Interventions for managing medication‐related osteonecrosis of the jaw
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
1 ((((medication or bisphosphonate or drug) and (osteonecrosis or necrosis) and jaw*)):ti,ab) AND (INREGISTER)
2 (MRONJ or BRONJ or BONJ:ti,ab) AND (INREGISTER)
3 (#1 or #2) AND (INREGISTER)
4 ((osteonecrosis or "bone necrosis"):ti,ab) AND (INREGISTER)
5 (osteochemonecro*:ti,ab) AND (INREGISTER)
6 (#4 or #5) AND (INREGISTER)
7 ((jaw* or jawbone* or mandib* or maxill* or (alveolar and bone*)):ti,ab) AND (INREGISTER)
8 ((diphosphonate* or bisphosphonate* or aminobisphosphonate* or alendronate or risedronate or pamidronate or "zoledronic acid" or ibandronate or "alendronic acid" or bevacizumab or denosumab or "etidronate disodium" or "ibandronic acid" or sirolimus or "sodium clodronate" or sorafenib or sunitinib or "tiludronic acid" or zoledronate or didronel or "clodronate disodium" or tiludronate or "risedronic acid" or "clodronic acid"):ti,ab) AND (INREGISTER)
9 ((Fosamax or Fosavance or Actonel or Aclasta or Zometa or Reclast or Didronel or Skelid or Bondronat or Bonviva or Aredia or Bonefos or Nexavar or Avastin or Prolia or Xgeva or Boniva or Atelvia or Rapamune or Rapamycin or Sutent or Zometa):ti,ab) AND (INREGISTER)
10 ((denosumab or prolia or ranmark or xgeva):ti,ab) AND (INREGISTER)
11 ((antivegf of avastin or bevacizumab):ti,ab) AND (INREGISTER)
12 ((aflibercept or eylea or "vegf trap" or zaltrap):ti,ab) AND (INREGISTER)
13 (("su 11248" or sunitinib or sunitinibum or sutent):ti,ab) AND (INREGISTER)
14 (("bms 907351" or bms907351 or cabozantinib or cometriq or "xl 184" or "xl 184" or xl184):ti,ab) AND (INREGISTER)
15 ((temsirolimus or torisel):ti,ab) AND (INREGISTER)
16 ((afinitor or certican or everolimus or everolimus or rad001 or "sdz rad" or votubia or zortress):ti,ab) AND (INREGISTER)
17 (#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) AND (INREGISTER)
18 (#6 and #7 and #17) AND (INREGISTER)
19 (#3 or #18) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh ^"Bisphosphonate‐associated osteonecrosis of the jaw"]
#2 ((medication or bisphosphonate or drug) near/4 (osteonecrosis or necrosis) near/3 jaw*)
#3 (MRONJ or BRONJ or BONJ):ti,ab
#4 {or #1‐#3}
#5 [mh ^Osteonecrosis]
#6 (osteonecro* or "bone necrosis"):ti,ab
#7 osteochemonecro*:ti,ab
#8 {or #5‐#7}
#9 [mh jaw]
#10 [mh ^"alveolar bone loss"]
#11 [mh ^"jaw diseases"]
#12 (jaw* or jawbone* or mandib* or maxill* or (alveolar near/4 bone*)):ti,ab
#13 {or #9‐#12}
#14 [mh diphosphonates]
#15 (diphosphonate* or bisphosphonate* or aminobisphosphonate* or alendronate or risedronate or pamidronate or "zoledronic acid" or ibandronate or "alendronic acid" or bevacizumab or denosumab or "etidronate disodium" or "ibandronic acid" or sirolimus or "sodium clodronate" or sorafenib or sunitinib or "tiludronic acid" or zoledronate or didronel or "clodronate disodium" or tiludronate or "risedronic acid" or "clodronic acid"):ti,ab
#16 (Fosamax or Fosavance or Actonel or Aclasta or Zometa or Reclast or Didronel or Skelid or Bondronat or Bonviva or Aredia or Bonefos or Nexavar or Avastin or Prolia or Xgeva or Boniva or Atelvia or Rapamune or Rapamycin or Sutent or Zometa):ti,ab
#17 [mh ^Denosumab]
#18 (denosumab or prolia or ranmark or xgeva):ti,ab
#19 [mh ^Bevacizumab]
#20 (antivegf of avastin or bevacizumab):ti,ab
#21 (aflibercept or eylea or "vegf trap" or zaltrap):ti,ab
#22 ("su 11248" or sunitinib or sunitinibum or sutent):ti,ab
#23 ("bms 907351" or bms907351 or cabozantinib or cometriq or "xl 184" or "xl 184" or xl184):ti,ab
#24 (temsirolimus or torisel):ti,ab
#25 [mh ^Everolimus]
#26 (afinitor or certican or everolimus or everolimus or rad001 or "sdz rad" or votubia or zortress):ti,ab
#27 {or #14‐#26}
#28 #8 and #13 and #27
#29 #4 or #28
Appendix 3. MEDLINE Ovid search strategy
1. Bisphosphonate‐associated osteonecrosis of the jaw/
2. ((medication or bisphosphonate or drug) adj4 (osteonecrosis or necrosis) adj3 jaw$).ti,ab.
3. (MRONJ or BRONJ or BONJ).ti,ab.
4. or/1‐3
5. Osteonecrosis/
6. (osteonecro$ or "bone necrosis").ti,ab.
7. osteochemonecro$.ti,ab.
8. or/5‐7
9. exp Jaw/
10. Alveolar bone loss/ci
11. Jaw diseases/ci
12. (jaw or jawbone$ or mandibl$ or maxill$ or (alveolar adj4 bone$)).ti,ab.
13. or/9‐12
14. exp Diphosphonates/
15. (diphosphonate$ or bisphosphonate$ or aminobisphosphonate$ or alendronate or risedronate or pamidronate or "zoledronic acid" or ibandronate or "alendronic acid" or bevacizumab or denosumab or "etidronate disodium" or "ibandronic acid" or sirolimus or "sodium clodronate" or sorafenib or sunitinib or "tiludronic acid" or zoledronate or didronel or "clodronate disodium" or tiludronate or "risedronic acid" or "clodronic acid").ti,ab
16. (Fosamax or Fosavance or Actonel or Aclasta or Zometa or Reclast or Didronel or Skelid or Bondronat or Bonviva or Aredia or Bonefos or Nexavar or Avastin or Prolia or Xgeva or Boniva or Atelvia or Rapamune or Rapamycin or Sutent or Zometa).ti,ab.
17. Denosumab/
18. (denosumab or prolia or ranmark or xgeva).ti,ab.
19. Bevacizumab/
20. (antivegf or avastin or bevacizumab).ti,ab.
21. (aflibercept or eylea or "vegf trap" or zaltrap).ti,ab.
22. ("su 11248" or sunitinib or sunitinibum or sutent).ti,ab.
23. ("bms 907351" or bms907351 or cabozantinib or cometriq or "xl 184" or "xl 184" or xl184).ti,ab.
24. (temsirolimus or torisel).ti,ab.
25. Everolimus/
26. (afinitor or certican or everolimus or everolimus or rad001 or "sdz rad" or votubia or zortress).ti,ab.
27. or/14‐26
28. 8 and 13 and 27
29. 4 or 28
The search will be done with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. ((medication or bisphosphonate or drug) adj4 (osteonecrosis or necrosis) adj3 jaw$).ti,ab.
2. (MRONJ or BRONJ or BONJ).ti,ab.
3. 1 or 2
4. "Bone necrosis"/
5. "Jaw osteonecrosis"/
6. (osteonecro$ or "bone necrosis").ti,ab.
7. osteochemonecro$.ti,ab.
8. or/4‐6
9. exp Jaw/
10. Alveolar bone loss/
11. Jaw disease/
12. (jaw or jawbone$ or mandibl$ or maxill$ or (alveolar adj4 bone$)).ti,ab.
13. or/9‐12
14. exp Bisphosphonic acid derivative/
15. (diphosphonate$ or bisphosphonate$ or aminobisphosphonate$ or alendronate or risedronate or pamidronate or "zoledronic acid" or ibandronate or "alendronic acid" or bevacizumab or denosumab or "etidronate disodium" or "ibandronic acid" or sirolimus or "sodium clodronate" or sorafenib or sunitinib or "tiludronic acid" or zoledronate ordidronel or "clodronate disodium" or tiludronate or "risedronic acid" or "clodronic acid").ti,ab.
16. (Fosamax or Fosavance or Actonel or Aclasta or Zometa or Reclast or Didronel or Skelid or Bondronat or Bonviva or Aredia or Bonefos or Nexavar or Avastin or Prolia or Xgeva or Boniva or Atelvia or Rapamune or Rapamycin or Sutent or Zometa).ti,ab.
17. Denosumab/
18. (denosumab or prolia or ranmark or xgeva).ti,ab.
19. Bevacizumab/
20. (antivegf or avastin or bevacizumab).ti,ab.
21. (aflibercept or eylea or "vegf trap" or zaltrap).ti,ab.
22. ("su 11248" or sunitinib or sunitinibum or sutent).ti,ab.
23. ("bms 907351" or bms907351 or cabozantinib or cometriq or "xl 184" or "xl 184" or xl184).ti,ab.
24. (temsirolimus or torisel).ti,ab.
25. Everolimus/
26. (afinitor or certican or everolimus or everolimus or rad001 or "sdz rad" or votubia or zortress).ti,ab.
27. or/14‐26
28. 8 and 13 and 27
29. 3 or 28
The above subject search was linked to adapted version of the Cochrane Crowd Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/
2. Controlled clinical study/
3. Random$.ti,ab.
4. randomization/
5. intermethod comparison/
6. placebo.ti,ab.
7. (compare or compared or comparison).ti.
8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
9. (open adj label).ti,ab.
10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
11. double blind procedure/
12. parallel group$1.ti,ab.
13. (crossover or cross over).ti,ab.
14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
15. (assigned or allocated).ti,ab.
16. (controlled adj7 (study or design or trial)).ti,ab.
17. (volunteer or volunteers).ti,ab.
18. trial.ti.
19. or/1‐18
20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
21. 19 not 20
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
bisphosphonates and jaw
osteonecrosis and jaw
necrosis and jaw
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
bisphosphonates and jaw
osteonecrosis and jaw or necrosis and jaw

Study flow diagram. Results of the search strategy for inclusion of studies in this review

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 1 MRONJ (incidence proportion).

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 2 MRONJ (incidence rate: MRONJ cases per patient‐year).

Comparison 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) versus a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions, Outcome 1 MRONJ (incidence proportion).
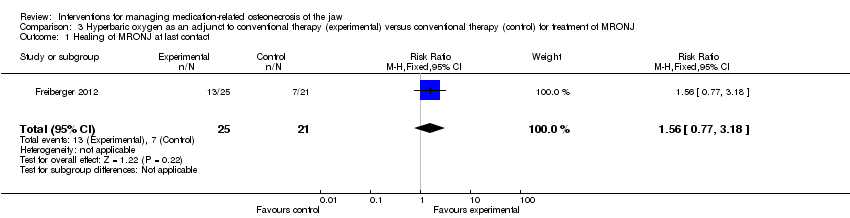
Comparison 3 Hyperbaric oxygen as an adjunct to conventional therapy (experimental) versus conventional therapy (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ at last contact.

Comparison 4 Autofluorescence‐guided bone surgery (experimental) versus tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ (defined as mucosal integrity) at 1 year.
| Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ | ||||||
| Population: prophylaxis of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard care (control) | Risk with dental examinations at three‐month intervals and preventive treatments (experimental) | |||||
| MRONJ (incidence proportion) (follow‐up: mean 32 months) | 233 per 1000 | 23 per 1000 | RR 0.10 | 253 | ⊕⊕⊝⊝ | Participants: high‐risk ( i.e. individuals with cancer exposed to intravenous zoledronic acid The outcome MRONJ was also reported as number of cases per patient‐year (incidence rate) rate ratio 0.18 (95% CI 0.04 to 0.74) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by two levels due to very serious risk of bias (high and unbalanced rate of crossovers after randomisation, high drop‐out rates due to high mortality, failure to adhere to the intention‐to‐treat principle, the mean follow‐up differed between experimental and control group). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions | ||||||
| Population: people treated with IV bisphosphonates who need dental extractions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with a standard dental extraction protocol without PRGF (control) | Risk with a dental extraction protocol with PRGF (experimental) | |||||
| MRONJ (incidence proportion) | 59 per 1000 | 5 per 1000 | RR 0.08 | 176 | ⊕⊝⊝⊝ | Participants: high risk, i.e. individuals with cancer exposed to IV zoledronic acid |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (high or unclear risk of selection bias, performance bias, detection bias, and attrition bias). IV = intravenous MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with conventional therapy (control) | Risk with hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) | |||||
| Healing of MRONJ (follow‐up: up to 24 months (outcome was measured at last follow‐up)) | 333 per 1000 | 520 per 1000 | RR 1.56 | 46 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, detection bias, and attrition bias; failure to adhere to the intention‐to‐treat principle). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with tetracycline fluorescence‐guided bone surgery (control) | Risk with autofluorescence‐guided bone surgery (experimental) | |||||
| Healing of MRONJ (follow‐up: 1 year) | 889 per 1000 | 933 per 1000 | RR 1.05 | 34 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, and detection bias). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| MRONJ stage | Description |
| AT RISK | No apparent necrotic bone in patients who have been treated with oral or intravenous bisphosphonates |
| STAGE 0 | No clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms |
| STAGE 1 | Exposed and necrotic bone or fistulas that probes to bone in patients who are asymptomatic and have no evidence of infection |
| STAGE 2 | Exposed and necrotic bone or fistulas that probes to bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage |
| STAGE 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e. inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) resulting in pathologic fracture, extraoral fistula, oral antral, or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor |
| From the American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update (Ruggiero 2014) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| 2 MRONJ (incidence rate: MRONJ cases per patient‐year) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | 0.18 [0.04, 0.74] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ at last contact Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ (defined as mucosal integrity) at 1 year Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.30] |

