Intervenciones de apoyo para adolescentes con asma a cargo de otros pacientes con asma o personas no especializadas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012331.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 19 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Kayleigh Kew (KK) wrote the Background and Methods sections of this review (based on the standard template), with clinical advice and input from Robin Carr (RC) and Iain Crossingham (IC). KK screened all references, extracted data for all studies and assessed risk of bias. Duplicate data extraction and risk of bias were provided by a member of the editorial team (Rebecca Normansell). KK performed the meta‐analyses, graded the evidence and led the write‐up, with support, feedback and input from RC and IC.
Sources of support
Internal sources
-
Kayleigh Kew, UK.
Supported by St George's, University of London
External sources
-
National Institute for Health Research (NIHR), UK.
Evidence to guide care in adults and children with asthma, 13/89/14
This project was supported by the NIHR, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health
Declarations of interest
KK: none.
RC: none that are relevant to the interventions considered in this review. RC is a part‐time Partnership General Practitioner (GP). He works as the long‐term conditions lead for the Oxfordshire Clinical Commissioning Group for respiratory illness and was the Medical Director of the Somerset chronic obstructive pulmonary disease service until October 2014. He received a salary from each of these employers. He organised primary care education for over 20 years and received honoraria from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca and Chiesi over the past 36 months for presenting lectures to primary care staff. He received travel reimbursement for attending a Cochrane Airways Group meeting in 2014, and again in 2015.
IC: none.
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group. We are very grateful to Elizabeth Stovold for designing the search strategy and to the Cochrane Airways Group staff for their editorial support.
We are very grateful for responses from study authors, Hyekyun Rhee and Nihaya Al‐Sheyab.
Rebecca Normansell was the Editor for this review and commented critically on both the protocol and the full review and assisted with duplicate data extraction.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 19 | Lay‐led and peer support interventions for adolescents with asthma | Review | Kayleigh M Kew, Robin Carr, Iain Crossingham | |
| 2016 Aug 26 | Lay‐led and peer support interventions for adolescents with asthma | Protocol | Kayleigh M Kew, Robin Carr, Iain Crossingham | |
Differences between protocol and review
We planned that IC and RC would share the duplicate extraction and risk of bias judgements, but this was done by a member of the editorial team owing to work commitments. It was not possible to carry out meta‐analyses for all outcomes or to carry out planned subgroup or sensitivity analyses.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Humans;
PICO
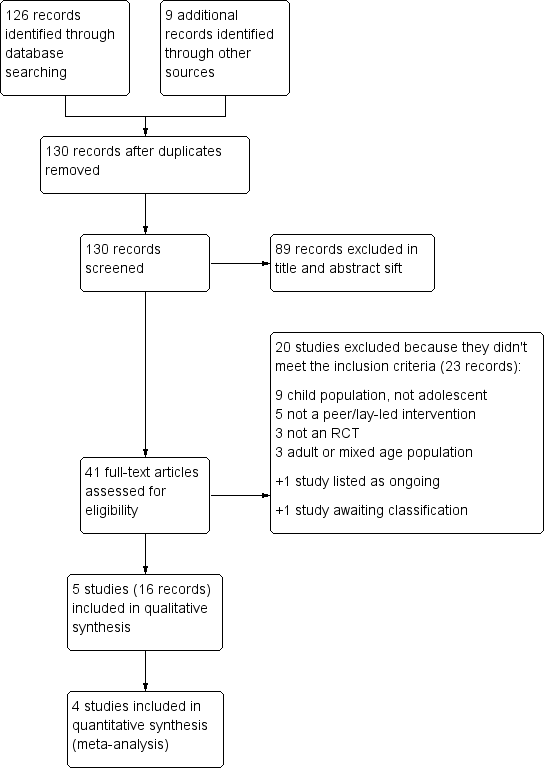
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Peer‐led vs control, Outcome 1 Change in asthma‐related quality of life (PAQLQ).

Comparison 1 Peer‐led vs control, Outcome 2 Asthma‐related quality of life (MCID).

Comparison 1 Peer‐led vs control, Outcome 3 Asthma control.
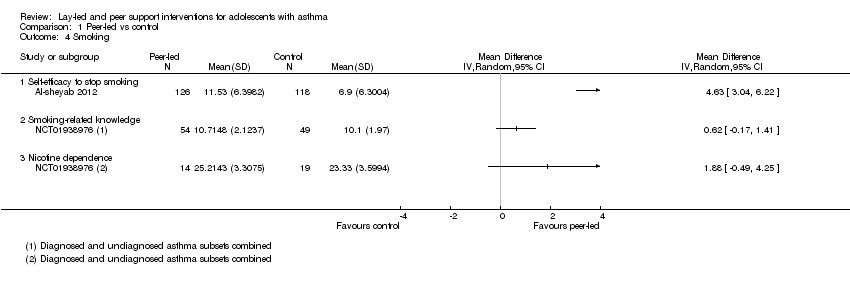
Comparison 1 Peer‐led vs control, Outcome 4 Smoking.
| Lay‐led and peer support interventions compared with usual care for adolescents with asthma | |||||
| Patient or population: adolescents with asthma Settings: school, day camp or primary care Intervention: lay‐led and peer support interventions Comparison: usual care/no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care/no intervention | Lay‐led or peer support intervention | ||||
| Asthma‐related quality of life (PAQLQ) 1 to 7 scale; higher = better 3 to 9 months | Mean change in control groups was 0.05 | Mean change in intervention groups was | 578 | ⊕⊕⊝⊝ | |
| Asthma‐related quality of life (MCID) 8 months | 123 per 1000 | 248 per 1000 | 251 | ⊕⊕⊝⊝ | |
| Asthma control Scale (range, score) ACT (5‐23) and ACQ (4‐16) 4 to 9 months | Not pooled. Two studies reported 2 different measures. Both effects favoured peer support, but neither result was statistically significant | 166 | ⊕⊕⊝⊝ | ||
| Unscheduled visits 9 months | Somewhat fewer mean visits per person in the intervention group than in the control group, but the data are skewed and are difficult to interpret | 84 | Not graded | ||
| Medication adherence 2.5 months | Skewed data reported non‐parametrically. Low baseline adherence (˜ 26%), which dropped further in both groups after the intervention, although it was less in the intervention group | 68 (1 RCT) | Not graded | Adherence to ICS was measured objectively with a dose counter | |
| Smoking 3 to 4 months | Mean self‐efficacy to stop smoking score in control group was 6.9 | Mean score in intervention groups was 4.63 better (3.04 to 6.22 better) | 244 | ⊕⊕⊝⊝ | SANDS subscale Range 0 to 16 |
| Mean smoking‐related knowledge score in control group was 10.1 | Mean score in intervention groups was 0.62 better (‐0.17 worse to 1.41 better) | 103 | Modified Tar‐Wars scale Range 0 to 13 | ||
| Mean nicotine dependence score in control group was 23.3 | Mean score in intervention groups was 1.88 better (‐0.49 worse to 4.25 better) | 33 | SANDS total Range 0 to 32 | ||
| Adverse events | No reports of adverse events, although only specifically mentioned in 1 study | ‐ | Not graded | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. | |||||
| aDowngraded for risk of bias. Outcome measured on a self‐rated scale. Likely to be affected by both performance and detection biases. bDowngraded for inconsistency (I2 = 71%). Random‐effects analysis used as planned, resulting in wide confidence intervals that just cross the line of no effect. Sensitivy analysis with a fixed‐effect model showed much tighter CIs around a mean difference of 0.16 (0.06 to 0.26). Not downgraded for imprecision. cConfidence intervals favour the intervention, but the effect is based on one study of 251 people (downgraded for imprecision). dTwo other studies reported the measure but did not plan a responder analysis (not downgraded for publication bias). eDowngraded for imprecision. Point estimates favoured the intervention, but lower confidence limits do not rule out possible harm. | |||||
| Study ID | Design | Observation | Age range, years | N | Intervention | Comparison | Country |
| Cluster OL | 3 months | 14 to 16 | 261 (4 clusters) | Triple A programme | No intervention | Jordan | |
| Cluster OL | 4 months | 12 to 13 | 433 (4 clusters) | Triple A programme + smoking pledge | Triple A programme alone | Jordan | |
| Individual OL | 2.5 months | 11 to 16 | 68 | Peer support + mp3 messaging | Attention control | USA | |
| Individual SB | 9 months | 13 to 17 | 112 | Peer‐led asthma camp | Adult‐led asthma camp | USA | |
| Cluster OL | 8 months | 12 to 16 | 272 (6 clusters) | Triple A programme | No intervention | Australia | |
| OL = open‐label; SB: single‐blind. Other details such as mean age, healthcare setting, measures of asthma severity, frequency and duration of sessions and baseline social support are described in the text (Included studies). | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in asthma‐related quality of life (PAQLQ) Show forest plot | 3 | Mean Difference (Random, 95% CI) | 0.40 [‐0.02, 0.81] | |
| 2 Asthma‐related quality of life (MCID) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Asthma control Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Smoking Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Self‐efficacy to stop smoking | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Smoking‐related knowledge | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nicotine dependence | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

