Intervenciones para el tratamiento de la osteonecrosis del maxilar inferior relacionada con la medicación
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization of patients with MRONJ to treatment groups was performed after informed consent, but before the initial staging examination using a series of 70 opaque envelopes containing the assignment." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The subjects and staff were not blinded to therapy because of the impracticality of providing sham HBO; however, the oral‐maxillofacial surgeon was not told the subjects’ assignments before the initial staging examination." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Lesion scores at the time of last contact were assigned by the study team, including the oral‐maxillofacial surgeon." No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate: at the 12‐ and 18‐month evaluations 50% and 63%, respectively, of participants were lost to follow‐up. High and unbalanced rate of crossovers: after randomization 5 participants switched from the control to the HBO group; 1 participant assigned to the HBO group declined HBO treatment and was switched to the control group. Data analysis: as‐treated, not by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Serum measurements of bone turnover and molecular measures of osteoclast signalling were not reported. These are not primary outcomes and will be "reported separately". All other outcome variables listed in the Methods and the study protocol were reported. |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
Follow‐up examinations: mucosal healing was monitored at 3, 7, and 14 days postoperatively; monitoring for MRONJ was continued at 21, 30, 60, 90, and 120 days, and 6 months, followed by visits every 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study cohort was divided randomly into two groups of 50 subjects" Generation of randomisation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, the personnel were not blinded. Because an extra 15 mL blood sample was obtained from the participants in the PRGF group, the participants were most likely not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | It is not reported whether outcome was monitored by an independent and blinded outcome assessor. Outcome was most likely assessed by the surgeon who had performed the dental extraction. |
| Incomplete outcome data (attrition bias) | Unclear risk | Completeness or loss to follow‐up was not reported. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the Methods were all reported. |
| Methods |
| |
| Participants |
| |
| Interventions | All participants: professional oral hygiene session 1 week before surgery; antibiotics for 6 days starting the evening before surgery
| |
| Outcomes |
Follow‐up examinations: mucosal healing was monitored at 3, 7, and 14 days postoperatively; monitoring for MRONJ was continued at 21, 30, 60, and 90 days, and 6 months, followed by visits every 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was assigned by a computer‐randomization program to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, the personnel and participants were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | At least during the mucosal healing period, due to the nature of the intervention, blinding of outcome assessors is not possible. |
| Incomplete outcome data (attrition bias) | Unclear risk | Completeness or loss to follow‐up was not reported. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the Methods were all reported. |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were prospectively examined before the start of therapy with zoledronic acid and were randomly allocated into two groups." Generation of randomisation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported |
| Blinding of participants and personnel (performance bias) | High risk | "treatment was not possible to be blinded" |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | High and unbalanced rate of crossovers: "36 patients, who were randomized to participate in group B did not want to be part of a close follow‐up and were then regrouped in group A." "At the end of this study, 153 (93.3%) patients of group A and 79 (87.8%) patients from group B have died." Data analysis: as‐treated, not by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the Methods were all reported. |
| Methods |
| |
| Participants |
| |
| Interventions |
In all participants, a tension‐free wound closure was achieved using mucoperiostal flaps. All participants remained in hospital for 4 days after the operation. Participants received routine postoperative instructions and the same postoperative analgesic drug therapy. Antibiotic treatment involved the administration of ampicillin/sulbactam 2000 mg/1000 mg (or clindamycin in case of hypersensitivity to penicillin or a penicillin allergy) intravenously while in hospital and then orally for a further 6 days after discharge from the hospital. | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Over a period of 12 months, the study population was prospectively referred for the treatment of MRONJ and divided randomly into two study groups" Generation of randomisation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 4 participants (2 each in the TF and the AF group) died after T2 (8 weeks after the operation) and 2 participants in the AF group failed to attend the 1‐year follow‐up (T4). No participant was lost for assessment of the primary endpoint at T2 (8 weeks after the operation). |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the Methods were all reported. |
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT |
RCT: randomised clinical trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Does teriparatide reverse osteonecrosis of the jaw in patients treated with either bisphosphonates or denosumab? A randomised, controlled trial |
| Methods | Interventional, randomised, parallel assignment, blinded |
| Participants | Target sample size: 68 All sexes eligible for study, 18 years and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: subcutaneous teriparatide injections (20 μg daily), plus calcium (600 mg tablet daily) and vitamin D (1000 IU tablet daily) supplementation for 8 weeks Control: placebo saline injections, plus calcium (600 mg tablet daily) and vitamin D (1000 IU tablet daily) supplementation for 8 weeks |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | September 2012 |
| Contact information | |
| Notes | Prof. Ebeling was contacted. The study results will be available in the second half of 2017. |
| Trial name or title | Assessment of platelet rich fibrin efficiency on healing delay and on jawbone osteochemonecrosis provoked by bisphosphonates (OCN/PRF) |
| Methods | Interventional, randomised, parallel assignment, open label |
| Participants | 270 participants are required to validate the expected objectives in this study All sexes eligible for study, 18 years and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: bone curettage + PRF insertion Control: bone curettage alone without PRF insertion |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2011 |
| Contact information | e.gerard@chr‐metz‐thionville.fr |
| Notes |
| Trial name or title | Prospective randomized study: assessment of PRF efficacy in prevention of jaw osteonecrosis after tooth extraction (PRF) |
| Methods | Interventional; randomised; parallel assignment, blinded |
| Participants | Cohort of 100 participants: control group 50 participants and experimental 50 participants All sexes eligible for study; 50 years and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: tooth extraction and insertion of PRF (non‐traumatic tooth extraction with antibiotics (amoxicillin clavulanate combination). Insertion of PRF membrane in tooth‐extraction site) Control: no PRF (non‐traumatic extraction with antibiotic without PRF insertion) |
| Outcomes | Number of participants with jaw osteonecrosis after tooth extraction |
| Starting date | January 2014 |
| Contact information | |
| Notes |
| Trial name or title | Study to the effect of teriparatide formulation Forteo versus Teribone on bisphosphonate‐related osteonecrosis of the jaw in osteoporosis patients |
| Methods | Interventional, parallel, randomised, open study |
| Participants | 15 female participants >= 20 years of age Inclusion criteria:
Exclusion criteria:
|
| Interventions | Forteo (teriparatide) vs Teribone (teriparatide) |
| Outcomes |
|
| Starting date | August 2012 |
| Contact information | [email protected]‐u.ac.jp |
| Notes |
1. P1NP, N‐terminal propeptide of type 1 collagen Procollagen I Intact N‐Terminal
2. Beta‐CTX, Beta‐carboxy‐terminal telopeptide of type 1 collagen (beta‐CrossLaps)
3. OCN: osteochemonecrosis
4. Cicatrisation: formation of scar tissue at a wound site by fibroblasts
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| Analysis 1.1  Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 1 MRONJ (incidence proportion). | ||||
| 2 MRONJ (incidence rate: MRONJ cases per patient‐year) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | 0.18 [0.04, 0.74] | |
| Analysis 1.2 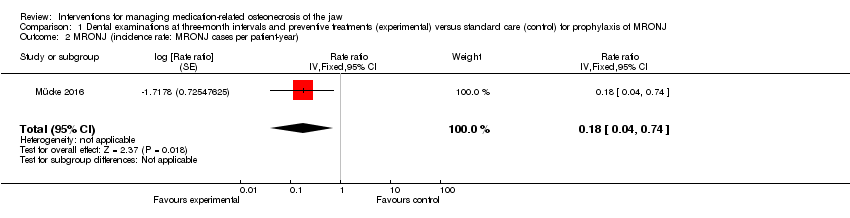 Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 2 MRONJ (incidence rate: MRONJ cases per patient‐year). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.51] |
| Analysis 2.1 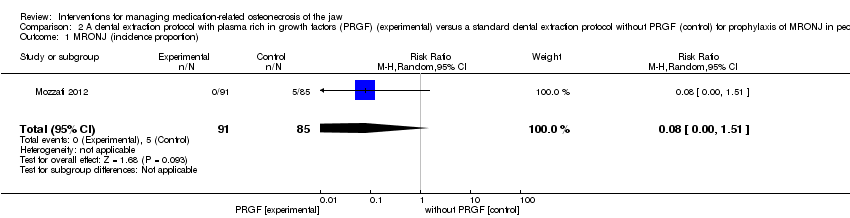 Comparison 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) versus a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions, Outcome 1 MRONJ (incidence proportion). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ at last contact Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| Analysis 3.1 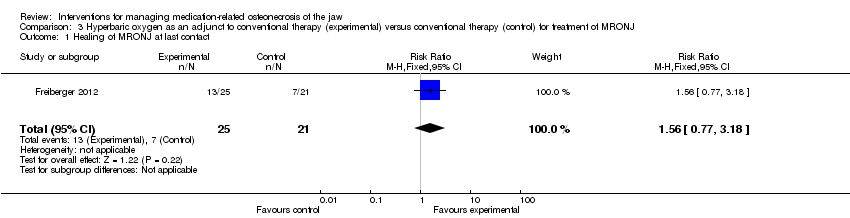 Comparison 3 Hyperbaric oxygen as an adjunct to conventional therapy (experimental) versus conventional therapy (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ at last contact. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ (defined as mucosal integrity) at 1 year Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.30] |
| Analysis 4.1  Comparison 4 Autofluorescence‐guided bone surgery (experimental) versus tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ (defined as mucosal integrity) at 1 year. | ||||

Study flow diagram. Results of the search strategy for inclusion of studies in this review

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 1 MRONJ (incidence proportion).

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 2 MRONJ (incidence rate: MRONJ cases per patient‐year).

Comparison 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) versus a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions, Outcome 1 MRONJ (incidence proportion).
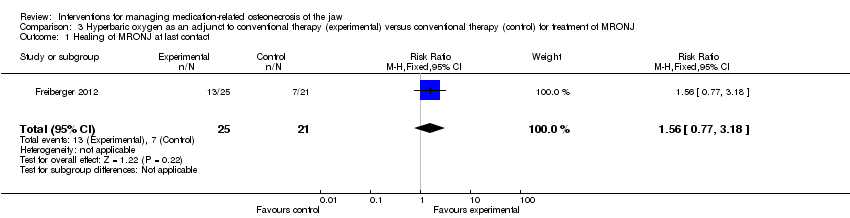
Comparison 3 Hyperbaric oxygen as an adjunct to conventional therapy (experimental) versus conventional therapy (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ at last contact.

Comparison 4 Autofluorescence‐guided bone surgery (experimental) versus tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ (defined as mucosal integrity) at 1 year.
| Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ | ||||||
| Population: prophylaxis of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard care (control) | Risk with dental examinations at three‐month intervals and preventive treatments (experimental) | |||||
| MRONJ (incidence proportion) (follow‐up: mean 32 months) | 233 per 1000 | 23 per 1000 | RR 0.10 | 253 | ⊕⊕⊝⊝ | Participants: high‐risk ( i.e. individuals with cancer exposed to intravenous zoledronic acid The outcome MRONJ was also reported as number of cases per patient‐year (incidence rate) rate ratio 0.18 (95% CI 0.04 to 0.74) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by two levels due to very serious risk of bias (high and unbalanced rate of crossovers after randomisation, high drop‐out rates due to high mortality, failure to adhere to the intention‐to‐treat principle, the mean follow‐up differed between experimental and control group). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions | ||||||
| Population: people treated with IV bisphosphonates who need dental extractions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with a standard dental extraction protocol without PRGF (control) | Risk with a dental extraction protocol with PRGF (experimental) | |||||
| MRONJ (incidence proportion) | 59 per 1000 | 5 per 1000 | RR 0.08 | 176 | ⊕⊝⊝⊝ | Participants: high risk, i.e. individuals with cancer exposed to IV zoledronic acid |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (high or unclear risk of selection bias, performance bias, detection bias, and attrition bias). IV = intravenous MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with conventional therapy (control) | Risk with hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) | |||||
| Healing of MRONJ (follow‐up: up to 24 months (outcome was measured at last follow‐up)) | 333 per 1000 | 520 per 1000 | RR 1.56 | 46 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, detection bias, and attrition bias; failure to adhere to the intention‐to‐treat principle). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with tetracycline fluorescence‐guided bone surgery (control) | Risk with autofluorescence‐guided bone surgery (experimental) | |||||
| Healing of MRONJ (follow‐up: 1 year) | 889 per 1000 | 933 per 1000 | RR 1.05 | 34 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, and detection bias). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| MRONJ stage | Description |
| AT RISK | No apparent necrotic bone in patients who have been treated with oral or intravenous bisphosphonates |
| STAGE 0 | No clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms |
| STAGE 1 | Exposed and necrotic bone or fistulas that probes to bone in patients who are asymptomatic and have no evidence of infection |
| STAGE 2 | Exposed and necrotic bone or fistulas that probes to bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage |
| STAGE 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e. inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) resulting in pathologic fracture, extraoral fistula, oral antral, or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor |
| From the American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update (Ruggiero 2014) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| 2 MRONJ (incidence rate: MRONJ cases per patient‐year) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | 0.18 [0.04, 0.74] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ at last contact Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ (defined as mucosal integrity) at 1 year Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.30] |

