Interventions for managing medication‐related osteonecrosis of the jaw
Abstract
Background
Medication‐related osteonecrosis of the jaw (MRONJ) is a severe adverse reaction experienced by some individuals to certain medicines commonly used in the treatment of cancer and osteoporosis (e.g. bisphosphonates, denosumab and antiangiogenic agents) and involves the progressive destruction of bone in the mandible or maxilla. Depending on the drug, its dosage, and the duration of exposure, the occurrence of this adverse drug reaction may be rare (e.g. following the oral administration of bisphosphonate or denosumab treatments for osteoporosis, or antiangiogenic agent‐targeted cancer treatment) or common (e.g. following intravenous bisphosphonate for cancer treatment). MRONJ is associated with significant morbidity, adversely affects quality of life (QoL), and is challenging to treat.
Objectives
To assess the effects of interventions versus no treatment, placebo, or an active control for the prophylaxis of MRONJ in people exposed to antiresorptive or antiangiogenic drugs.
To assess the effects of non‐surgical or surgical interventions (either singly or in combination) versus no treatment, placebo, or an active control for the treatment of people with manifest MRONJ.
Search methods
Cochrane Oral Health’s Information Specialist searched the following databases: Cochrane Oral Health’s Trials Register (to 23 November 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 10), MEDLINE Ovid (1946 to 23 November 2016), and Embase Ovid (23 May 2016 to 23 November 2016). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on language or publication status when searching the electronic databases; however, the search of Embase was restricted to the last six months due to the Cochrane Embase Project to identify all clinical trials and add them to CENTRAL.
Selection criteria
We included randomised controlled trials (RCTs) comparing one modality of intervention with another for the prevention or treatment of MRONJ. For 'prophylaxis of MRONJ', the primary outcome of interest was the incidence of MRONJ; secondary outcomes were QoL, time‐to‐event, and rate of complications and side effects of the intervention. For 'treatment of established MRONJ', the primary outcome of interest was healing of MRONJ; secondary outcomes were QoL, recurrence, and rate of complications and side effects of the intervention.
Data collection and analysis
Two review authors independently screened the search results, extracted the data, and assessed the risk of bias in the included studies. For dichotomous outcomes, we reported the risk ratio (RR) (or rate ratio) and 95% confidence intervals (CI).
Main results
We included five RCTs (1218 participants) in the review. Three trials focused on the prophylaxis of MRONJ. Two trials investigated options for the treatment of established MRONJ. The RCTs included only participants treated with bisphosphonates and, thus, did not cover the entire spectrum of medications associated with MRONJ.
Prophylaxis of MRONJ
One trial compared standard care with regular dental examinations in three‐month intervals and preventive treatments (including antibiotics before dental extractions and the use of techniques for wound closure that avoid exposure and contamination of bone) in men with metastatic prostate cancer treated with zoledronic acid. The intervention seemed to lower the risk of MRONJ: RR 0.10; 95% CI 0.02 to 0.39 (253 participants; low‐quality evidence). Secondary outcomes were not evaluated.
As dentoalveolar surgery is considered a common predisposing event for developing MRONJ, one trial investigated the effect of plasma rich in growth factors (PRGF) for preventing MRONJ in people with cancer undergoing dental extractions. There was insufficient evidence to support or refute a benefit of PRGF on MRONJ incidence when compared with standard treatment (RR 0.08, 95% CI 0.00 to 1.51; 176 participants; very low‐quality evidence). Secondary outcomes were not reported. In another trial comparing wound closure by primary intention with wound closure by secondary intention after dental extractions in people treated with oral bisphosphonates (700 participants), no cases of intraoperative complications or postoperative MRONJ were observed. QoL was not investigated.
Treatment of MRONJ
One trial analysed hyperbaric oxygen (HBO) treatment used in addition to standard care (antiseptic rinses, antibiotics, and surgery) compared with standard care alone. HBO in addition to standard care did not significantly improve healing from MRONJ compared with standard care alone (at last follow‐up: RR 1.56; 95% CI 0.77 to 3.18; 46 participants included in the analysis; very low‐quality evidence). QoL data were presented qualitatively as intragroup comparisons; hence, an effect estimate of treatment on QoL was not possible. Other secondary outcomes were not reported.
The other RCT found no significant difference between autofluorescence‐ and tetracycline fluorescence‐guided sequestrectomy for the surgical treatment of MRONJ at any timepoint (at one‐year follow‐up: RR 1.05; 95% CI 0.86 to 1.30; 34 participants included in the analysis; very low‐quality evidence). Secondary outcomes were not reported.
Authors' conclusions
Prophylaxis of MRONJ
One open‐label RCT provided some evidence that dental examinations in three‐month intervals and preventive treatments may be more effective than standard care for reducing the incidence of MRONJ in individuals taking intravenous bisphosphonates for advanced cancer. We assessed the certainty of the evidence to be low.
There is insufficient evidence to either claim or refute a benefit of either of the interventions tested for prophylaxis of MRONJ (i.e. PRGF inserted into the postextraction alveolus during dental extractions, and wound closure by primary or secondary intention after dental extractions).
Treatment of MRONJ
Available evidence is insufficient to either claim or refute a benefit for hyperbaric oxygen therapy as an adjunct to conventional therapy. There is also insufficient evidence to draw conclusions about autofluorescence‐guided versus tetracycline fluorescence‐guided bone surgery.
PICO
Plain language summary
Interventions for managing medication‐related osteonecrosis (severe bone damage) of the jaw
Review question
What are the effects of different interventions to either prevent or treat medication‐related osteonecrosis of the jaw compared with each other or compared with no treatment or an inactive intervention ('placebo')?
Background
Medication‐related osteonecrosis of the jaw (MRONJ) is severe bone damage in the jaw bone that occurs in some people as an adverse reaction to certain medicines commonly used in the treatment of cancer and osteoporosis (a disease that makes bones fragile). It is a painful condition that can be difficult to treat. MRONJ occurs rarely in people taking some medicines for osteoporosis. However, in people receiving these drugs at higher doses for cancer‐related conditions, the risk of MRONJ may be higher and has been reported to occur in up to 5 in 100 individuals. It is essential to obtain better treatments for people who have MRONJ. It is also important to identify effective preventive measures to reduce the risk of MRONJ.
Study characteristics
Working with Cochrane Oral Health, we searched for studies that had been published up to November 2016. We found three studies that focused on the prevention of MRONJ and two studies that tested treatments for MRONJ. The studies involved 1218 adults, with the smallest study having 40 participants and the largest study having 700 participants. Most study participants were women, but one study was of men with prostate cancer receiving bisphosphonate infusions (given by drip into a vein). All studies included only participants treated with bisphosphonates (used to support treatment and reduce risk of fracture and bone pain), although several other drugs are also known to induce MRONJ.
Key results
One study provided low‐quality evidence that dental examinations at three‐month intervals and preventive treatments (antibiotics before dental extractions and the use of techniques for wound closure that avoid exposure and contamination of bone) are more effective than standard care for reducing the number of cases with MRONJ in a group of people receiving intravenous bisphosphonates for cancer‐related conditions. In the experimental group (which received preventive care consisting of antibiotics and specific wound closure), fewer people developed MRONJ (2 participants per 100 who underwent close monitoring) compared with the control group (23 participants per 100 who had standard care).
There was insufficient evidence to conclude that the use of the other interventions investigated would reduce the risk of MRONJ or would improve healing of MRONJ.
Quality of evidence
The quality of evidence was low or very low. This was due to limitations in how the studies were designed and run. For example, some participants changed groups during the study, some participants did not finish the study, and the outcomes were measured at different follow‐up times.
Authors' conclusions
Summary of findings
| Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ | ||||||
| Population: prophylaxis of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard care (control) | Risk with dental examinations at three‐month intervals and preventive treatments (experimental) | |||||
| MRONJ (incidence proportion) (follow‐up: mean 32 months) | 233 per 1000 | 23 per 1000 | RR 0.10 | 253 | ⊕⊕⊝⊝ | Participants: high‐risk ( i.e. individuals with cancer exposed to intravenous zoledronic acid The outcome MRONJ was also reported as number of cases per patient‐year (incidence rate) rate ratio 0.18 (95% CI 0.04 to 0.74) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by two levels due to very serious risk of bias (high and unbalanced rate of crossovers after randomisation, high drop‐out rates due to high mortality, failure to adhere to the intention‐to‐treat principle, the mean follow‐up differed between experimental and control group). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions | ||||||
| Population: people treated with IV bisphosphonates who need dental extractions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with a standard dental extraction protocol without PRGF (control) | Risk with a dental extraction protocol with PRGF (experimental) | |||||
| MRONJ (incidence proportion) | 59 per 1000 | 5 per 1000 | RR 0.08 | 176 | ⊕⊝⊝⊝ | Participants: high risk, i.e. individuals with cancer exposed to IV zoledronic acid |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (high or unclear risk of selection bias, performance bias, detection bias, and attrition bias). IV = intravenous MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with conventional therapy (control) | Risk with hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) | |||||
| Healing of MRONJ (follow‐up: up to 24 months (outcome was measured at last follow‐up)) | 333 per 1000 | 520 per 1000 | RR 1.56 | 46 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, detection bias, and attrition bias; failure to adhere to the intention‐to‐treat principle). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with tetracycline fluorescence‐guided bone surgery (control) | Risk with autofluorescence‐guided bone surgery (experimental) | |||||
| Healing of MRONJ (follow‐up: 1 year) | 889 per 1000 | 933 per 1000 | RR 1.05 | 34 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, and detection bias). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
Background
Description of the condition
Medication‐related osteonecrosis of the jaw (MRONJ) is a severe adverse reaction experienced by some individuals to certain medicines commonly used in the treatment of cancer and osteoporosis (e.g. bisphosphonates, denosumab and antiangiogenic agents) and involves the progressive destruction of bone in the mandible or maxilla.
Osteonecrosis of the jaw (ONJ) associated with bisphosphonate treatment was first reported in 2003 (Marx 2003; Migliorati 2003; Ruggiero 2007; Sigua‐Rodriguez 2014). Subsequently, ONJ was observed in individuals who took denosumab, an antiresorptive medication unrelated to the bisphosphonate class (Bone 2017). A growing number of case reports currently suggest that ONJ is also associated with antiangiogenic agents such as bevacizumab, aflibercept, sunitinib, temsirolimus, and everolimus (Ruggiero 2014; Zhang 2016). The condition formerly referred to as 'bisphosphonate‐related ONJ' has been renamed 'medication‐related ONJ' due to the growing number of ONJ cases associated with non‐bisphosphonate treatments (Ruggiero 2014).
The exact mechanisms underlying MRONJ remain unknown. Interestingly, MRONJ is primarily limited to the maxillofacial region. In contrast to other skeletal bones, jaw bones (the alveolar process and periodontium) have relatively high vascularity, bone turnover, and remodelling because of continuous mechanical stress, which may make them vulnerable to the adverse effects of drugs. Proposed hypotheses that attempt to explain the localisation of MRONJ exclusively to the jaws include altered bone remodelling, angiogenesis inhibition, constant microtrauma, suppression of innate or acquired immunity, and possible effects of inflammation or infection (Ruggiero 2014).
According to the case definition provided by the American Society for Bone and Mineral Research and the American Association of Oral and Maxillofacial Surgeons, people may be considered to have MRONJ if all of the following characteristics are present: (i) current or previous treatment with antiresorptive or antiangiogenic agents, (ii) exposed or necrotic bone in the maxillofacial region that did not heal (by primary or secondary intent) within eight weeks after identification by a healthcare provider, (iii) no history of radiation therapy to the jaws, and (iv) no evidence of metastatic disease to the jaws (Ruggiero 2007; Sigua‐Rodriguez 2014). MRONJ has been divided into four stages based on clinical symptoms. Stage 0 describes individuals with prodromal disease (unexposed variant). Bone exposure is common in individuals with stage 1 to 3 MRONJ without infection (stage 1), with infection (stage 2), or with infection as well as a pathological fracture or fistula, or evidence of osteolysis extending to the inferior border of the mandible or sinus floor (stage 3) (Table 1) (Ruggiero 2007; Ruggiero 2014; Sigua‐Rodriguez 2014; Vescovi 2012a).
| MRONJ stage | Description |
| AT RISK | No apparent necrotic bone in patients who have been treated with oral or intravenous bisphosphonates |
| STAGE 0 | No clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms |
| STAGE 1 | Exposed and necrotic bone or fistulas that probes to bone in patients who are asymptomatic and have no evidence of infection |
| STAGE 2 | Exposed and necrotic bone or fistulas that probes to bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage |
| STAGE 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e. inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) resulting in pathologic fracture, extraoral fistula, oral antral, or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor |
From the American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update (Ruggiero 2014)
The frequency of MRONJ is highly variable and ranges from very rare (less than 1/10,000) to common (1/100 or more), depending on the drug, treatment indication (cancer versus osteoporosis), dose, and duration of treatment (Dodson 2015). For example, in randomised controlled trials (RCTs) and a meta‐analysis the incidence of MRONJ in individuals with cancer exposed to IV zoledronic acid was between 0.3 and 5% (Coleman 2011; Lopez‐Olivo 2012; Mauri 2009; Morgan 2010). The reported risk of MRONJ in individuals with cancer treated with denosumab ranged from 0.7% to 1.9% (Boquete‐Castro 2016; Qui 2014; Ruggiero 2014). A meta‐analysis that compared the safety of denosumab and zoledronic acid in individuals with bone metastases did not reveal a significant difference in the risk of MRONJ between the denosumab and zoledronic acid groups (Chen 2016).
Among individuals with osteoporosis, who receive substantially lower doses of bisphosphonates or denosumab than those with cancer, MRONJ is rare and the incidence may not be substantially greater than the natural background incidence of the condition. In people receiving bisphosphonates to treat osteoporosis, incidence estimates range from less than 0.1 to 0.7 cases per 10,000 patient years of exposure (Chamizo Carmona 2013; Grbic 2010). In a recent report studying people exposed to denosumab for treatment of osteoporosis, the incidence of MRONJ was 5.2 per 10,000 patient‐years (Bone 2017). The risk for MRONJ among people with osteoporosis treated with bisphosphonates or denosumab approximates the risk for MRONJ that is observed in placebo groups (Bone 2017; Grbic 2010). The risk of MRONJ among people exposed to antiresorptive medications for the treatment of osteoporosis is approximately 100‐fold smaller than the risk in people with cancer (Ruggiero 2014).
Evidence supporting the association of antiangiogenic medications with the development of MRONJ is primarily based on case reports. The frequency of MRONJ in people receiving antiangiogenic agents is not known accurately and reliably. Analysis of the United States Food and Drug Administration’s Adverse Event Reporting System database showed that the intravenous BPs were associated with the highest risk for MRONJ, denosumab was associated with risk comparable to bisphosphonates used for osteoporosis, and the antiangiogenic agents were associated with the lowest risk for MRONJ (Zhang 2016). In a combined analysis of three phase III trials the incidence of MRONJ in people exposed to the angiogenesis inhibitor bevacizumab was 0.2% (Guarneri 2010). The incidence was substantially higher in those exposed to both zoledronic acid and bevacizumab (Guarneri 2010).
The treatment of MRONJ is challenging, and an effective and appropriate therapy that substantially improves the outcome remains to be identified. The median time to resolution of osteonecrosis symptoms may be up to 12 months and depends on the specific therapeutic intervention (Hinson 2015). Additional information on the natural history of MRONJ comes from a report of individuals with multiple myeloma who were prospectively observed for a minimum of 3.2 years following diagnosis (Badros 2008). MRONJ resolved in 62% of cases, resolved and then recurred in 12%, and did not heal in 26%.
Antiresorptive medications associated with MRONJ
Bisphosphonates are osteotropic agents with antiresorptive activity that are used in a wide spectrum of indications such as the treatment and prevention of osteoporosis, as well as the treatment of Paget's disease, multiple myeloma, and malignancy‐associated hypercalcaemia. Bisphosphonates bind to bone hydroxyapatite and specifically inhibit the activity of osteoclasts, the bone‐resorbing cells. Bone turnover is thereby reduced, which results in an increase in the mineral density of the bone and a reduction in serum calcium (Chestnut 2001; Guyatt 2002; Ruggiero 2007; Sigua‐Rodriguez 2014). Bisphosphonates have a long retention time in bone, and effects may persist for some time after treatment has been stopped. There are two major risk categories for bisphosphonate‐related ONJ: (i) low risk in individuals without cancer treated with oral bisphosphonates (e.g. alendronic acid, clodronic acid, etidronic acid, ibandronic acid, and risedronic acid) or intravenous bisphosphonates (e.g. ibandronic acid and zoledronic acid) for osteoporosis, Paget’s disease, osteopenia, and osteogenesis imperfecta; and (ii) high risk in individuals with cancer treated with intravenous bisphosphonates (e.g. zoledronic acid, pamidronic acid, and ibandronic acid) for multiple myeloma and bone metastases (Bagan 2009; Ruggiero 2014; Vescovi 2012a). Additional parameters affecting the development of bisphosphonate‐related ONJ include the duration of bisphosphonate exposure, age, comedication, comorbidity, smoking, and oral health/oral hygiene (Bamias 2005; Dimopoulos 2006; Katsarelis 2015; Ruggiero 2014; Sigua‐Rodriguez 2014).
Denosumab, a potent antiresorptive agent, is used to treat osteoporosis in postmenopausal women and in men who have an increased risk of fracture. The recommended dose is 60 mg administered as a single subcutaneous injection once every 6 months. Denosumab is also used to prevent bone complications in adults with bone metastases from solid tumours and to treat a type of bone cancer called giant cell tumour of bone. The recommended maintenance dose for the latter indications is much higher, 120 mg every 4 weeks. Denosumab is a monoclonal antibody, which has been designed to attach to an antigen called RANK ligand (RANKL). By attaching to and blocking RANKL, denosumab reduces the formation and activity of osteoclasts, the cells in the body that are involved in breaking down bone tissue (Katsarelis 2015; Pageau 2009; Ruggiero 2014; Xu 2013). The exact pathophysiological mechanisms of denosumab‐related ONJ are currently unknown.
Antiangiogenic medications associated with MRONJ
Antiangiogenic agents are increasingly used as anticancer drugs for the treatment of renal cell carcinomas, gastrointestinal tumours, and other solid tumours. The drugs interfere with the formation of new blood vessels by inhibiting angiogenesis signalling cascades, such as vascular endothelial growth factor signalling (bevacizumab and aflibercept), mechanistic target of rapamycin signalling (temsirolimus and everolimus), or receptor tyrosine kinase signalling (sunitinib). MRONJ is a known, rare side effect of these agents, possibly resulting from their interaction with wound healing or osteoclast differentiation and survival (Patel 2015; Ruggiero 2014). Drug approval authorities (US Food and Drug Administration, European Medicines Agency) have included drug safety warnings in the drug labels of bevacizumab, aflibercept, and sunitinib regarding the risk of MRONJ.
Description of the intervention
Interventions for the prevention of MRONJ in at‐risk individuals or the management of MRONJ in individuals with manifest disease may include the following.
Prophylaxis of MRONJ
A range of dental prophylactic measures may be used alone or in combination. A primary means of prevention is the completion of all dental treatment (such as restorative therapy, root canal treatment, periodontitis therapy, or tooth extraction) before the commencement of antiresorptive or antiangiogenic therapy or as soon as possible following the commencement of antiresorptive or antiangiogenic therapy to ensure that treatment is completed within the specified ‘time frame’ for the intended agent. Antibiotic prophylaxis or antiseptic mouthwash (e.g. chlorhexidine) may be used. Individuals may take part in a preventive recall programme, or be provided with information regarding antiresorptive or antiangiogenic therapy risks, professional teeth cleaning, effective oral hygiene, and the importance of limiting or ceasing oral health risk behaviours (such as smoking and drug and alcohol use), or both. Surgical interventions may use a non‐traumatic surgical technique (i.e. surgical treatment designed to minimise tissue damage). The use of plasma rich in growth factors (PRGF) may promote bone and adjacent soft tissue regeneration in post‐extraction defects, thereby reducing the risk of MRONJ. To minimise wound exposure to bacteria, reconstructive surgical techniques for wound closure can be used. Some specific dental extraction methods recommend the discontinuation of antiresorptive or antiangiogenic agents before dentoalveolar surgery.
Treatment of MRONJ
For individuals with established MRONJ, the objective is to control infection, minimise necrosis progression, and promote tissue healing (Bagan 2009; Rollason 2016; Ruggiero 2014; Sigua‐Rodriguez 2014; Vescovi 2006; Vescovi 2012a). The standard medical care of MRONJ is currently anti‐infective treatment with systemic antibiotics or oral antiseptic rinses (e.g. chlorhexidine), or both, and surgical debridement or resection (Ruggiero 2014).
Non‐surgical treatment options
Healing may be stimulated by oral pentoxifylline and α‐tocopherol (vitamin E) in addition to antimicrobial therapy. Other options are adjunct hyperbaric oxygen (HBO) therapy, which involves breathing pure oxygen in a pressurised room or tube, or topical ozone therapy (OT) to improve healing. Low‐level laser therapy (LLLT) is also considered a promising adjunctive treatment method for MRONJ. The lasers most commonly used for biomodulation in bone are argon, carbon dioxide, helium/neon, and neodymium‐doped yttrium‐aluminium‐garnet. The use of (autologous) platelet‐rich plasma (PRP) has been suggested to enhance postsurgical wound healing. PRP is commonly used in a gel formulation, which is formed by mixing PRP (derived from the centrifugation of autologous whole blood) with thrombin and calcium chloride. PRP gel contains higher amounts of fibrinogen, platelets, and growth factors than whole blood. Moreover, bone may be restored by teriparatide, a recombinant form of parathyroid hormone. Teriparatide is approved for the treatment of osteoporosis but is used off‐label for other indications such as fracture healing, dental stability, and ONJ. Recombinant human bone morphogenetic proteins (rhBMPs), which also have the ability to induce osteogenesis, are another treatment option to enhance bone healing in MRONJ. After sequestrectomy, a carrier/scaffold (absorbable collagen sponge) that contains rhBMP is placed into the defect.
Surgical treatment options
Surgical treatments include sequestrectomy, debridement, resection, immediate reconstruction. Surgical treatment may also include extraction of teeth within exposed necrotic bone.
How the intervention might work
Prophylaxis of MRONJ
Controlling risk factors for MRONJ may represent an effective prophylaxis for MRONJ. MRONJ is a complication that can develop spontaneously after dentoalveolar surgery in combination with antiresorptive agents. Therefore, the completion of necessary elective dentoalveolar surgery before the start of this therapy may help reduce the risk of MRONJ (Ruggiero 2007; Ruggiero 2014). Another known risk factor is infection (Katsarelis 2015; Ruggiero 2014). Dental prophylaxis, caries control, and conservative restorative dentistry are expected to minimise the number of bacteria and eliminate the ports of entry for bacteria, thereby reducing the risk of infection. Regular dental evaluations during antiresorptive or antiangiogenic therapy may help to recognise significant risks at an early stage and enable prompt measures to be taken to counter them (Ruggiero 2014; SDCEP 2017). If surgery is necessary, for example, during bisphosphonate therapy, wound exposure to bacteria may be controlled by antibiotic prophylaxis, antiseptic mouthwash, or both. Choosing of surgical procedures that help minimise bone exposure or trauma to the jaws may reduce the risk of MRONJ. Platelet‐derived growth‐factor preparations, such as PRP and PRGF, applied at the surgical site may accelerating wound healing and reduce the time of increased infection risk. Stopping antiresorptive drugs prior to an invasive dental procedure (drug holiday) could be useful for prevention of MRONJ. Due to the pharmacokinetics, the antiresorptive effect of bisphosphonates and denosumab is maintained for several weeks or months. This would require cessation of antiresorptive therapy for at least two months to significantly reduce the risk of MRONJ during invasive dental procedures (Ruggiero 2014; Damm 2013).
Treatment of MRONJ
Treatment objectives for people with a defined diagnosis of MRONJ are to control infection of the soft and hard tissues, and minimise the progression or occurrence of bone necrosis to optimise wound healing. Stage‐dependent strategies to treat MRONJ have been proposed (Ruggiero 2014), which can be classified into non‐surgical and surgical treatment.
Non‐surgical treatment options
Non‐surgical management includes, for example, drug treatment with teriparatide, which is a recombinant form of parathyroid hormone that stimulates osteoblasts to increase bone density when used intermittently. Alternative options are treatment with pentoxifylline and α‐tocopherol in combination with anti‐microbial therapy, OT, HBO, and LLLT (Vescovi 2012a). Pentoxifylline and α‐tocopherol have been used to treat osteoradionecrosis for many years. Pentoxifylline, a methylxanthine derivative and phosphodiesterase inhibitor, improves blood flow by increasing erythrocyte flexibility and vasodilatation, and modulates immunological activity; α‐tocopherol has antioxidant properties (Epstein 2010); pentoxifylline and α‐tocopherol may play a role in encouraging wound healing and reducing scarring; ozone has antimicrobial and wound‐healing properties, and OT as an adjunct treatment has been hypothesised to induce the repair of tissues by cleansing osteonecrotic lesions, which leads to mucosal healing (Petrucci 2007; Ripamonti 2011). HBO has been shown to be effective in addition to conventional therapies to treat osteoradionecrosis (Bennett 2016). HBO has been proven to stimulate new blood vessel growth within the damaged tissues and to improve the availability of oxygen for wound healing. Thus, HBO has been hypothesised to be a useful adjunctive treatment for MRONJ (Freiberger 2009). Phototherapy with a low‐intensity laser is used as an adjunctive therapy for treating several diseases including wounds. The laser light used with LLLT lies within the red visible and near infrared wavelengths, promoting biological effects, such as inflammation and angiogenesis; it also increases the inorganic matrix, which may support wound healing (Martins 2012; Vescovi 2006). Platelet‐derived growth‐factor preparations, such as PRP and PRGF, are applied at the surgical site as an adjuvant to stimulate regeneration of osseous and epithelial tissues, thereby accelerating wound healing. Platelet‐derived growth‐factors are proposed to support angiogenesis and to improve bone formation by enhancing osteoblast formation and activity (Lee 2007; Lopez‐Jornet 2016). rhBMP is used in surgical procedures to improve bone formation and remodelling during bone healing by enhancing the effects of osteoblast formation and activity (Gerard 2014).
Surgical treatment options
Surgical treatments may include a more conservative approach, such as sequestrectomy and surgical debridement or aggressive therapies, such as resections of affected bone with reconstruction. One of the advantages of using a more conservative surgical approach like sequestrectomy is that a better healing should be expected since the periosteum and unaffected bone are conserved (Eckardt 2011; Stanton 2009; Comas‐Calonge 2017).
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important to maintain on the Cochrane Library (Worthington 2015). This review was identified as a new priority title by the oral and maxillofacial surgery expert panel (Cochrane Oral Health priority review portfolio).
Among the drugs associated with MRONJ, bisphosphonates are by far the most widely used for a wide range of clinical indications. For example, bisphosphonates can be used in breast cancer and prostate cancer, which have the highest sex‐related incidence rates worldwide. Osteoporosis, another common indication for bisphosphonates, is estimated to affect 200 million women worldwide: approximately one‐tenth of women aged 60 years, one‐fifth of women aged 70 years, two‐fifths of women aged 80 years, and two‐thirds of women aged 90 years (Kanis 2007). Moreover, several other drugs (denosumab, antiangiogenic medications) have recently been associated with MRONJ. MRONJ may occur as a common side effect, particularly in individuals with cancer, depending on the drug and the dosage used. Therefore, the population at risk for MRONJ is large and expanding, and the public health implications may be substantial.
MRONJ significantly affects quality of life (QoL) and the decline in QoL correlates with MRONJ stage (Kyrgidis 2012; Miksad 2011). The following factors contribute to impairment of QoL: (i) infected and painful necrotic jaw bone; (ii) ulcerated, painful, and swollen oral mucosa; (iii) chronic sinus tracts and facial disfigurement; (iv) impaired speech, swallowing, and eating; and (v) frequent medical and dental evaluations and treatments (Migliorati 2010). Rehabilitation after a complete cure of MRONJ is often protracted. A further aggravating circumstance is a high risk of recurrence, which is higher than in other diseases of the jaw bone. Thus, it is important to develop strategies to prevent or manage MRONJ. Preventative dentistry may be shown to decrease the incidence of MRONJ, in which case the implementation of preventive strategies will become an important consideration for individuals, clinicians, and policy makers (Dimopoulos 2006; Ripamonti 2009). Epidemiological studies have shown that the risk of MRONJ increases with a longer duration of treatment and with higher drug doses. Effective measures to prevent and treat MRONJ may significantly improve the risk‐benefit balance, in particular for people requiring long‐term or high‐dose therapy.
However, there is uncertainty regarding how to prevent MRONJ before and during bisphosphonate therapy and how to manage manifest MRONJ (Lopez‐Jornet 2010). As a consequence, current recommendations are contradictory in certain respects (Ruggiero 2014; SDCEP 2017). This review complements and extends the previous Cochrane review by Rollason 2016, which focused on interventions for treating ONJ associated with bisphosphonate drugs.
Objectives
To assess the effects of interventions versus no treatment, placebo, or an active control for the prophylaxis of MRONJ in people exposed to antiresorptive or antiangiogenic drugs.
To assess the effects of non‐surgical or surgical interventions (either singly or in combination) versus no treatment, placebo, or an active control for the treatment of people with manifest MRONJ.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing one modality of intervention with another for the prevention or treatment of MRONJ. We excluded quasi‐randomised and non‐RCTs, as well as case studies, case series (or those of case series design), and cross‐sectional studies. We did not exclude studies on the basis of language, publication status or date of publication.
Types of participants
To assess preventive strategies, we included participants who were treated with known risk medications and who had not yet developed MRONJ before assignment to the experimental or control group.
To assess interventions to treat MRONJ, we included people who had developed clinically apparent MRONJ. Case definition included exposure to risk drug and the presence of necrotic bone or fistulae that probes to bone.
We applied no restrictions regarding participant sex, age, initial health status, and pre‐existing conditions, or type of ONJ‐related drug (e.g. alendronic acid, clodronic acid, etidronic acid, ibandronic acid, incadronic acid, olpadronic acid, pamidronic acid, risedronic acid, tiludronic acid, zoledronic acid, denosumab, bevacizumab, aflibercept, sunitinib, temsirolimus, or everolimus), dose, or duration of therapy. To comply with the MRONJ case definition (Ruggiero 2014), we did not include participants with a history of head and neck radiation therapy.
Types of interventions
For prophylaxis of MRONJ
Any intervention (before or after commencement of antiresorptive or antiangiogenic drug therapy) that aims at prevention of MRONJ. Examples of interventions discussed in the literature include the following.
-
Completion of all necessary dental treatment before the commencement of antiresorptive or antiangiogenic agents or as soon as possible following commencement of antiresorptive or antiangiogenic agents
-
Antibiotic prophylaxis or antiseptic mouthwash
-
Preventive recall programme and provision of information for patients
-
Non‐traumatic surgery (i.e. surgical treatment designed to minimise tissue damage), reconstructive techniques for wound closure to minimise wound exposure to bacteria, and specific dental extraction protocols
-
Supportive measures to accelerate wound healing after surgery, such as platelet‐rich plasma (PRP) and plasma rich in growth factors (PRGF)
-
Cessation of therapy with antiresorptive or antiangiogenic agents (‘drug holiday’) before invasive dental procedures
For treatment of MRONJ
Any intervention (non‐surgical, surgical, or a combination of both) that aims to treat clinically manifest MRONJ. Examples of interventions discussed in the literature include the following.
-
Non‐surgical
-
Antiseptic mouthwashes
-
Antibiotic and antifungal therapy
-
Parathyroid hormone and teriparatide
-
Pentoxifylline and α‐tocopherol
-
Ozone therapy (OT)
-
Hyperbaric oxygen therapy (HBO)
-
Laser therapy (low‐level laser therapy (LLLT))
-
Platelet‐derived growth‐factor preparations, such as PRP and PRGF
-
Recombinant human bone morphogenetic proteins (rhBMPs)
-
-
Surgical
-
Surgical debridement, sequestrectomy
-
Jaw bone resection
-
Extraction of teeth within exposed necrotic bone
-
Comparisons: any single or combined experimental intervention versus control. The control arm consisted of participants receiving no treatment, placebo, or an active control (e.g. standard care).
Types of outcome measures
Primary outcomes
Prophylaxis of MRONJ
Incidence of MRONJ
Two related measures are often used to describe the incidence of MRONJ: incidence proportion (cumulative incidence) and incidence rate of MRONJ. As the incidence rate of MRONJ peaks after two to four years of exposure to bisphosphonates or denosumab in individuals with cancer (Henry 2011; Nakamura 2015; Saad 2012), we had originally planned to include only trials with a follow‐up period of at least three years for the primary outcome. However, we found that the three‐year follow‐up threshold was not applicable as a strict selection criterion for the following reasons: a large proportion of individuals with metastatic cancer (i.e. those most likely to be affected by MRONJ) may die before reaching a three‐year follow‐up. Moreover, follow‐up periods were reported inconsistently between studies (mean follow‐up versus range, follow‐up period of the total study population versus that for each study arm separately, follow‐up per protocol versus follow‐up period as observed).
Treatment of MRONJ
Healing of MRONJ
There is no standardised scale for the assessment of MRONJ healing. Healing of MRONJ may be defined based on clinical examination, imaging findings, or both. Wound healing may be defined as absolute area healed per day, percentage of initial area healed per day, and advance of the wound margin towards the wound centre per day. Wound healing may also be defined as the time taken for mucosa to completely cover necrotic tissue and exposed bone (‘cure period’). Number of participants with resolution of MRONJ (defined as mucosal healing with covering of the area of exposed bone) within a prespecified period of time (e.g. one year) may also be used to describe the healing of MRONJ. Follow‐up time should be at least one year for this primary outcome.
Secondary outcomes
Prophylaxis of MRONJ
-
Quality of life (QoL)
-
Time‐to‐event
-
Rate of complications and side effects of the intervention
Treatment of MRONJ
-
QoL
-
Recurrence
-
Rate of complications and side effects of the intervention
For the outcome 'complications', if the intervention involved interruption/delay of antiresorptive or antiangiogenic treatments, progression of the underlying disease (e.g. fracture in osteoporosis or disease progression in cancer), these were considered to be complications of the intervention.
For QoL measures, we reported whether validated scales were used. Non‐validated scales were not excluded a priori. QoL had to have been measured at baseline and at least once during follow‐up.
Search methods for identification of studies
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for RCTs. Due to the Cochrane Crowd Project, which aimed to identify all clinical trials on the Embase database and add them to CENTRAL, only recent months of the Embase database were searched. Please see the Cochrane Oral Health website for more information.
Electronic searches
Cochrane Oral Health’s Information Specialist searched the following databases for relevant trials:
-
Cochrane Oral Health’s Trials Register (searched 23 November 2016) (see Appendix 1);
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) in the Cochrane Library (searched 23 November 2016) (see Appendix 2);
-
MEDLINE Ovid (1946 to 23 November 2016) (see Appendix 3);
-
Embase Ovid (23 May 2016 to 23 November 2016) (see Appendix 4).
The subject strategies for databases were modelled on the search strategy designed for MEDLINE Ovid in Appendix 3. This was combined with subject strategy adaptations of the Highly Sensitive Search Strategy designed by Cochrane for identifying RCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c. (Lefebvre 2011)).
Searching other resources
The following trial registries were searched for ongoing studies:
-
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov; searched 23 November 2016) (see Appendix 5);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 23 November 2016) (see Appendix 6).
We asked experts in the field to help identify unpublished literature and searched the reference lists of potential clinical trials in an attempt to identify any study not found by the other searches.
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (NB, HH) independently assessed the titles and abstracts of each paper identified by the review search strategy. We excluded only clearly irrelevant records at this stage. Following this, we obtained the full text of potentially relevant studies and assessed these for eligibility based on the inclusion criteria as outlined above. In the event that the two review authors could not reach a consensus, another review author (OZ) acted as arbiter. We maintained a detailed log of study eligibility and reasons for exclusion, and recorded these in 'Characteristics of excluded studies' tables.
Data extraction and management
Two review authors (NB, HH) independently collected details from the included trials using a structured form. If necessary, a third review author (OZ) was consulted to resolve inconsistencies. We extracted the following details and entered them into 'Characteristics of included studies' tables in Review Manager 5 (RevMan 5) (RevMan 2014).
-
Methods
-
Trial design
-
Duration of study
-
Sample size calculation
-
Country of origin
-
Year of publication
-
Language of the original publication
-
Category (i.e. prophylaxis or treatment of MRONJ)
-
Funding
-
Registration in a public trials registry
-
-
Participants
-
Number of participants
-
Age
-
Sex
-
Condition treated with antiresorptive or antiangiogenic agents
-
Inclusion criteria
-
Exclusion criteria
-
-
Interventions (i.e. the type of intervention and procedural information)
-
Outcomes
-
Primary outcomes
-
Secondary outcomes
-
We planned to contact study authors to ask for further information or clarification of their data if necessary.
Assessment of risk of bias in included studies
Two review authors (NB, HH) independently assessed the risk of bias in the included studies according to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the included trials for risk of bias (high, low, or unclear) in the following key domains:
-
random sequence generation (allocation bias);
-
allocation concealment (allocation bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessors (detection bias);
-
incomplete outcome data (attrition bias);
-
selective reporting (reporting bias).
'Unclear’ indicates either a lack of information or uncertainty over the potential for bias. We completed a 'Risk of bias' table for each study and presented the results graphically by study and by domain over all studies. If the risk of bias was not clear because of a lack of detail in the studies, we planned to contact the study authors to request further information.
We categorised overall risk of bias by outcome as shown in the table below.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
We used RevMan 5 (RevMan 2014) to perform the analyses.
For continuous data, we planned to calculate the mean differences and 95% confidence intervals (CI). We planned to report continuous outcomes as means and standard deviations. When studies used different instruments to measure the same construct, we planned to use the standardised difference in means in the analysis to combine the data.
For dichotomous outcomes, we calculated risk ratios (RR) along with 95% CI from cumulative incidence data. In cases of reported incidence rates, the rate ratio was the effect measure of choice.
To summarise time‐to‐event data, we planned to use methods of survival analysis and we planned to express the intervention effect as a hazard ratio, along with 95% CI.
Where insufficient information was reported to enable effect measures to be calculated, we provided a narrative report of the summary measures.
Unit of analysis issues
The individual participant was the unit of analysis.
If there was a choice of timepoints for a primary outcome, we selected the timepoint closest to 3 years for prophylaxis and 1 year for treatment. We avoided multiple testing of the effect at each of the timepoints.
Dealing with missing data
We attempted, where feasible, to contact authors from the primary studies to obtain missing data. We used the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions to estimate the missing standard error of the log rate ratio (Higgins 2011).
Assessment of heterogeneity
To identify and measure the statistical heterogeneity of the data, we planned to use the I² statistic (Higgins 2003). This value (percentage) defines the variability in effect estimates between studies that is beyond what would be expected by chance. The I² value can be categorised as not important (0% to 40%), moderate heterogeneity (30% to 60%), substantial heterogeneity (50% to 90%), and very substantial heterogeneity (75% to 100%) (Higgins 2003). We also planned to use graphical displays, such as Galbraith plots, if appropriate. Galbraith plots enable the display of several estimates of the same quantity having different standard errors; this is why they provide a useful way of checking for the presence of heterogeneity (Anzures‐Cabrera 2010; Copas 2009). Clinical diversity (i.e. variability in the participants, interventions, and outcomes studied) may contribute to statistical heterogeneity. If a sufficient number of studies was included, we planned to explore heterogeneity by conducting subgroup analyses. If there was substantial evidence for between‐study heterogeneity, we planned to use a random‐effects meta‐analysis.
Assessment of reporting biases
If there had been sufficient studies, we would have assessed publication bias using methods based on a funnel plot, such as Egger's test (Egger 1997). However, all publication bias methods were characterised by a relatively low power and could not be assumed to prove or exclude publication bias (Higgins 2011).
Data synthesis
We followed the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions for the statistical analysis of results (Higgins 2011). If the studies had been sufficiently similar with respect to the participants included, interventions compared, and outcomes and timepoints reported, we would have conducted meta‐analyses. We would have used a random‐effects or fixed‐effect meta‐analysis as appropriate to combine quantitative data. For comparisons in which a meta‐analysis could not be carried out, we have provided a narrative report of the summary measures and treatment effects.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity (i.e. differences associated with the participants, interventions, or outcomes across the included studies) may contribute to statistical heterogeneity (i.e. differences in the effects of interventions). If a sufficient number of studies were included, we planned to explore heterogeneity by conducting subgroup analyses in any case (i.e. whether statistical heterogeneity was present or not). To assess the effect of particular aspects of the studies on the primary and secondary outcome variables, we had planned to conduct the following subgroup analyses: medication dose or dose intensity (i.e. unit dose of medication administered per unit time); medication type (e.g. nitrogenous or non‐nitrogenous bisphosphonate) or compound; stage and type of disease (e.g. cancer or non‐cancer); and risk factors (e.g. multimorbidity, age, smoker). If we had included at least 10 studies, we would have investigated these effects using a meta‐regression analysis.
In the case of significant statistical heterogeneity, we would have attempted to identify the source of the heterogeneity with subgroup analyses.
Sensitivity analysis
If there had been sufficient RCTs for meta‐analyses, we would have performed a sensitivity analysis to check the robustness of results when omitting studies with high or unclear risk of bias or to investigate whether the meta‐analysis result was heavily determined by outlier studies. We would have used the Galbraith plot to detect potential outliers.
Presentation of main results
We have developed a 'Summary of findings' table for each comparison, and have presented summary information for the primary and secondary outcomes.
Following GRADE methods and using GRADEPro software (GRADEPro 2014), two review authors (NB and OZ) assessed the quality of evidence with reference to the overall risk of bias of the included studies, directness of the evidence, consistency of the results, precision of the estimates, and risk of publication bias. Factors that may lead to downgrading of evidence in the GRADE approach are: (a) risk of bias, (b) inconsistency between studies, (c) indirectness, (d) imprecision, and (e) likely publication bias. Factors that may lead to upgrading are: (a) large effect size, (b) dose‐response gradient, (c) if all plausible confounding would reduce a demonstrated effect, and (d) if all plausible confounding would suggest a spurious effect when the actual results show no effect. We assessed the quality of the body of evidence for each comparison and outcome as high, moderate, low, or very low.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The search retrieved 1105 references after de‐duplication. After screening the titles and abstracts, we excluded all but 23 references from further evaluation. We examined the full text of the remaining 23 articles and found that eight references relating to five studies met the prespecified inclusion criteria and were therefore included in this review. We identified four additional studies that are ongoing and listed these under Characteristics of ongoing studies. We excluded 11 full‐text articles for reasons noted in the Characteristics of excluded studies table. The flow diagram (Figure 1) displays the study selection process.

Study flow diagram. Results of the search strategy for inclusion of studies in this review
Included studies
We included five studies in this review (Freiberger 2012; Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016). For details, see the Characteristics of included studies table. Three studies focused on the prophylaxis of MRONJ (Mozzati 2012; Mozzati 2013; Mücke 2016). Two trials investigated options for the treatment of MRONJ (Freiberger 2012; Ristow 2016). The trials varied in sample size between 40 (Ristow 2016) and 700 participants (Mozzati 2013). In total, 1218 participants were included in this review. More women than men took part in the studies, with the exception of one study (Mücke 2016), which recruited only men with prostate cancer.
Prophylaxis of MRONJ
Mücke 2016 involved 253 men with prostate cancer and bone metastases who received treatment with intravenous zoledronic acid. This study was conducted at the University of Munich, Germany, from 2008 to 2014. All participants had baseline assessments and treatments, if necessary, before the start of bisphosphonate therapy. Participants in the control group were monitored and treated when deemed necessary by the participant's dentist and were re‐evaluated once per year. In the experimental group, the participants were closely monitored and treated when necessary at 12‐week intervals. Thiry‐six of 126 participants randomly allocated to the experimental group refused close monitoring and changed to the control group. The primary outcome was the incidence of MRONJ. The major diagnostic criterion of MRONJ was non‐healing exposed bone in the mandible or maxilla for longer than eight weeks. The incidence of MRONJ was calculated as the incidence rate (i.e. the number of people developing MRONJ per patient‐years) and incidence proportion (i.e. number of people developing MRONJ relative to the number people in the study group). Follow‐up was at least two years in the control group and at least one year in the experimental group. The effect on QoL was not investigated. Time‐to‐event data were not provided.
Mozzati 2012 included 176 individuals with cancer treated with intravenous bisphosphonates who underwent dental extractions. Participants recruited from January 2005 to December 2009 at the University of Torino, Italy, were randomly allocated to the experimental group treated with PRGF, which was inserted into the postextraction alveolus, or the control group without PRGF. All participants had a professional oral hygiene session one week before surgery and antibiotics for six days starting the evening before surgery. Surgical care included anaesthesia by alveolar nerve block, no intraligamentous or intrapapillary infiltrations, mucosal flap, and suturing to enable healing via primary intention. After surgery, the participants were monitored (at 3, 7, 14, 21, 30, 60, 90, and 120 days, and thereafter every 6 months) for clinical signs of MRONJ, such as pain, swelling, and non‐healing exposed necrotic bone or fistulae, or both, with connection to the bone. Follow‐up was between 24 and 60 months. The primary outcome was the development of MRONJ. Intraoperative complications and time‐to‐event were recorded. QoL was not investigated.
Another RCT by Mozzati et al. prospectively compared two surgical protocols with different degrees of invasiveness for tooth extraction in people undergoing treatment with oral bisphosphonates (Mozzati 2013). Conditions treated with bisphosphonates were osteoporosis, rheumatoid arthritis, and Paget's disease. A total of 700 participants recruited from January 2005 to April 2011 at the University of Torino, Italy, were randomly assigned to delicate surgery and wound closure by primary intention or non‐traumatic avulsion and wound closure by secondary intention. In the first group, surgical extraction was carried out via an intrasulcular incision and mobilisation of a mucoperiosteal flap. In the second group, extraction was carried out without detachment of full‐thickness flaps, and sockets were filled with absorbable haemostatic gelatin sponges. After surgery, participants were monitored (at 3, 7, 14, 21, 30, 60, and 90 days, and thereafter every 6 months) for clinical signs of MRONJ, such as pain, swelling, non‐healing exposed necrotic bone or fistulae, or both, with connection to the bone. Follow‐up was between 12 and 72 months. The primary outcome was the success rate, defined as the proportion of participants without clinical signs of postoperative MRONJ. Intraoperative complications were recorded. QoL was not investigated.
Treatment of MRONJ
Freiberger 2012 tested HBO as an adjunct to routine surgery and antibiotics in the treatment of MRONJ caused by bisphosphonate use. A cohort size of 70 participants was planned for the study. From July 2006 to December 2010, the trial screened 133 people for eligibility, and 49 people with MRONJ were randomised to receive standard care with or without HBO. MRONJ in these people was related to the use of zoledronic acid, pamidronic acid, or alendronic acid for the treatment of multiple myeloma, breast cancer, osteoporosis, or other indications. Treatment for MRONJ included surgical debridement at the discretion of the referring surgeon and antibiotics for any sign of local infection. Participants in the HBO group received 40 HBO sessions at 2 atmospheres of pressure for 2 hours each over 4 weeks. The study participants had scheduled follow‐up visits at 3, 6, 12, and 18 months, and received 14 months of weekly status checks by telephone or email. Eighteen participants completed the full 24‐month observation period. After randomisation, six participants changed from their allocated treatment arm to the alternative trial arm. For the primary outcome, oral lesions were scored by size and number, and a change in lesion scores compared with the baseline condition was used to grade the primary outcome. Possible outcome categories were healed (defined as gingival coverage with no exposed bone), improved, unchanged, or worse. Secondary outcomes were QoL (Duke Health Profile instrument), laboratory measures of bone turnover, and molecular indicators of osteoclast activation, such as RANK, RANKL, OPG, and pAKt. The rate of complications was not reported. The trial received financial support from the Novartis pharmaceutical company.
Ristow 2016 compared autofluorescence‐guided bone resection with tetracycline fluorescence‐guided bone resection for the treatment of MRONJ. Forty participants suffering from MRONJ due to the use of antiresorptive medication (bisphosphonates with or without denosumab) for the treatment of cancer (85%) or osteoporosis (15%) were included. The major challenge in bone surgery in MRONJ is the delineation between necrotic and viable bone to ensure complete removal of necrotic bone while preserving as much vital bone as possible. In this open‐label trial, 20 randomly assigned control participants received preoperative doxycycline, which is incorporated into viable bone and is visualised with a certified medical lamp intraoperatively. Twenty participants in the experimental group received ampicillin/sulbactam (or clindamycin 600 mg in case of hypersensitivity to penicillin or a penicillin allergy) preoperatively without doxycycline labelling. Autofluorescence of vital bone, which was induced with a special fluorescence lamp (provided for the study by the manufacturer), was used to visualise vital bone intraoperatively. The primary outcome was success rate, defined as the absence of a MRONJ site after surgery (i.e. full mucosal coverage at eight weeks after surgery). Secondary endpoints were mucosal integrity at the remaining measurement timepoints, loss of sensitivity (numbness) of the alveolar nerve (Vincent sign), subjective pain, and signs of infection. Participants were monitored at 10 days, 8 weeks, 6 months, and 1 year after surgery. QoL was not investigated. Rate of complications was not reported.
Excluded studies
After evaluation of the full‐text articles, we excluded 11 studies because they were not RCTs (Asaka 2016; Bonacina 2011; Bramati 2015; Coviello 2012; Dimopoulos 2009; DE Iuliis 2014; Lee 2014; Montebugnoli 2007; Pelaz 2014; Vescovi 2010; Vescovi 2012a). Four studies are ongoing and study results are not yet available (ACTRN12612000950864; NCT01526915; NCT02198001; UMIN000009132). See the Characteristics of excluded studies and Characteristics of ongoing studies.
Risk of bias in included studies
See 'Risk of bias' in the included studies as a graphical overview in Figure 2. See Characteristics of included studies tables for more details about our 'Risk of bias' assessments.
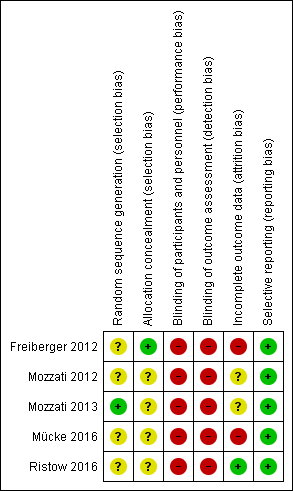
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
In all five trials, participants were randomly divided into two groups (Freiberger 2012; Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016). The authors of four trials did not mention the generation of randomisation sequence and we therefore rated the level of risk as unclear (Freiberger 2012; Mozzati 2012; Mücke 2016; Ristow 2016). The method of sequence generation was noted in only one study: the participants were assigned by a computer randomisation programme, and we judged the level of risk to be low (Mozzati 2013). Freiberger 2012 did not report the method of sequence generation but reported the concealment of allocation using a series of 70 opaque envelopes containing the assignment (judged as low risk). Allocation concealment was not reported for the other studies, and we rated the risk level as unclear (Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016).
Blinding
Personnel were not blinded in all studies, either because of the nature of the intervention (Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016) or because blinding was deemed impractical (Freiberger 2012). Outcome assessors were not blinded in three studies (Freiberger 2012; Mücke 2016; Ristow 2016). Although not reported, masking of outcome assessors was most likely not present in the other two studies (Mozzati 2012; Mozzati 2013). Therefore, we considered the level of risk for performance and detection bias to be high for all studies.
Incomplete outcome data
We assessed the level of risk as unclear in two studies because completeness or loss to follow‐up was not reported (Mozzati 2012; Mozzati 2013).
We judged attrition bias to be high in Freiberger 2012 and Mücke 2016. Although a clear description of losses and withdrawals was given, data analysis was performed as‐treated and not by intention‐to‐treat, and both studies had a high and unbalanced rate of crossovers between study arms. Neither study reported data in a format that would have enabled us to recalculate effects on an intent‐to‐treat basis.
In Ristow 2016, some participants were lost for the assessment of secondary endpoints. However, no participants were lost for the assessment of the primary endpoint; hence, we rated attrition bias as low (Ristow 2016).
Selective reporting
Outcomes defined in the methods sections of the papers (Freiberger 2012; Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016) and the study protocol at ClinicalTrials.gov (Freiberger 2012) were completely reported with the exception of one study. Freiberger 2012 did not report some predefined secondary outcomes, such as the results of serum measurements of bone turnover and molecular measures of osteoclast signalling. However, the authors stated that these results will be presented separately (Freiberger 2012). Altogether, we considered the risk of reporting bias to be low for all studies (Freiberger 2012; Mozzati 2012; Mozzati 2013; Mücke 2016; Ristow 2016).
Effects of interventions
See: Summary of findings for the main comparison Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ; Summary of findings 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions; Summary of findings 3 Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ; Summary of findings 4 Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ
Prophylaxis of MRONJ
Regular dental examinations at three‐month intervals and preventive treatments versus standard care for the prophylaxis of MRONJ in men with metastatic prostate cancer and intravenous zoledronic acid
We identified one study with 253 participants that explored the preventive effect of a prophylactic treatment to reduce MRONJ in men with metastatic prostate cancer treated with zoledronic acid (Mücke 2016). The study compared regular dental examinations at three‐month intervals and preventive treatments (including antibiotics before dental extractions, and the use of techniques for wound closure that avoid exposure and contamination of bone) versus standard care (i.e. monitoring and treatment if necessary at the discretion of the participant's dentist).
Incidence of MRONJ
Our primary outcome, incidence of MRONJ, was reported as incidence rate per year and incidence proportion. MRONJ was defined as the non‐healing of exposed bone in the mandible or maxilla for longer than eight weeks without any change in the stage of disease. Mean follow‐up time was 28.8 months. Regular dental examinations at three‐month intervals and preventive treatments showed a lower risk ratio (RR) for MRONJ (0.10; 95% CI 0.02 to 0.39) compared to standard care when dental extractions were performed. There was also a significant difference in the number of MRONJ cases per patient‐years (rate ratio 0.18; 95% CI 0.04 to 0.74). We rated the quality of the evidence for the primary outcome to be low. See summary of findings Table for the main comparison, Analysis 1.1, and Analysis 1.2.
Plasma rich in growth factors inserted into the postextraction alveolus in addition to standardised medical and surgical care versus standardised medical and surgical care alone for MRONJ prophylaxis in individuals treated with intravenous bisphosphonates who underwent dental extractions
One RCT reported the effect of PRGF for preventing MRONJ in 176 participants with cancer undergoing dental extractions (Mozzati 2012).
Incidence of MRONJ
The diagnosis of MRONJ was based on clinical examination and radiographic examinations. Clinical signs of MRONJ were pain, swelling, and non‐healing exposed necrotic bone or fistulae, or both, with connection to the bone. The study group had a total follow‐up period of 24 to 60 months. At the last contact, no participants in the PRGF group (N = 91) but five participants in the control group (N = 85) developed MRONJ. The RR was 0.08 (95% CI 0.00 to 1.51). We rated the quality of the evidence for the primary outcome to be very low. See summary of findings Table 2 and Analysis 2.1.
Rate of complications and side effects of the intervention
No intraoperative complications were observed in either of the groups.
Delicate surgery and closure by primary intention versus non‐traumatic tooth avulsion and closure by secondary intention for the prophylaxis of MRONJ in individuals treated with oral bisphosphonates who underwent dental extractions
One RCT with 700 participants compared wound closure by primary intention with wound closure by secondary intention after dental extractions in individuals treated with oral bisphosphonates (Mozzati 2013).
Incidence of MRONJ
The participants were regularly monitored for clinical signs of MRONJ: pain, swelling, and non‐healing exposed necrotic bone or fistulae, or both, with connection to the bone. In both study arms, no case of postoperative MRONJ was observed.
Rate of complications and side effects of the intervention
No intraoperative complications were observed in either of the two groups.
Treatment of MRONJ
We identified two RCTs assessing the effect of different treatment protocols in people with manifest MRONJ (Freiberger 2012; Ristow 2016).
Hyperbaric oxygen therapy in addition to standard care (antiseptic rinses, antibiotics, surgery) versus standard care
One RCT with 49 participants analysed the healing of MRONJ using HBO treatment in addition to standard care (antiseptic rinses, antibiotics, surgery) (Freiberger 2012). All participants terminated bisphosphonate administration before or at the time of consent, with the exception of one who continued bisphosphonate administration for one month after the initial examination.
Healing of MRONJ
Oral lesions were graded by size and number, and staged by clinical severity. The last contact was intended to be 24 months after consent; however, only 18 participants completed the full 24‐month observation period. Healing was defined as gingival coverage with no exposed bone. HBO in addition to standard care did not significantly improve healing from MRONJ at any of the investigated timepoints (at last follow‐up: RR 1.56; 95% CI 0.77 to 3.18). We rated the quality of the evidence for the primary outcome to be very low. See summary of findings Table 3 and Analysis 3.1.
Quality of life
QoL was measured using the Duke Health Profile, a 17‐question generic self‐reporting instrument with six health domains (physical, mental, social, general, perceived health, and self‐esteem) and four dysfunction measurements (anxiety, depression, pain, and disability) (Freiberger 2012). QoL assessments were recorded at the time of the initial interview and at six months. Only within‐group comparisons for each domain were provided based on a dichotomous classification (‘improved’, ‘no change, or worse’). Because no score values were provided, we were unable to make a between‐group analysis.
Autofluorescence‐guided bone surgery versus tetracycline fluorescence‐guided bone surgery in individuals with MRONJ referred for surgical treatment
One RCT with 40 participants compared autofluorescence‐guided and tetracycline fluorescence‐guided bone surgery for the treatment of MRONJ (Ristow 2016).
Healing of MRONJ
The primary endpoint reported by Ristow 2016 was success rate. Success was defined as the absence of a MRONJ site after surgery, specified as the maintenance of full mucosal coverage (mucosal integrity) after surgery at the time of the evaluation. All measurements were acquired at five specific timepoints: preoperatively, and 10 days, 8 weeks, 6 months, and 1 year after surgery. There was no significant difference between the autofluorescence‐ and the tetracycline fluorescence‐guided groups at any of the timepoints (at one‐year follow‐up: RR 1.05; 95% CI 0.86 to 1.30). We rated the quality of the evidence for the primary outcome to be very low. See summary of findings Table 4 and Analysis 4.1.
Discussion
At present, the mechanisms of MRONJ are not well known, and the prevention and treatment of MRONJ remains challenging. Thus, it is important to identify effective strategies for managing this well‐known complication of antiresorptive medication.
Summary of main results
We identified three randomised controlled trials (RCTs), each evaluating different interventions, for the prevention of MRONJ (Mozzati 2012; Mozzati 2013; Mücke 2016). There is low‐quality evidence that dental examinations at three‐month intervals and preventive treatments are more effective than standard care in reducing the incidence proportion and the incidence rate of MRONJ in individuals taking intravenous bisphosphonates for advanced cancer and bone metastases. After evaluation of the available evidence, it has not been possible to either claim or refute a benefit of PRGF, inserted into the postextraction alveolus during dental extractions, for the prevention of MRONJ. The available evidence was also insufficient to support either a more‐invasive (delicate surgery and wound closure by primary intention) or less‐invasive (non‐traumatic avulsion and wound closure by secondary intention) surgical strategy for the prophylaxis of MRONJ after dental extractions. Two RCTs evaluating the effect of platelet‐rich fibrin in the prevention of MRONJ after tooth extraction are ongoing (NCT01526915; NCT02198001).
We identified two RCTs that evaluated specific methods to improve the healing of MRONJ, namely hyperbaric oxygen (HBO) therapy and fluorescence‐guided bone surgery (Freiberger 2012; Ristow 2016). There was insufficient evidence to either claim or refute a benefit of HBO as an adjunct to conventional therapy for improved healing of MRONJ. There was also insufficient evidence to support either auto‐fluorescence‐guided bone surgery or tetracycline fluorescence‐guided bone surgery for improved healing of MRONJ. The small sample size may have contributed to a lack of measurable effect. Two ongoing trials are currently investigating teriparatide for the treatment of MRONJ (ACTRN12612000950864; UMIN000009132).
Overall completeness and applicability of evidence
The types of interventions evaluated in the included RCTs varied widely and so we were not able to combine data from different studies.
The RCTs included only people with bisphosphonates for the treatment of cancer or osteoporosis, or both. Although one study allowed the participation of individuals treated with denosumab, no people treated with this antiresorptive medication were included (Ristow 2016). None of the trials investigated the association between MRONJ and antiangiogenic medications. Thus, the included RCTs do not cover the entire spectrum of medications associated with MRONJ.
One trial recruited a highly selective group of participants (i.e. men with prostate cancer receiving zoledronic acid for the treatment of bone metastases) (Mücke 2016). The applicability of the results of this study to other populations is unclear.
A subgroup of individuals, namely those with a history of head and neck radiation therapy, were generally excluded from the included trials due to the widely accepted case definition of MRONJ. Although exclusion of these individuals may be useful in reducing the heterogeneity of the study populations and in controlling for an important influencing variable, this may have impaired the overall completeness of evidence.
Quality of the evidence
We judged the overall quality of the evidence to be low or very low, meaning that we are uncertain about the estimates of effect.
All included studies were assessed to have a high risk of bias overall, as they had at least one domain rated at high risk. All of the trials were open label. Due to their nature, some interventions could not be blinded to participants or surgeons. None of the trials blinded the outcome assessors. Altogether, a lack of blinding confers a high risk of bias. In one trial, the length of follow‐up differed between comparison groups, which may have biased the results of the study (Mücke 2016). A high and unbalanced rate of crossovers after randomisation between the comparison groups in two trials may also have conferred a high risk of bias (Freiberger 2012; Mücke 2016). We also downgraded the quality of evidence due to imprecision (most studies included relatively few participants (Freiberger 2012; Ristow 2016) or had few events (Mozzati 2012; Mozzati 2013) and thus have wide 95% confidence intervals around the effect estimates).
Potential biases in the review process
The methods we used in the review were established and documented in advance of the review being undertaken. We were not influenced by prior knowledge of the study results when making judgements regarding study eligibility. We made no subsequent changes to the types of studies and types of participants to be included in the review as specified in the protocol, with one exception. For trials investigating the effects of interventions for the prophylaxis of MRONJ, we originally required a follow‐up period of at least three years. The three‐year follow‐up threshold, however, turned out not to be a feasible selection criterion (see Primary outcomes). We consider this change to the inclusion criteria to be well justified, however, and we do not believe that we have introduced a relevant selection bias.
Cochrane Oral Health Information Specialist (Anne Littlewood) conducted comprehensive searches of journal and conference databases to ensure that all published and unpublished trials were identified. We did not limit the searches to a particular language. Two review authors independently extracted the trials that met the inclusion criteria. The study authors were contacted where necessary to ascertain if any newer data were available following publication.
Agreements and disagreements with other studies or reviews
Several systematic reviews addressing the prophylaxis and treatment of MRONJ have been published (Bermúdez‐Bejarano 2017; Diniz‐Freitas 2016; El‐Rabbany 2017; Fliefel 2015; Khan 2015; Lopez‐Jornet 2016; Rollason 2016; Rupel 2014; Silva 2016; Spanou 2015).
The most recent systematic review and meta‐analysis on the effectiveness of treatments for MRONJ was conducted by El‐Rabbany 2017. In their analysis, the review authors included non‐RCTs and prospective cohort studies as well as RCTs. The review concluded that surgical treatment is more effective than medical treatment for resolving MRONJ. The review authors admit that this conclusion was based on studies that had a medium‐to‐high risk of bias and low statistical power.
Another recent literature review focused on the role of antibiotics in the prophylaxis and treatment of MRONJ (Bermúdez‐Bejarano 2017). The review included all types of trial designs including case series. Sparse clinical data and a lack of RCTs made it impossible to definitively identify the most appropriate modality for each of the clinical situations studied.
A systematic review by Diniz‐Freitas 2016 focused on strategies to prevent MRONJ in people undergoing dental extractions. As no eligible RCTs were identified, the analysis was based on case series and cohort studies. No conclusive scientific evidence was available regarding the efficacy of MRONJ prevention strategies in people treated with antiresorptive or antiangiogenic drugs subjected to tooth extraction.
Interventions for treating specifically bisphosphonate‐related ONJ were evaluated in a Cochrane Review by Rollason 2016. The review, which considered only RCTs, identified one trial. This trial was also included in our analysis and evaluated the effect of HBO therapy adjunct to standard care (Freiberger 2012). Unlike our review, Rollason 2016 investigated 'improvement of osteonecrosis' as another primary outcome in addition to 'healing of osteonecrosis'. Rollason 2016 found that participants in the HBO group improved more than the standard‐care group at the three‐month follow‐up. We did not consider the outcome 'improvement' in our review for the following reason. The outcome 'improvement' is less well defined than the outcome 'healing'. Improvement of MRONJ may not be stable. Whether MRONJ has improved at a given time point may not correlate with the true effectiveness outcome, namely healing of MRONJ. Consistent with our findings, Rollason 2016 did not observe a clear difference between the HBO and control groups for the outcome 'healed'. The authors concluded that this single RCT could not confirm or refute the effectiveness of HBO therapy. The authors stated that there is a lack of evidence from RCTs to guide the treatment of bisphosphonate‐related ONJ.
All these reviews agree that high‐quality research is required before conclusive statements can be made regarding strategies for the prevention or treatment of MRONJ.

Study flow diagram. Results of the search strategy for inclusion of studies in this review

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 1 MRONJ (incidence proportion).

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 2 MRONJ (incidence rate: MRONJ cases per patient‐year).

Comparison 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) versus a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions, Outcome 1 MRONJ (incidence proportion).
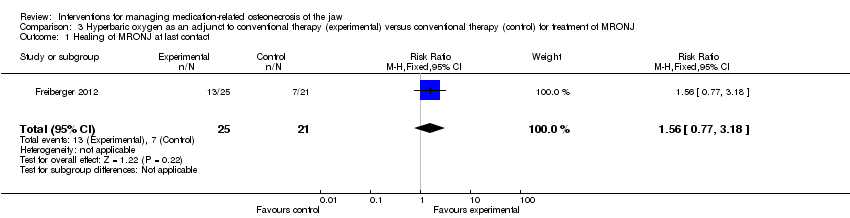
Comparison 3 Hyperbaric oxygen as an adjunct to conventional therapy (experimental) versus conventional therapy (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ at last contact.

Comparison 4 Autofluorescence‐guided bone surgery (experimental) versus tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ (defined as mucosal integrity) at 1 year.
| Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ | ||||||
| Population: prophylaxis of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard care (control) | Risk with dental examinations at three‐month intervals and preventive treatments (experimental) | |||||
| MRONJ (incidence proportion) (follow‐up: mean 32 months) | 233 per 1000 | 23 per 1000 | RR 0.10 | 253 | ⊕⊕⊝⊝ | Participants: high‐risk ( i.e. individuals with cancer exposed to intravenous zoledronic acid The outcome MRONJ was also reported as number of cases per patient‐year (incidence rate) rate ratio 0.18 (95% CI 0.04 to 0.74) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by two levels due to very serious risk of bias (high and unbalanced rate of crossovers after randomisation, high drop‐out rates due to high mortality, failure to adhere to the intention‐to‐treat principle, the mean follow‐up differed between experimental and control group). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions | ||||||
| Population: people treated with IV bisphosphonates who need dental extractions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with a standard dental extraction protocol without PRGF (control) | Risk with a dental extraction protocol with PRGF (experimental) | |||||
| MRONJ (incidence proportion) | 59 per 1000 | 5 per 1000 | RR 0.08 | 176 | ⊕⊝⊝⊝ | Participants: high risk, i.e. individuals with cancer exposed to IV zoledronic acid |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (high or unclear risk of selection bias, performance bias, detection bias, and attrition bias). IV = intravenous MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with conventional therapy (control) | Risk with hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) | |||||
| Healing of MRONJ (follow‐up: up to 24 months (outcome was measured at last follow‐up)) | 333 per 1000 | 520 per 1000 | RR 1.56 | 46 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, detection bias, and attrition bias; failure to adhere to the intention‐to‐treat principle). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with tetracycline fluorescence‐guided bone surgery (control) | Risk with autofluorescence‐guided bone surgery (experimental) | |||||
| Healing of MRONJ (follow‐up: 1 year) | 889 per 1000 | 933 per 1000 | RR 1.05 | 34 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, and detection bias). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| MRONJ stage | Description |
| AT RISK | No apparent necrotic bone in patients who have been treated with oral or intravenous bisphosphonates |
| STAGE 0 | No clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms |
| STAGE 1 | Exposed and necrotic bone or fistulas that probes to bone in patients who are asymptomatic and have no evidence of infection |
| STAGE 2 | Exposed and necrotic bone or fistulas that probes to bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage |
| STAGE 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e. inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) resulting in pathologic fracture, extraoral fistula, oral antral, or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor |
| From the American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update (Ruggiero 2014) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| 2 MRONJ (incidence rate: MRONJ cases per patient‐year) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | 0.18 [0.04, 0.74] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ at last contact Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ (defined as mucosal integrity) at 1 year Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.30] |

