainly given orally (200 mg/d); intravenous iron was administered in three trials (Christodoulakis 2005; Kettelhack 1998; Kosmadakis 2003). We were unable to determine whether iron should be given intravenously or orally for the best effect, or if it is necessary at all as long as iron deficiency is not proven preoperatively.
Furthermore, the applicability of the results is mainly limited because the cause of anaemia was not analysed preoperatively. Besides the reticulocyte counts, parameters of iron metabolism such as transferrin, ferritin, etc. were defined as secondary outcomes in six trials (Christodoulakis 2005; Heiss 1996; Kosmadakis 2003; Larson 2001; Na 2011; Qvist 1999). None of the studies stratified their intervention related to the cause of anaemia.
According to the current meta‐analysis of all trials, there are still insufficient primary and secondary outcome data to stratify the analysis of these outcomes for gender, different participant populations, or age (e.g. elderly patients aged > 65 years).
Quality of the evidence
We assessed the quality of the evidence for all primary and secondary outcomes using the GRADE approach (Guyatt 2008; Guyatt 2011; Schünemann 2009). This assessment was based on evaluation of the risk of bias (study limitations), publication bias, imprecision, inconsistency, and indirectness of the evidence (summary of findings Table 1).
For the primary outcome need for allogeneic RBC transfusion, the overall quality of evidence was moderate, meaning there might be an important bias in our estimate of the effect of rHuEPO + iron on this outcome. The risk of publication bias remained unclear, but not serious. We found no pre‐published study protocols without consecutive publication. Due to missing study protocols of already published trials, we cannot rule out selective outcome reporting (Figure 4, see funnel plot for publication bias in primary outcome). Based on small confidence intervals in the trials, and an adequate number of events as well as participants, the clinical decision‐making thresholds did not cross the line between in favour of treatment or control treatment, therefore we did not downgrade the quality of evidence for imprecision. Inconsistency of effect estimates, or heterogeneity of evidence, was illustrated by a wide variance of point estimates and large I2 values.
The high I2 value for the primary outcome may represent substantial heterogeneity. Assuming that the intervention effects would differ between the included studies, we used the random‐effects model and subgroup analysis to explore heterogeneity. Due to the minimal numeric and clinically important differences between high and low dose, we did not present the subgroups of low and high dose for the primary outcome.
The quality of evidence for presurgical haemoglobin concentration was low due to the findings mentioned above regarding evidence quality and to study limitations in sensitivity analysis.
We assessed the overall quality of evidence for the secondary outcome number of transfused allogeneic RBC units as moderate (summary of findings Table 1). Imprecision was present because the width of the confidence interval was consistent with both important benefit and harm.
The quality of evidence for the outcomes mortality and adverse events was moderate, downgraded because of imprecision due to a small number of events leading to wide confidence intervals.
The quality of the evidence for the outcome length of hospital stay was low. Inconsistency or heterogeneity was supported by a wide variance of point estimates, high I2 values, and heterogeneity of effect estimates. The quality of the evidence was also downgraded due to imprecision with a width of confidence interval including both no effect and appreciable harm.
Potential biases in the review process
The strengths of this review lie in the robust and comprehensive methodology employed to search the literature in important databases and assess and analyse all relevant trials. We followed the recommendations specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Search strategies were generated by the review group and approved by Cochrane Anaesthesia. The review group consists of several experts in the field (CvH, LK) who are in contact with those continuing clinical research in the field. Screening of the literature search was performed independently by at least two review authors. The meta‐analysis was done following the best clinical and systematic review methodology according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). A clinician and methodologist of the review group were involved during all stages of the review process.
Furthermore, when there was insufficient reporting of data to allow inclusion and assessment of trials in different languages, we contacted authors directly to obtain the necessary data or translated the study review into English (e.g. Gerasimov 2012).
Agreements and disagreements with other studies or reviews
The results of this systematic review are in line with recent recommendations and findings of national and international guidelines in preoperative anaemia (German S3 Guideline 2018; Goodnough 2011; Kozek‐Langenecker 2017). Regardless of the type of surgery, the German GRADE methodology‐based guideline identified 14 RCTs for the outcome haemoglobin concentration, and 13 RCTs for the outcome transfusion requirements after treatment of preoperative anaemia with rHuEPO (German S3 Guideline 2018). Similar to our findings, the authors of that guideline reported a reduced risk (odds ratio 0.32, 95% CI 0.20 to 0.50; P < 0.001) for anaemic patients undergoing cardiac‐ or non‐cardiac surgery receiving RBC transfusion when rHuEPO with iron supplementation was given preoperatively (German S3 Guideline 2018). Additionally, an MD of 0.96 (95% CI 0.55 to 1.37) for presurgical haemoglobin concentration after rHuEPO treatment with iron was identified in the meta‐analysis of anaemic patients before cardiac‐ or non‐cardiac surgery (German S3 Guideline 2018). However, similar to our findings, the German guideline group suggested the treatment of preoperative anaemia with rHuEPO only after the diagnostics have identified the cause of anaemia (e.g. renal insufficiency) due to the quality of the evidence (German S3 Guideline 2018). If concomitant iron deficiency was present, treatment with rHuEPO in combination with iron was recommended (German S3 Guideline 2018). In the recommendations for the "management of severe perioperative bleeding", the European Society of Anaesthesiology confirms a diagnosis of the cause of anaemia and appropriate therapy (Kozek‐Langenecker 2017).
When participants in cardiac‐ and non‐cardiac surgery were merged, two meta‐analyses identified a reduced risk in RBC transfusion after preoperative administration of rHuEPO in combination with iron compared to placebo with or without iron (Cho 2019; Kei 2019): the RR of RBC transfusion was 0.57 (95% CI 0.46 to 0.71; participants = 4179; RCTs = 25) without increase in mortality (RR 1.31, 95% CI 0.8 to 2.16) or incidence of severe adverse events (e.g. deep vein thrombosis) (RR 1.28, 95% CI 0.95 to 2.31) (Kei 2019); and (RR 0.59, 95% CI 0.47 to 0.73; participants = 4750; studies = 28) without any difference in thromboembolic events (RR 1.02, 95% CI 0.78 to 1.33; studies = 28) (Cho 2019). However, both meta‐analyses included studies of participants who were not preoperatively anaemic, but who received rHuEPO and iron (Cho 2019; Kei 2019).
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 rHuEPO versus control treatment, outcome: 1.1 Transfusion.

Forest plot of comparison: 1 rHuEPO versus control treatment, outcome: 1.1 Transfusion.

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.2 Haemoglobin concentration immediately prior to surgery (g/dL).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.3 Number of RBC units transfused per participant (intraoperative to 5 days postoperative).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.4 Mortality (within 30 days after surgery).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.5 Adverse events (number of participants with any adverse event).

Forest plot of comparison: 1 rHuEPO versus control treatment with or without iron, outcome: 1.6 Length of hospital stay.

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 1: Transfusion (measured in participants during or up to 5 days after operation)

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 2: Haemoglobin concentration immediately prior to surgery (g/dL)

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 3: Number of RBC units transfused per participant (intraoperative to 5 days postoperative)

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 4: Mortality (within 30 days after surgery)

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 5: Adverse events (number of participants with any adverse event)

Comparison 1: rHuEPO versus control treatment with or without iron, Outcome 6: Length of hospital stay
| rHuEPO with iron compared to control treatment with or without iron in preoperative anaemic adults undergoing non‐cardiac surgery | |||||
| Patient or population: adult participants, irrespective of gender and type of non‐cardiac surgery, with mild or severe preoperative anaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Risk with control treatment | Risk with rHuEPO + iron | ||||
| Transfusion (measured in participants during or up to 5 days after operation) | 444 per 1000 | 231 per 1000 | RR 0.55 | 1880 | ⊕⊕⊕⊝ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | The mean Hb ‐ rHuEPO high 500 to 600 IU/kg BW ranged across control groups from 10.7 to 12.4 g/dL. | MD 1.87 (1.26 higher to 2.49 higher) | ‐ | 852 | ⊕⊕⊝⊝ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | The mean Hb ‐ rHuEPO low 150 to 300 IU/kg BW ranged across control groups from 11.9 to 12.1 g/dL. | MD 0.11 (0.46 lower to 0.69 higher) | ‐ | 334 | ⊕⊕⊝⊝ |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | The mean number of RBC units ranged across control groups from 1.28 to 2.69. | MD 0.09 lower | ‐ | 1420 | ⊕⊕⊕⊝ |
| Mortality (within 30 days after surgery) | 45 per 1000 | 54 per 1000 | RR 1.18 | 230 | ⊕⊕⊕⊝ |
| Adverse events (number of participants with any adverse event) | 88 per 1000 | 81 per 1000 | RR 0.93 | 1722 | ⊕⊕⊕⊝ |
| Length of hospital stay | The mean length of hospital stay ranged across control groups from 7 to 13. | MD 1.07 lower (4.12 lower to 1.98 higher) | ‐ | 293 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded 1 level due to inconsistency. Unexplained inconsistency, with widely different point estimates. | |||||
| Outcome | Statistical method | All studies | Trials with low risk of bias for random sequence generation, blinding, incomplete outcome data | ||
|---|---|---|---|---|---|
| Studies | Effect estimate (95% CI) | Studies | Effect estimate (95% CI) | ||
| Transfusion (measured in participants during or up to 5 days after operation) | Risk ratio | 12 | RR 0.55 (0.38; 0.80) | 5 | RR 0.79 (0.61; 1.01) |
| Hb concentration preoperatively (directly before surgery) | Mean difference | 7 | MD 0.97 (0.07; 1.87) | 2 | MD −0.31 (−0.69; 0.08) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | Mean difference | 3 | MD 1.87 (1.26; 2.49) | 0 | ‐ |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | Mean difference | 4 | MD 0.11 (−0.46; 0.69) | 2 | MD 0.15 (−1.28; 1.58) |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | Mean difference | 6 | MD −0.09 (−0.23; 0.05) | 3 | MD −0.09 (−0.27; 0.09) |
| Mortality (within 30 days after surgery) | Risk ratio | 2 | RR 1.18 (0.39; 3.63) | 1 | RR 1.25 (0.35; 4.52) |
| Adverse events (number of participants | Risk ratio | 10 | RR 0.97 (0.70; | 5 | RR 0.92 (0.63; |
| Length of hospital stay | Mean difference | 3 | MD −1.07 (−4.12; 1.98) | 2 | MD 0.65 (0.05; |
| BW: body weight; CI: confidence interval; Hb: haemoglobin; IU: international unit; IV: inverse variance; MD: mean difference; MH: Mantel‐Haenszel; RBC: red blood cells; rHuEPO: recombinant human erythropoietin; RR: risk ratio | |||||
| Outcome | Statistical heterogeneity (I2) | Fixed‐effect (95% CI) | Random‐effects (95% CI) |
|---|---|---|---|
| Transfusion (measured in participants during or up to 5 days after operation) | 84% | RR 0.52 (0.45, 0.59) | RR 0.55 (0.38, 0.45) |
| Hb concentration preoperatively (directly before surgery) | 97% | MD 1.58 (1.47, 1.70) | MD 0.97 (0.07, 1.87) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO high (500 to 600 IU/kg BW) | 69% | MD −0.11 (−0.39, 0.17) | MD 0.11 (−0.46, 0.69) |
| Subgroup Hb concentration immediately prior to surgery (g/dL) rHuEPO low (150 to 300 IU/kg BW) | 89% | MD 1.94 (1.81, 2.07) | MD 1.87 (1.26, 2.49) |
| Number of RBC units transfused per participant (intraoperative to 5 days postoperative) | 0% | MD −0.09 (−0.23, 0.05) | MD −0.09 (−0.23, 0.05) |
| Mortality (within 30 days after surgery) | 0% | RR 1.19 (0.39, 3.63) | RR 1.18 (0.39, 3.63) |
| Adverse events (number of participants with any adverse event) | 0% | RR 0.93 (0.68, 1.28) | RR 0.97 (0.70, 1.34) |
| Length of hospital stay | 87% | MD 0.59 (−0.00, 1.18) | MD −1.07 (−4.12, 1.98) |
| BW: body weight; CI: confidence interval; Hb: haemoglobin; IU: international unit; MD: mean difference; RBC: red blood cells; rHuEPO: recombinant human erythropoietin; RR: risk ratio | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Transfusion (measured in participants during or up to 5 days after operation) Show forest plot | 12 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.80] |
| 1.2 Haemoglobin concentration immediately prior to surgery (g/dL) Show forest plot | 7 | 1186 | Mean Difference (IV, Random, 95% CI) | 0.97 [0.07, 1.87] |
| 1.2.1 rHuEPO low 150 to 300 IU/kg body weight | 4 | 334 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.46, 0.69] |
| 1.2.2 rHuEPO high 500 to 600 IU/kg body weight | 3 | 852 | Mean Difference (IV, Random, 95% CI) | 1.87 [1.26, 2.49] |
| 1.3 Number of RBC units transfused per participant (intraoperative to 5 days postoperative) Show forest plot | 6 | 1420 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 1.4 Mortality (within 30 days after surgery) Show forest plot | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.39, 3.63] |
| 1.5 Adverse events (number of participants with any adverse event) Show forest plot | 10 | 1722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 1.6 Length of hospital stay Show forest plot | 3 | 293 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐4.12, 1.98] |

