La prise de décision partagée pour les personnes souffrant d'asthme
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel, open‐label, cluster RCT Length of observation: 22 months Setting: 74 general practices in Michigan and New York, USA | |
| Participants | Population: 74 physicians were randomised and 69 completed the trial. It is not clear how many were randomised to each group, but the study states that 637 children were recruited in total, and outcome data were available for 472. Baseline characteristics Baseline data were reported for the whole population rather than for each group. 60% of physicians and 70% of children were male. Physician and child ages were reported in brackets rather than as a mean per group. 30% of families were Latino/Hispanic (15%) or African American (15%). Inclusion criteria:Physician criteria: primary specialty of general paediatrics; licensed no earlier than 1960; providing direct patient care; if board‐specialised, certified only in paediatrics; willing to take part in the interactive seminar if randomised to the treatment group. Child criteria: 1 to 12 years of age; diagnosis of asthma made by a physician; no other chronic disorders with pulmonary complications; at least 1 emergency medical visit for asthma in the previous year. An emergency visit was a hospitalisation, emergency department (ED) visit, or physician office visit on an emergency basis defined as administration of epinephrine subcutaneously or bronchodilators by aerosol. Exclusion criteria: none in addition to inclusion criteria | |
| Interventions | Intervention: shared decision‐making seminars for clinicians Interactive seminar based on self‐regulation theory to guide physicians in NAEPP care and to engage in interactive conversations with patients to derive information for making therapeutic decisions, create a supportive atmosphere, reinforce self‐management, give a view of the long‐term therapeutic plan, and build patients’ confidence in controlling symptoms and using medicines. Materials included brief lectures from respected asthma specialists; a video depicting effective clinician teaching and communications behaviour; case studies presenting troublesome clinical problems; a protocol by which physicians could assess their own behaviour regarding patient communications; and review of messages to communicate and materials to use when teaching patients. Resources: The seminar comprised 2 face‐to‐face group meetings, each lasting 2 ½ hours, held over a 2‐ to 3‐week period. Control: usual care Physicians in the control group were randomly assigned a date corresponding to 1 of the 3 seminar time points, to determine when follow‐up interviews of their patients should begin. | |
| Outcomes | Physician survey (items related to using clinical practice methods/medicines, encouraging self‐management, and providing patient teaching and communications). Analysis of data illustrated close correlation between physician and parent descriptions of behaviour. Questions on the parent interview form related to symptom status of the child, medicines prescribed, use of healthcare services for asthma (ED visits, hospitalisations, physician office visits), parents’ observations and opinions of physicians’ teaching and communications behaviours, other aspects of the clinician–patient interaction | |
| Notes | Trial registration: not reported Funding: supported by MD/Family Partnership ‐ Education in Asthma Management grant number HL‐44976 from the Lung Division of the National Heart, Lung, and Blood Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized, controlled study design" but no description of how this was done |
| Allocation concealment (selection bias) | High risk | "Names of patients meeting criteria were selected by the investigators at random from the roster provided by physicians", which may have introduced recruitment bias within practices, even if practices were themselves randomised adequately to groups |
| Blinding of participants and personnel (performance bias) | High risk | "Patients and their parents were blind to physicians’ involvement in the intervention." "A potential source of bias in the study was that physicians would give positive reports of their behavior to be consistent with good clinical and communications practices. To guard against such bias, data were collected from parents of patients regarding physician behavior as a means of corroborating physician reports." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Patients and parents were blind so outcomes measured by them can be considered low risk of bias. Outcomes measured or self‐assessed by the physicians taking part in the study are at high risk of detection bias." "A potential source of bias in the study was that physicians would give positive reports of their behavior to be consistent with good clinical and communications practices. To guard against such bias, data were collected from parents of patients regarding physician behavior as a means of corroborating physician reports." Patients and parents were blinded to their physician's participation in the intervention. Depends who is reporting the outcome, and to whom. Will be assessed separately when GRADE is applied |
| Incomplete outcome data (attrition bias) | High risk | "Data were collected from physicians at baseline, and 69 (93%) provided follow‐up data 5 months after the program. Data were also collected from 637 of their patients at baseline, and in a 22‐month window after the intervention, 472 (74%) of this number provided follow‐up data." Unclear how many were randomised to each group and whether dropout was balanced, but nonetheless quite high attrition overall |
| Selective reporting (reporting bias) | High risk | Study does not report methods fully, for example, number of people assigned to each group and participant flow. In terms of data, uncertainty regarding the number of participants per group means that data are difficult to analyse reliably in meta‐analyses. Some data relevant to this review are presented narratively. We did not identify a study protocol or trial registration |
| Other bias | Low risk | None noted |
| Methods | Study design: parallel, individually randomised, open‐label RCT Length of observation: 6 months Setting: 3 primary care practices (1 urban, 2 suburban) in Philadephia, USA | |
| Participants | Population: 60 families were randomised to the online portal for SDM (30) or to the control group (30). Baseline characteristics Mean age was 8.3 years (SD 1.9) in the intervention group and 8.2 years (SD 1.9) in the control group. 43% of the intervention group and 40% of the control group were white. In the intervention group, 60% had mild asthma, 37% moderate, and 3% severe. In the control group, 47% had mild asthma, 47% moderate, and 6% severe. Inclusion criteria: Eligible participants were children aged 6 to 12 years with persistent asthma who received care at a study site, along with their parent or legal guardian. We enrolled English‐speaking parents/guardians who served as the primary member of their household involved in communicating with the doctor’s office and had consistent computer and Internet access. Exclusion criteria: At clinicians’ discretion, parents of children whose asthma was not a primary or current health concern were excluded, as were those not currently taking a controller medication. | |
| Interventions | Intervention: shared decision‐making portal MyAsthma, developed with input from families and clinicians with the goal of fostering ongoing SDM, provided decision support to both clinicians and parents. The clinician interface appeared in the electronic health record (EHR), and the parent interface appeared within MyChart, the EHR vendor’s patient portal. Features include identification of parents’ concerns and goals for asthma treatment; monthly symptom tracking, drug side effects, goal progress; educational content; and asthma care plan. Parents were encouraged via email to complete monthly portal surveys. Answers informed guideline‐based decision support for parents and clinicians, directing them to speak to one another if asthma was not well controlled, or if side effects occurred, or to continue current therapy. Control: usual care + decision support Families in the control group did not have access to the portal; however, clinicians caring for control group children had access to a clinician‐focused decision support system proven effective in fostering guideline‐based care. | |
| Outcomes | Families completed surveys at enrolment and at 3 and 6 months. Feasibility assessed as % of participants in intervention group completing the monthly portal survey. Acceptability of asthma care measured at 6 months on 11‐point Likert scale. Clinical outcomes were numbers of asthma ED visits, hospitalisations, and specialist and GP visits over the 6‐month study (parental report validated when possible by chart review); number of prescriptions assessed through EHR; and number of days of missed school (child) or work (parent) over past month. Parent Patient Activation Measure. Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF) as quality of life measure. ACT as control measure | |
| Notes | Trial registration: NCT01715389 Funding: supported by the Chair’s Initiative Grant and the William Wikoff Smith Endowed Chair in Pediatric Genomics from Children’s Hospital of Philadelphia, and by award number K23HD059919 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomization sequence was generated by the study coordinator (SLM). Randomization was stratified by practice and by whether the child had mild or moderate versus severe persistent asthma." |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes were used to ensure blinding of study staff to treatment condition before enrolment and randomization." |
| Blinding of participants and personnel (performance bias) | High risk | 'Parents either had access to the portal or not so it was not possible to blind them to treatment allocation. This knowledge may have affected clinician and parent behaviour during the study and potentially biased outcomes." |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were generally parent rated, which would introduce high risk of detection bias. Resource use outcomes and prescription refills would be less subject to detection biases. |
| Incomplete outcome data (attrition bias) | Low risk | 4 families in the intervention group (13.3%) and 3 in the control group (10%) could not be reached via phone or email. These families were not included in the analysis, but dropout was judged to be low and balanced enough that outcomes are unlikely to have been biased. |
| Selective reporting (reporting bias) | High risk | Some outcomes listed in the protocol that were of interest to this review were not fully reported in the paper or on clinicaltrials.gov (e.g. satisfaction with asthma care between groups, total scores on the ITG‐ASF and ACT). |
| Other bias | Unclear risk | Study authors noted: "The study population was a convenience sample based largely on clinician recommendation and was not designed to be representative of all children with asthma in the care network." This does not necessarily introduce bias. |
| Methods | Study design: parallel, cluster‐randomised, single‐blind RCT Acronym: PELICAN Length of observation: 9 months Setting: 5 outpatient clinics in Holland | |
| Participants | Population: 33 children were randomised within the 5 clusters to the intervention group (15) or the control group (18) Baseline characteristics 66.7% of the intervention group and 57.1% of the control group were male. Mean age was 8.4 years (SD 1.7) in the intervention group and 8.7 years (SD 1.7) in the control group. 93.3% of the intervention group and 100% of the control group were white. In the intervention group, mean FEV1 was 111%; 80% were on ICS; mean PAQLQ was 6.35 (1.17); and ACQ 0.5 (0.6). In the control group, mean FEV1 was 101%; 57% were on ICS; mean PAQLQ was 6.02 (0.89); and ACQ 0.8 (1.4). Inclusion criteria: Children had physician‐diagnosed asthma, were 6 to 12 years of age, and used asthma medication (i.e. bronchodilators and/or inhaled corticosteroids) for at least 6 weeks during the previous year. Exclusion criteria: comorbid conditions that significantly influence health‐related quality of life, not able to attend a regular school class (as an indicator of normal intelligence), and insufficient skills in speaking and/or reading the Dutch language | |
| Interventions | Intervention: shared decision‐making online tool Nurse‐led patient‐centred care via an online tool. First, children completed the PAQLQ and selected 1 to 3 personal asthma problems, which were forwarded to the nurse. Then at the consultation, the nurse discussed with the child and parent which problem to prioritise, discussed details of the problem and chose a treatment goal through shared decision‐making, formulated a SMART goal (specific, measurable, acceptable, realistic, and time‐bound), brainstormed solutions together and documented an action plan, discussed results at the next visit, and repeated if necessary. Nurses were trained in the process during a 2‐hour meeting before the study. Control: enhanced usual care Besides usual care, the intervention group also received recommendations based on the Pelican outcome by a practice nurse. Described as enhanced usual care as seen more regularly than would be the case in practice. | |
| Outcomes | Primary: quality of life (PAQLQ). Secondary: asthma control (ACQ), symptoms and medication via a diary, cost‐effectiveness, caregiver quality of life (PACQLQ), process outcomes | |
| Notes | Trial registration: NCT01109745 Funding: Dutch Lung Foundation (previously Dutch Asthma Foundation), NutsOhra foundation, and a grant from the Nijmegen Centre of Evidence‐Based Practice (RadboudUMC grant) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned children…in a 1:1 ratio using minimization software (Minim) that forced a balance between study arms for age (6–8 vs. 9–11 years old) and asthma control (ACQ score <1 vs greater than or equal to 1)" |
| Allocation concealment (selection bias) | Unclear risk | Not described but states that individual practices managed allocation to groups, which may not have adequately controlled for selection biases |
| Blinding of participants and personnel (performance bias) | High risk | "Children, parents, and nurses were aware of treatment allocation." |
| Blinding of outcome assessment (detection bias) | High risk | "This was a single‐blinded study. The analyses presented in this manuscript were based on blinded data. The study code was broken after the analyses were concluded." Study does not specify who was blinded. Outcome assessment and several outcomes were patient‐rated, which would introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) | High risk | Protocol states: "The primary analysis is an intention‐to‐treat analysis, however both explanatory and intention‐to‐treat analyses will be performed." "A total of 33 children started with the study, 15 in the intervention group and 18 in the usual care group. One child was lost to follow‐up during the study and three children had too many missing data of the primary outcome, leaving 29 children for the analysis." All dropouts were from the usual care group. |
| Selective reporting (reporting bias) | Low risk | Outcomes relative to the review that were defined in the trial registration were reported but could not be included in meta‐analyses owing to non‐parametric methods. |
| Other bias | High risk | The 33 children recruited were significantly fewer than the 170 planned, which (1) meant the study was underpowered and (2) may reflect the feasibility of the intervention. "112 general practices was invited to participate of which 28 practices did not respond and 73 other practices refused participation for reasons such as lack of time, participation in other research projects, too few pediatric asthma patients or no affinity. Of the 11 practices that decided on participation, two practices were withdrawn due to lack of sufficient participants." |
| Methods | Study design: parallel, individually randomised, open‐label RCT Acronym: BOAT Length of observation: 52 weeks and 104 weeks Setting: 5 clinical Kaiser Permanante (a not‐for‐profit health plan) sites in the USA | |
| Participants | Population: 612 adults were randomised to a shared decision‐making intervention (204), clinical decision‐making (204), or a usual care group (204). Baseline characteristics 43.6% of the SDM group was male, 44.1% of the CDM group, and 42.6% of the usual care group. Mean age was 45.7 (SD 13.3) in the SDM group, 46.9 (SD 12.1) in the CDM group, and 45.1 (SD 12.4) in the usual care group. Most participants were white (62.8% SDM, 60.8% CDM, 62.3% usual care). Most participants' asthma symptoms were poorly or very poorly controlled (85.8% SDM, 82.9 CDM, 83.2 usual care). Other characteristics presented included education level, family income, smoking, controller medication use, recent hospitalisation, symptom frequency, and categories of FEV1 % predicted. Inclusion criteria: patients whose asthma was not well controlled, and whose adherence to their asthma regimen was likely to be inadequate. KP members, aged 18 to 70 years, with evidence suggestive of poorly controlled asthma, were identified at 5 clinical sites using computerised records of overuse of rescue medications (a controller/[controller + rescue medication] ratio < 0.5 and at least 3 beta‐agonist dispensings in the past year) or a recent asthma‐related ED visit or hospitalisation. Exclusion criteria: intermittent asthma (brief exacerbations or symptoms less than once/week), primary diagnosis of chronic obstructive pulmonary disease or emphysema, insufficient pulmonary function reversibility (for ex‐/current smokers and those without regular controller use), regular use of oral corticosteroids, current asthma care management | |
| Interventions | Intervention: shared decision‐making Sessions followed the same structure as clinical decision‐making but with the following added: description of SDM approach, identification and summary of patient goals and preferences, discussion of options and relative merits in terms of patients' goals and preferences, and negotiation of a treatment decision. Five sessions; 2 face‐to‐face and 3 over the phone at 3, 6, and 9 months. Intervention delivered to participants by 16 nurses, respiratory therapists, pharmacists, nurse practitioners, and physicians' assistants, most of whom were already asthma care managers. Specific training in shared decision‐making was provided. Control 1: clinical decision‐making Sessions included the following: building rapport, schedule for sessions, symptom/medication/triggers assessed, asthma understanding assessed and improved, spirometry reviewed, asthma severity and control determined using GINA, adherence problems addressed, new regimen recommended based on guidelines, prescription, action plan, inhaler technique instruction and asthma diary given, follow‐up appointment set. Five sessions; 2 face‐to‐face and 3 over the phone at 3, 6, and 9 months. Intervention delivered to participants by 16 nurses, respiratory therapists, pharmacists, nurse practitioners, and physicians' assistants, most of whom were already asthma care managers. Specific training in clinical decision‐making was provided. Control 2: usual care Usual care based on a stepped‐care approach to pharmacotherapy with the aim of long‐term asthma control, as recommended by the National Asthma Education Prevention Program’s Expert Panel Report 2. At some sites, clinicians had the option to refer patients to an asthma care management program similar to but less structured than the clinician decision‐making intervention. | |
| Outcomes | Primary: adherence to controller medications, better asthma‐related quality of life, lower health care utilisation for acute symptoms than among patients who received usual care (no asthma care management). Secondary: short‐acting beta‐agonist (SABA) use, lung function, asthma control | |
| Notes | Trial registration: NCT00149526; NCT00217945 Funding: supported by National Institutes of Health grants R01 HL69358 and R18 HL67092 Notes: Adherence was measured using a continuous medication acquisition (CMA) index for each year, calculated as the total days’ supply acquired in a given year divided by 365 days (30–32). The index represents the proportion of the prescribed medication supply acquired by the patient during each 365‐day period, and may potentially overestimate, but not underestimate, actual use. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐based adaptive randomization algorithm was used to ensure concealment from randomization staff and better‐than‐chance balance among the three groups on age (18–34, 35–50, and 51–70 yr), sex, race/ethnicity, hospitalisation in the prior two years (yes/no), and frequency of asthma controller use in the past week (none,1–3, ≥4 d)." |
| Allocation concealment (selection bias) | Low risk | "computer‐based adaptive randomization algorithm was used to ensure concealment from randomization staff" |
| Blinding of participants and personnel (performance bias) | High risk | Study investigators and participants could not be kept blind to treatment allocation owing to the nature of the interventions. |
| Blinding of outcome assessment (detection bias) | High risk | Most outcomes would be subject to some form of detection bias by knowledge of treatment allocation, particularly self‐rated outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Similar proportions of participants in each group were followed up at 12 months (89.2% in the SDM group, 88.2% in the CDM group, and 92.6% in the usual care group). Attendance was similar in SDM and CDM groups for all time points except 9 months, where fewer people in the CDM group (59.3%) than the SDM group (75.5%) attended. It is assumed that attendance at the session resulted in gathering of appropriate outcome data at this time point. |
| Selective reporting (reporting bias) | High risk | Several outcomes are not reported fully for year 2 (including adherence and asthma control), and only results for the symptom subscale are given for the quality of life measure, rather than the total score. |
| Other bias | Low risk | None noted |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; CDM: clinician decision‐making; CMA: continuous medication acquisition; ED: emergency department; EHR: electronic health record; FEV1: forced expiratory volume in one second; GINA: Global Initiative for Asthma; GP: general practitioner; ICS: inhaled corticosteroid; ITG‐AST: Integrated Therapeutics Group ‐ Child Asthma Short Form; NAEPP: National Asthma Education and Prevention Program; PACQLQ: Pediatric Asthma Caregiver's Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; RCT: randomised controlled trial; SD: standard deviation; SDM: shared decision‐making; SMART: specific, measurable, acceptable, realistic, and time‐bound (goal).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Population does not match the inclusion criteria: mixed respiratory population; only 17% had asthma and results are not given separately | |
| Intervention does not match the inclusion criteria: Focus is asthma education, self‐management, and empowerment, rather than shared decision‐making. | |
| Intervention does not match the inclusion criteria: case management/discharge planning from emergency department. Emphasis is not on shared decision‐making. | |
| Intervention does not match the inclusion criteria: Main emphasis is on communication skills. Not enough information about the intervention to include confidently (only abstracts, no full publication identified) | |
| Intervention does not match the inclusion criteria: Focus is on supporting physicians' decisions, not on sharing decisions with patients | |
| Intervention does not match inclusion criteria; broad intervention in which shared decision‐making was not the primary focus | |
| Not an RCT: single group assignment | |
| Intervention does not match the inclusion criteria: patient‐centred education following ED visit, not decision‐making | |
| Intervention does not match the inclusion criteria: video to educate asthma families to follow an action plan. Some emphasis on communication but not strictly on shared decision‐making with a clinician | |
| Intervention does not match the inclusion criteria: testing different methods of disseminating a shared decision‐making intervention, rather than assessing whether it works | |
| Intervention does not match the inclusion criteria: child‐centred care and empowerment to self‐manage asthma, not shared decision‐making |
ED: emergency department; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel randomised controlled trial |
| Participants | Convenience sample of participants 18 to 65 years, with diagnosis of mild to severe asthma, and prescribed inhaled corticosteroids, alone or in combination with long‐acting β2‐agonists |
| Interventions | Asthma eduction plus decision aid vs asthma education alone |
| Outcomes | Knowledge of asthma; decisional conflict; appropriate use of asthma pharmacotherapy; asthma control |
| Notes | Funding: Principal investigator and co‐investigator received a grant from the Allergy, Genes and Environment Network for funding of the research (reference number for the project: 11CKT2). Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Publication: peer‐reviewed journal article |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Rationale and design of a comparative effectiveness trial of home‐ and clinic‐based self‐management support coaching for older adults with asthma |
| Methods | Pragmatic randomised controlled trial with 3 arms |
| Participants | 425 adults with asthma aged ≥ 60, based in New York |
| Interventions | 1. Intervention delivered in primary care 2. Intervention delivered at home 3. Usual care "In the intervention, care coaches use a novel screening tool to identify the specific barriers to asthma control and self‐management they experience. Once identified, the coach and patient choose from a menu of actions to address it. The intervention emphasizes efficiency, flexibility, shared decision making and goal setting, communication strategies appropriate for individuals with limited cognition and literacy skills, and ongoing reinforcement and support. Additionally, we introduced asthma‐specific enhancements to the electronic health records of all participating clinical practices, including an asthma severity assessment, clinical decision support, and a patient‐tailored asthma action plan." |
| Outcomes | Patients will be followed for 12 months and interviewed at baseline, and at 3, 6, and 12 months; data on emergency department visits and hospitalisations will be obtained through the New York State Statewide Planning and Research Cooperative System. |
| Starting date | Unclear |
| Contact information | Alex D Federman ‐ Division of General Internal Medicine, Icahn School of Medicine at Mount SInai, New York |
| Notes |
| Trial name or title | Goal‐setting intervention in patients with active asthma |
| Methods | Two‐armed, single‐blind, multi‐centre, cluster‐randomised controlled feasibility trial |
| Participants | Planned recruitment: 80 |
| Interventions | "Patients in the intervention arm will be asked to complete a novel goal‐setting tool immediately prior to an asthma review consultation. This will be used to underpin a focused discussion about their goals during the asthma review. A tailored management plan will then be negotiated to facilitate achieving their prioritised goals. Patients in the control arm will receive a usual care guideline‐based review of asthma." |
| Outcomes | "Data on quality of life, asthma control and patient confidence will be collected from both arms at baseline and 3 and 6 months post‐intervention. Data on health services resource use will be collected from all patient records 6 months pre‐ and post‐intervention. Semi‐structured interviews will be carried out with healthcare staff and a purposive sample of patients to elicit their views and experiences of the trial. The outcomes of interest in this feasibility trial are the ability to recruit patients and healthcare staff, the optimal method of delivering the intervention within routine clinical practice, and acceptability and perceived utility of the intervention among patients and staff." |
| Starting date | Overall trial start date: 01/09/2012 Overall trial end date: 30/11/2013 |
| Contact information | Dr Gaylor Hoskins ‐ Nursing Midwifery and Allied Health Professions (NMAHP) Research Unit, Iris Murdoch Building, University of Stirling |
| Notes |
| Trial name or title | Assessment of shared decision‐making aids in asthma |
| Methods | Randomised, parallel, double‐blind study (investigators and outcome assessors blinded) |
| Participants | Planned enrolment: 51 Men or women, aged 18 to 65 years, with current diagnosis of mild to severe asthma (details of asthma eligibility given on clinicaltrials.gov) People with COPD or recent asthma education (last 6 months) excluded |
| Interventions | Patient decision aid that participants read and fill before being provided education on asthma. The decision aid is a 12‐page A3 booklet entitled "Should I take asthma inhaled controller medication to optimize asthma control?" Control group received no intervention. |
| Outcomes | Primary outcomes: asthma knowledge measured by QCALF score and decisional conflict measured by DCS score (both as change from baseline to 2 months) |
| Starting date | March 2013 ‐ Study authors confirmed that study was undergoing amendments at the time of writing of this review. |
| Contact information | Louis‐Philippe Boulet, MD, Centre de Recherche de l'Institut Universitaire de Cardiologie et de Pneumologie de Quebec |
| Notes |
| Trial name or title | Comparative effectiveness of asthma interventions within a practice‐based research network |
| Methods | Unclear if randomised. A centralised database will be created with the goal of facilitating comparative effectiveness research on asthma outcomes specifically for this study. Patient and community level analysis will include results from patient surveys, focus groups, and asthma patient density mapping. Community variables such as income and housing density will be mapped for comparison. |
| Participants | This study will include 95 practices, 171 schools, and more than 30,000 asthmatic patients. |
| Interventions |
|
| Outcomes | Hospitalisations and emergency department visits; improved adherence to medication; improved quality of life; reduced school absenteeism; improved self‐efficacy; |
| Starting date | Unclear |
| Contact information | [email protected] ‐ Carolinas Physicians Network, Carolinas HealthCare System, Charlotte, NC |
| Notes |
ACSS: Asthma Control Scoring System; COPD: chronic obstructive pulmonary disease; DCS: Decisional Conflict Scale; EMR: electronic medical record; QCALF: self‐administered French scale assessing four domains of asthma knowledge.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life improvement (AQLQ responders) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Shared decision‐making versus usual care, Outcome 1 Quality of life improvement (AQLQ responders). | ||||
| 2 Quality of life scores (ITG‐ASF) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Shared decision‐making versus usual care, Outcome 2 Quality of life scores (ITG‐ASF). | ||||
| 2.1 ITG‐ASF night‐time symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ITG‐ASF daytime symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 ITG‐ASF functional limitation scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life scores (mini‐AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Shared decision‐making versus usual care, Outcome 3 Quality of life scores (mini‐AQLQ). | ||||
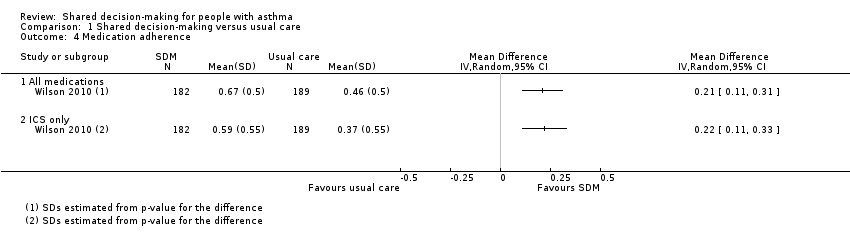
| 4 Medication adherence Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.4 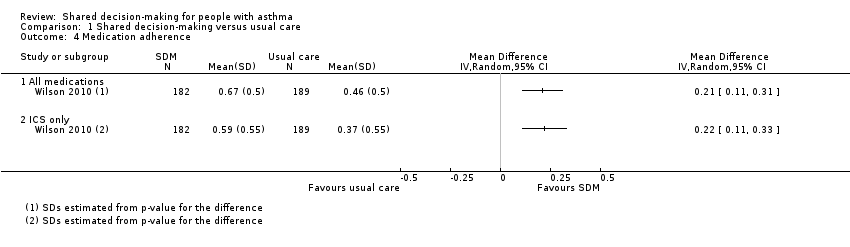 Comparison 1 Shared decision‐making versus usual care, Outcome 4 Medication adherence. | ||||
| 4.1 All medications | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 ICS only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations of asthma Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Shared decision‐making versus usual care, Outcome 5 Exacerbations of asthma. | ||||
| 5.1 Requiring hospital admission | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Requiring ED visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Requiring specialist visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Requiring GP visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma well controlled Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Shared decision‐making versus usual care, Outcome 6 Asthma well controlled. | ||||
| 6.1 ACQ < 1 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 ACT > 22 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 ATAQ = 0 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.
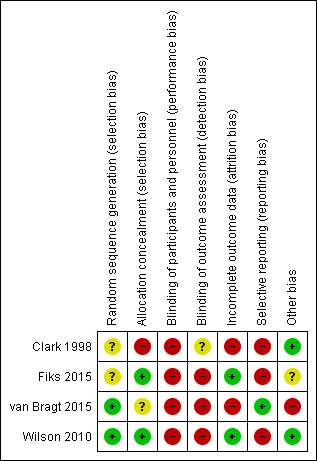
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Shared decision‐making versus usual care, Outcome 1 Quality of life improvement (AQLQ responders).

Comparison 1 Shared decision‐making versus usual care, Outcome 2 Quality of life scores (ITG‐ASF).

Comparison 1 Shared decision‐making versus usual care, Outcome 3 Quality of life scores (mini‐AQLQ).
Comparison 1 Shared decision‐making versus usual care, Outcome 4 Medication adherence.

Comparison 1 Shared decision‐making versus usual care, Outcome 5 Exacerbations of asthma.
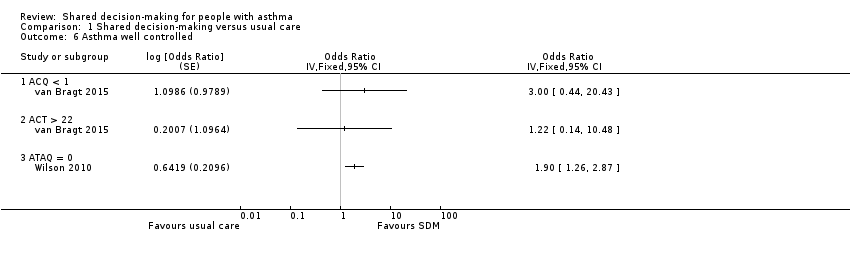
Comparison 1 Shared decision‐making versus usual care, Outcome 6 Asthma well controlled.
| Shared decision‐making compared with usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | ||
| Risk with usual care | Risk with shared decision‐making | ||||||
| Asthma‐related quality of life (follow‐up: 6 to 24 months) | AQLQ responders | 556 per 1000 | 704 per 1000 | OR 1.90 | 371 | ⊕⊕⊕⊝ | Participants achieving > 0.5‐point improvement (MCID for this scale) |
| ITG‐ASF daytime symptom scale | Mean ITG‐ASF daytime symptom score was 12 | MD 4 higher | ‐ | 53 | ⊕⊝⊝⊝ | Higher score = Better quality of life The same study also reported mean night‐time symptom scale and functional limitation scale (see Analysis 1.2). | |
| Mini‐AQLQ | Mini‐AQLQ score was 5.5 | MD 0.4 higher | ‐ | 371 | ⊕⊕⊝⊝ | Higher score = Better quality of life. MCID 0.5 | |
| Parent/patient satisfaction | Presentation on forest plot not possible; summarised narratively in text and Table 2 | ‐ | ‐ | ‐ | |||
| Medication adherence (follow‐up: 12 to 24 months) | ICS only | The ICS adherence was 0.59 | MD 0.22 higher | ‐ | 371 | ⊕⊕⊕⊝ | Adherence calculated using continuous medication acquisition (CMA) from pharmacy data. Maximum score 1. The same study reported all‐medication adherence (see Analysis 1.4). |
| Exacerbations of asthma (follow‐up: 6 months) | Requiring ED visit | 222 per 1,000 | 77 per 1,000 | OR 0.29 | 53 | ⊕⊕⊝⊝ | The same study reported exacerbations requiring hospital admission, "specialist visits", and GP visits (see Analysis 1.5). |
| Asthma control (follow‐up: 12 to 24 months) | Asthma well controlled; ATAQ = 0 | No control group risk presented | Not estimable | OR 1.90 | 371 (1 RCT) | ⊕⊕⊕⊝ | Lower score = Better asthma control A different small study reported asthma control on ACT and ACQ (see Analysis 1.6). |
| Adverse events (all) | Included trials did not measure or report any adverse events. | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence. | |||||||
| aRisk of performance and detected bias. Downgraded once. bOne study. Confidence intervals include possible harm and benefit of intervention. Downgraded once. cOnly quality of life subscales reported. Downgraded once for indirectness. dAlthough the mean difference for this scale lies below the MCID, the responder analysis suggests that significantly more people achieved the MCID change with the intervention. No downgrade. eAdherence calculated using continuous medication acquisition from pharmacy data. This is a proxy measure and may overestimate true adherence. Downgraded once. fOne study. Confidence intervals very wide and include possible harm and benefit of intervention. Downgraded twice. | |||||||
| Study ID | Country | Population | Age (years) | Design | Intervention | Aimed at | Control |
| USA | 74 physicians; 637 children | 1 to 12 | Cluster RCT | SDM seminars | HCPs | Usual care | |
| USA | 60 families | 6 to 12 | Individual RCT | SDM portal | HCPs and patients/parents | Usual care + decision support | |
| Holland | 33 children | 6 to 12 | Cluster RCT | SDM online tool | HCPs and patients/parents | Enhanced usual care | |
| USA | 612 adults | 18 to 65 | Individual RCT | SDM structured sessions | HCPs | 1. Guideline‐led decision‐making 2. Usual care | |
| HCP: healthcare provider; RCT: randomised controlled trial; SDM: shared decision‐making. | |||||||
| Was/did the clinician: | SDM | Control | P value (GEEa) |
| Reassuring and encouragingb | 4.63 | 4.42 | 0.006 |
| Look into how family managed | 3.98 | 3.69 | 0.02 |
| Describe how child should be fully | 71.% | 59% | 0.007 |
| Describe at least 1 of 3 goals: | 75% | 64% | 0.07 |
| Give information to relieve specific | 4.1 | 3.9 | 0.007 |
| Enable family to know how to make | 4.3 | 4.2 | 0.07 |
| aGEE method to assess "Time2" (follow‐up) scores with baseline scores and group assignment as covariates in regression models. NB: A total of 472 parents were followed up; numbers in each group are not given. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life improvement (AQLQ responders) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Quality of life scores (ITG‐ASF) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 ITG‐ASF night‐time symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ITG‐ASF daytime symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 ITG‐ASF functional limitation scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life scores (mini‐AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Medication adherence Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 All medications | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 ICS only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations of asthma Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Requiring hospital admission | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Requiring ED visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Requiring specialist visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Requiring GP visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma well controlled Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 6.1 ACQ < 1 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 ACT > 22 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 ATAQ = 0 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

