Toma de decisiones compartida para los pacientes con asma
Resumen
Antecedentes
El asma es una enfermedad inflamatoria crónica que afecta las vías respiratorias y es frecuente tanto en adultos como en niños. Se caracteriza por síntomas que incluyen sibilancias, disnea, opresión torácica y tos. A los pacientes con asma se los puede ayudar a controlar la enfermedad mediante la toma de decisiones compartida (TDC). La TDC incluye al menos a dos participantes (el médico y el paciente) y el intercambio mutuo de información, incluidos los valores y las preferencia del paciente, para lograr un consenso acerca del tratamiento favorecido que culmina en una acción acordada. El autocuidado efectivo es particularmente importante para los pacientes con asma, y la TDC puede mejorar los resultados clínicos y la calidad de vida al educar a los pacientes y motivarlos a participar activamente en su propia salud.
Objetivos
Evaluar los posibles beneficios y los efectos perjudiciales de la toma de decisiones compartida para los adultos y los niños con asma.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Trials Register), que contiene estudios identificados en varias fuentes, incluyendo CENTRAL, MEDLINE y Embase. También se hicieron búsquedas en registros de ensayos clínicos y se verificaron las listas de referencias de los estudios incluidos. Las búsquedas más recientes se han realizado el 29 de noviembre de 2016.
Criterios de selección
Se incluyeron estudios controlados con asignación al azar individual o grupal y de diseño paralelo realizados para comparar una intervención de TDC para adultos y niños con asma versus una intervención de control. Se incluyeron los estudios disponibles como informes de texto completo, los publicados solo como resúmenes y los datos no publicados, y no se aplicaron restricciones sobre el lugar, la fecha ni el idioma de publicación. Se incluyeron intervenciones dirigidas a los profesionales de la asistencia sanitaria o a los pacientes, sus familias o cuidadores, o ambos. Se incluyeron estudios que comparaban la intervención versus atención habitual o una intervención mínima de control, y los que comparaban una intervención de TDC versus otra intervención activa. Se excluyeron los estudios de las intervenciones que incluían componentes múltiples diferentes de la intervención de TDC a menos que el grupo de control también recibiera estas intervenciones.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron las búsquedas, extrajeron los datos de los estudios incluidos y evaluaron el riesgo de sesgo. Los resultados primarios fueron la calidad de vida relacionada con el asma, la satisfacción del paciente/padre y la adherencia a la medicación. Los resultados secundarios incluyeron las exacerbaciones del asma, el control del asma, la aceptabilidad/factibilidad desde la perspectiva de los profesionales de la asistencia sanitaria y todos los eventos adversos. Se calificó y presentó la evidencia en una tabla de “Resumen de los hallazgos”.
No fue posible agrupar ninguno de los datos de resultado extraídos debido a la heterogeneidad clínica y metodológica, aunque los hallazgos se presentaron en diagramas de bosque cuando fue posible. Los datos asimétricos se describieron de forma narrativa.
Resultados principales
Se incluyeron cuatro estudios que compararon la TDC versus control y que incluían a un total de 1342 participantes. Tres estudios incluyeron a niños con asma y a sus cuidadores, y uno incluyó a adultos con asma. Tres estudios se realizaron en los Estados Unidos y uno en los Países Bajos La duración del ensayo fue entre seis y 24 meses. Un ensayo administró la intervención de TDC al médico, y tres ensayos administraron la intervención de TDC directamente al participante. Dos estudios pediátricos incluyeron el uso de un portal en línea, seguido de consultas cara a cara. Un estudio administró una intervención de TDC o una intervención de toma de decisiones clínicas a través de una combinación de consultas cara a cara y llamadas telefónicas. El estudio final asignó al azar a médicos pediátricos generales para recibir un programa de seminario que promovía la aplicación de los principios de TDC. Todos los ensayos fueron no enmascarados, aunque un estudio, que administró la intervención a los médicos, declaró que los participantes desconocían la participación de los médicos en el ensayo. Hubo inquietudes acerca del sesgo de selección y de desgaste y el informe selectivo, y se observó que un estudio reclutó significativamente pocos participantes. Los cuatro estudios incluidos usaron diferentes enfoques para medir la fidelidad/adherencia a la intervención y para informar sobre los resultados del estudio.
Un estudio que incluyó a adultos con asma controlado de forma deficiente informó una mejoría en la calidad de vida (CdV) para el grupo de TDC comparado con el grupo de control, mediante el Asthma Quality of Life Questionnaire (AQLQ) para la evaluación (diferencia de medias [DM] 1,90; intervalo de confianza [IC] del 95%: 1,24 a 2,91), aunque otros dos ensayos no identificaron un beneficio. La satisfacción paciente/padre con el rendimiento de los pediatras fue mayor en el grupo de TDC en un ensayo que incluyó a niños. La adherencia a la medicación fue mejor en el grupo de TDC en dos estudios ‐ uno que incluyó a adultos y uno que incluyó a niños (toda la adherencia a la medicación: DM 0,21; IC del 95%: 0,11 a 0,31; número medio de prescripciones controladas de medicación durante 26 semanas: 1,1 en el grupo de TDC (n = 26) y 0,7 en el grupo de control (n = 27)). En un estudio, las tasas de visitas relacionadas con el asma fueron inferiores en el grupo de TDC que en el grupo de atención habitual (1,0/y versus 1,4/y; p = 0,016), aunque otros dos estudios no informaron una diferencia en las exacerbaciones ni en las prescripciones para los ciclos cortos de esteroides orales. Finalmente, un estudio describió mejores probabilidades de informar ningún problema de asma en el grupo de TDC que en el grupo de atención habitual (odds ratio [OR] 1,90; IC del 95%: 1,26 a 2,87), aunque otros dos estudios que informaron el control del asma no identificaron un beneficio con la TDC. No se encontró información acerca de la aceptabilidad de la intervención para el profesional de la asistencia sanitaria ni información sobre los eventos adversos. En términos generales, la confianza en los resultados del estudio varió de muy baja a moderada, y los resultados se disminuyeron debido al riesgo de sesgo, la imprecisión y la imposibilidad para generalizar la evidencia.
Conclusiones de los autores
Las diferencias apreciables entre los cuatro ensayos controlados aleatorios (ECA) incluidos indican que no es posible proporcionar conclusiones generales significativas. Los estudios individuales demostraron algunos beneficios de la TDC sobre el control, en cuanto a la calidad de vida; la satisfacción del paciente y los padres; la adherencia a la medicación prescrita; la reducción en las visitas a la asistencia sanitaria relacionada con el asma; y la mejoría en el control del asma. La confianza en los hallazgos de estos estudios individuales varía de moderada a muy baja, y es importante observar que los estudios no midieron ni informaron los eventos adversos.
Los ensayos futuros deben tener el poder estadístico suficiente y una duración suficiente para detectar diferencias en los resultados importantes para los pacientes como las exacerbaciones y las hospitalizaciones. El uso de resultados centrales del asma y de escalas validadas, de ser posible, facilitaría el metanálisis futuro. Los estudios realizados en ámbitos de menores ingresos y que incluyan una evaluación económica serían de interés. Los investigadores deben registrar sistemáticamente los eventos adversos, aunque no se prevea ninguno. Los estudios identificados hasta la fecha no han incluido adolescentes; los ensayos futuros deben considerar su inclusión. También se recomienda la medición y el informe de la fidelidad de la intervención.
PICO
Resumen en términos sencillos
¿La toma de decisiones compartida entre el paciente y el profesional de la asistencia sanitaria puede ayudar a los pacientes con asma?
Antecedentes a la pregunta
El asma es una enfermedad a largo plazo que es frecuente en adultos y niños. Los pacientes con asma a menudo presentan sibilancias, tos y tienen dificultad para respirar. La toma de decisiones compartida significa involucrar completamente a los individuos con asma en las decisiones acerca de la atención. Por lo general, incluye al paciente y al médico o la enfermera, y las características clave incluyen la posibilidad de compartir la información para ayudar a los individuos con asma a tomar las mejores decisiones para sí mismos. Al incluir a individuos con asma en el proceso de toma de decisiones, se espera un mejor control del asma lo cual les causará menos problemas.
Pregunta de la revisión
Se deseaba examinar la evidencia sobre la toma de decisiones compartida para los pacientes con asma comparada con la atención estándar del asma, o una manera diferente de tomar decisiones de asistencia sanitaria. Se deseaba saber si la toma de decisiones compartida tiene un efecto sobre la calidad de vida, las crisis asmáticas, la satisfacción del paciente con la atención, el control del asma, la adherencia a los planes de medicación y los efectos no deseados.
Características de los estudios
Se revisó la evidencia hasta noviembre 2016. Se encontraron cuatro estudios, con 1342 personas, que intentaron responder a esta pregunta. Todos los participantes tenían asma; los participantes de tres estudios eran niños y los de un estudio eran adultos. Tres estudios se realizaron en los Estados Unidos y uno en los Países Bajos; los estudios duraron de seis meses a dos años. Diferentes estudios usaron diferentes métodos de toma de decisiones compartida, que incluyeron discusiones cara a cara, llamadas telefónicas y mensajes en línea.
Resultados clave
Debido a que estos estudios se realizaron de diferentes maneras, no fue posible combinar los resultados. Se encontró evidencia de estudios individuales que indicaban que la toma de decisiones compartida puede mejorar la calidad de vida y el control del asma y puede reducir las visitas de asistencia sanitaria para el asma. La toma de decisiones compartida también puede ayudar a los pacientes a tomar el inhalador para el asma más regularmente debido a la mejor comprensión de por qué necesitan hacerlo. El hecho de pasar por este proceso puede dar lugar a que los pacientes se sientan más satisfechos con la atención, debido a que pueden sentirse motivados acerca de la elección de alternativas. Sin embargo, todos estos resultados fueron informados por diferentes estudios, y algunos estudios revelaron un beneficio de la toma de decisiones compartida, mientras que otros no. Es importante mencionar que ninguno de estos estudios consideró si la toma de decisiones compartida causa efectos secundarios no deseados. Los cuatro estudios midieron la eficacia en la administración o la recepción de la intervención de toma de decisiones compartida aunque lo hicieron de diferentes maneras.
Calidad de la evidencia
No existe mucha seguridad en cuanto a la calidad de la evidencia presentada en esta revisión. Hubo preocupación en cuanto al número pequeño de estudios y acerca de las diferencias en la forma en que se diseñaron los estudios incluidos. Además, los participantes sabían en qué grupo estaban (es decir toma de decisiones compartida o atención estándar), lo cual puede haber afectado a cómo respondieron a las preguntas acerca del asma durante el ensayo.
Mensajes a domicilio
Alguna evidencia indica que la toma de decisiones compartida podría ayudar a los pacientes con asma, aunque no existe seguridad en cuanto a si es útil. En los estudios futuros, los estudios más amplios que incluyan a adolescentes mientras observan los efectos secundarios, los efectos perjudiciales y los beneficios deberían resultar útiles para responder esta pregunta.
Authors' conclusions
Summary of findings
| Shared decision‐making compared with usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | ||
| Risk with usual care | Risk with shared decision‐making | ||||||
| Asthma‐related quality of life (follow‐up: 6 to 24 months) | AQLQ responders | 556 per 1000 | 704 per 1000 | OR 1.90 | 371 | ⊕⊕⊕⊝ | Participants achieving > 0.5‐point improvement (MCID for this scale) |
| ITG‐ASF daytime symptom scale | Mean ITG‐ASF daytime symptom score was 12 | MD 4 higher | ‐ | 53 | ⊕⊝⊝⊝ | Higher score = Better quality of life The same study also reported mean night‐time symptom scale and functional limitation scale (see Analysis 1.2). | |
| Mini‐AQLQ | Mini‐AQLQ score was 5.5 | MD 0.4 higher | ‐ | 371 | ⊕⊕⊝⊝ | Higher score = Better quality of life. MCID 0.5 | |
| Parent/patient satisfaction | Presentation on forest plot not possible; summarised narratively in text and Table 2 | ‐ | ‐ | ‐ | |||
| Medication adherence (follow‐up: 12 to 24 months) | ICS only | The ICS adherence was 0.59 | MD 0.22 higher | ‐ | 371 | ⊕⊕⊕⊝ | Adherence calculated using continuous medication acquisition (CMA) from pharmacy data. Maximum score 1. The same study reported all‐medication adherence (see Analysis 1.4). |
| Exacerbations of asthma (follow‐up: 6 months) | Requiring ED visit | 222 per 1,000 | 77 per 1,000 | OR 0.29 | 53 | ⊕⊕⊝⊝ | The same study reported exacerbations requiring hospital admission, "specialist visits", and GP visits (see Analysis 1.5). |
| Asthma control (follow‐up: 12 to 24 months) | Asthma well controlled; ATAQ = 0 | No control group risk presented | Not estimable | OR 1.90 | 371 (1 RCT) | ⊕⊕⊕⊝ | Lower score = Better asthma control A different small study reported asthma control on ACT and ACQ (see Analysis 1.6). |
| Adverse events (all) | Included trials did not measure or report any adverse events. | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence. | |||||||
| aRisk of performance and detected bias. Downgraded once. bOne study. Confidence intervals include possible harm and benefit of intervention. Downgraded once. cOnly quality of life subscales reported. Downgraded once for indirectness. dAlthough the mean difference for this scale lies below the MCID, the responder analysis suggests that significantly more people achieved the MCID change with the intervention. No downgrade. eAdherence calculated using continuous medication acquisition from pharmacy data. This is a proxy measure and may overestimate true adherence. Downgraded once. fOne study. Confidence intervals very wide and include possible harm and benefit of intervention. Downgraded twice. | |||||||
Background
Description of the condition
Asthma is a chronic disease that affects the airways. It is usually characterised by chronic inflammation of the airways, which causes wheeze, shortness of breath, chest tightness, cough, and variable airflow limitation (GINA 2016). Symptoms vary significantly in nature, frequency, and severity, both within and between individuals with a diagnosis of asthma. Day‐to‐day symptoms vary according to the presence of external stimuli (e.g. exercise, allergens), and people with asthma can experience flare‐ups or 'exacerbations', which are associated with significant morbidity and mortality worldwide (GINA 2016; Global Asthma Network 2014; NRAD 2014). Long‐term goals of asthma management include maintaining control of symptoms and minimising risk of exacerbations, airflow limitation, and treatment side effects (GINA 2016). Educating adults and children to self‐manage their asthma is widely recognised as integral to achieving these goals (Gibson 2002; Guevara 2003).
Description of the intervention
Shared decision‐making (SDM) should involve at least two participants (the medical practitioner and the patient) and is defined as mutual sharing of information to build consensus about preferred treatment that culminates in an agreed action (Charles 1997). Decisions about management of long‐term conditions are based on a multitude of factors, including relative efficacy and safety of treatments, costs, and palatability. Shared decision‐making provides a way of balancing these factors by considering the values and preferences of the patient and the opinions of healthcare providers. Légaré describes the three essential elements of SDM as follows (Légaré 2013).
-
Recognizing and acknowledging that a decision is required.
-
Knowing and understanding the best available evidence.
-
Incorporating the patient's values and preferences into all decisions.
For asthma, management guidelines increasingly acknowledge the role of "the patient and healthcare provider partnership" for a shared‐care approach (GINA 2016). Interventions provided to encourage patient‐centred care in clinical consultations across a range of conditions generally put the onus on the healthcare provider; some seek to offer a pathway for patients or parents to better engage in their asthma care; and others suggest a combination of these approaches (Dwamena 2012; Fiks 2015; Wilson 2010). Thus different approaches may have different aims and outcomes. Interventions aimed at changing healthcare provider behaviour might include open communications, efforts to identify and address patient and family concerns about asthma and its treatment, discussion of treatment preferences and barriers to implementation, shared development of treatment goals, and encouragement of active self‐assessment and self‐management (NHLBI/NAEPP 2007).
How the intervention might work
The potential benefit of SDM is dependent on the willingness and ability of both sides to interact, and this ability might depend on factors such as "ethnicity, literacy, understanding of health concepts (health literacy), numeracy, beliefs about asthma and medications, desire for autonomy, and the health care system" (GINA 2016). As such, SDM will not necessarily be equally acceptable to all patients or care‐givers and may not be applied in the same way across healthcare contexts. Benefits of SDM may be seen for individuals and more widely for health services and society as enhanced uptake of evidence‐based options and reduction in overuse of options that confer minimal benefit, thus reducing practice and geographic variations in care and avoiding unnecessary expenditures (Coulter 2011; Légaré 2014).
Preferences for an active, collaborative, or passive role in decision‐making vary among populations, but patient roles are often passive, and many patients report that they wish to be more involved (Caress 2005; Sleath 2011). Patient preferences for involvement in decision‐making are related to education level, perceptions of the healthcare provider, financial barriers to receiving appropriate care, and psychosocial factors, but preferences have not been strongly associated with demography or asthma severity (Adams 2001; Caress 2005). Nonetheless, evidence regarding how best to achieve SDM in practice is sparse, especially in paediatric asthma with regards to the child‐parent relationship and adapted emphasis on SDM as the child matures (Rivera‐Spoljaric 2014).
Researchers have highlighted organisational factors that may serve as a barrier to feelings of satisfaction among patients or families regarding the role they play in their asthma care, especially quality and duration of consultations, which vary substantially across healthcare contexts (Caress 2005). A narrative synthesis of the fast‐growing trend toward patient involvement in medicine has identified that the preparedness of service systems can enable successful SDM, alongside empowerment, patient education, communication for involvement, and staff training (Snyder 2016). It is possible that engaging in SDM may cause unintended harms, for example, by allowing a patient to choose an option without proper discussion of harms and benefits, so it is important that staff are appropriately trained, and that decision aids are used correctly (Coulter 2011).
Why it is important to do this review
Shared decision‐making (SDM) may improve clinical outcomes and quality of life by educating and empowering patients to be actively involved in their own health (Butz 2007; Wilson 2010). These interventions may be particularly beneficial in people with asthma, as self‐management behaviours are important for, and make SDM particularly relevant to, the population with asthma (Gibson 2002; Guevara 2003). The US Institute of Medicine has prioritised SDM, and Asthma UK has identified methods to "empower and enable people to take control of their own asthma" as a research priority (Asthma UK 2011; Institute of Medicine 2009).
A recent Cochrane review found 43 studies that tested effects of interventions to encourage patient‐centred care in clinical consultations, and found mixed results in terms of patient satisfaction, health behaviour, and health status (Dwamena 2012). Review authors suggested that complex interventions with condition‐specific materials aimed at both providers and patients might be promising, but acknowledge that evidence was limited at the time. Similarly, Légaré focused on interventions aimed at improving uptake of SDM by healthcare professionals across medical disciplines with a primary focus on how well this is adopted in practice (Légaré 2014). Review of available evidence for SDM in asthma will allow us to conduct wider searches of the asthma literature to find additional studies and to focus on important condition‐specific outcomes. Attention to clinical outcomes is particularly important, given the possible tension between SDM and adherence to clinical guidelines. Growth of SDM research means it is likely that new evidence will have been published since the time existing reviews were prepared.
Objectives
To assess benefits and potential harms of shared decision‐making for adults and children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that used individual or cluster randomisation. We planned to exclude cross‐over trials; however, we will include the first phase of cross‐over trials in future versions of the review. We did not identify any relevant cross‐over studies. We excluded non‐randomised studies because they would restrict our ability to imply causation of intervention effects and are more likely to be subject to selection biases and confounders. However, we summarised narratively any non‐randomised evidence identified by our searches and contrasted this summary with results presented in our discussion. We planned to include studies reported as full‐text articles, those published as abstracts only, and unpublished data.
Types of participants
We included studies of adults and children with a diagnosis of asthma, confirmed by a medical practitioner or by spirometry according to guidelines (e.g. GINA 2016). We excluded studies that included participants with other long‐term conditions, in particular, chronic obstructive pulmonary disease (COPD), unless researchers presented separate results for those with asthma. We also excluded studies looking at shared decision‐making (SDM) in asthma specifically for people with cognitive impairments, as these interventions are likely to have a different focus. If a study included a subset of eligible participants (e.g. a mixed population that includes participants with other health conditions), we included it only if we could analyse separately disaggregated data for eligible participants.
Types of interventions
We included studies that assessed SDM interventions for people with asthma. We included interventions aimed at healthcare professionals (specialists, general practitioners, nurses, pharmacists, etc.), patients and their families or care‐givers, or both. We included studies that compared the intervention against usual care or a minimal control intervention and those compared an SDM intervention versus another active intervention, such as clinical decision‐making. We excluded studies of interventions that involved multiple components other than the SDM intervention unless the control group also received these components.
Types of outcome measures
Primary outcomes
-
Asthma‐related quality of life (on a validated scale e.g. Asthma Quality of Life Questionnaire (AQLQ))
-
Patient/parent satisfaction
-
Medication adherence
Secondary outcomes
-
Exacerbations of asthma (leading to a course of oral corticosteroid (OCS) treatment or an unscheduled visit to a healthcare professional)
-
Asthma control (e.g. Asthma Control Questionnaire (ACQ))
-
Acceptability/feasibility from the perspective of healthcare professionals
-
Adverse events (all)
Reporting one or more of the outcomes listed here was not a criterion for inclusion of studies in this review.
Trial authors and editorial teams chose primary outcomes by consensus as those most likely to be relevant to the intervention under investigation and most important to patients and their families/care‐givers.
We prioritised extraction of any validated measures of patient/parent satisfaction, medication adherence, asthma control, and acceptability/feasibility but did not predefine accepted measures in advance, so as not to restrict analyses unnecessarily. If study authors used non‐validated measures, or used a mixture of validated and non‐validated measures across studies, we planned to assess which were sufficiently similar for pooling to make sense.
We planned to extract and analyse data from both parent and child perspectives as provided by paediatric studies.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP 1946 to date.
-
Weekly searches of Embase Ovid SP 1974 to date.
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO).
-
Monthly searches of the Allied and Complementary Medicine database (AMED EBSCO).
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register were identified through search strategies based on the scope of Cochrane Airways. We have presented in Appendix 1 details of these strategies, as well as a list of handsearched conference proceedings.See Appendix 2 for search terms used to identify studies for this review. We based our search terms for 'shared decision‐making' on those used in a Cochrane Review by Légaré (Légaré 2014).
We also conducted a search of ClinicalTrials.gov (http://ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; http://who.int/ictrp/en/). We searched all databases from their inception to the present, and we imposed no restriction on language of publication. We conducted the most recent searches on 29 November 2016.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references.
On 15 November 2016, we searched for errata or retractions from included studies published in full text on PubMed (http://ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (KK and RN) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications for all studies in the 'retrieve' category. Two review authors (KK and PM) independently screened full‐text articles and identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted a third person. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram (Figure 1) and Characteristics of excluded studies tables (Moher 2009).

Study flow diagram.
Data extraction and management
We used a data collection form piloted on one included study to record study characteristics and outcome data. One review author (KK) extracted the following study characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and dates of the study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (KK and RN) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if a study reported outcome data that were not useable in an analysis. We resolved disagreements by reaching consensus or by involving a third person. One review author (KK) transferred data into the Review Manager (RevMan) file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review versus data provided in the study reports.
Assessment of risk of bias in included studies
Two review authors (KK and RN) independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with a third person. We assessed the risk of bias of each included study according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for each study that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios and continuous data as mean differences. Had we been able to combine data presented on different scales, we planned to use standardised mean differences. We entered data presented as a scale with a consistent direction of effect.
We planned to undertake meta‐analyses only when this was meaningful (i.e. if treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When a single study reported multiple trial arms, we planned to include only the relevant arms. If we had combined two comparisons (e.g. two types of SDM vs usual care) in the same meta‐analysis, we planned to halve the control group to avoid double counting.
If both change from baseline and endpoint scores were available for continuous data, we planned to use change from baseline unless most studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end‐of‐study measurement.
If both an analysis that included only participants who completed the trial and an analysis that imputed data for participants who were randomly assigned but did not provide endpoint data (e.g. last observation carried forward) were available, we planned to use the latter.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of people admitted with one or more exacerbation, rather than number of exacerbations per person). We planned to meta‐analyse data from cluster RCTs only if available data had been adjusted (or could be adjusted) to account for clustering.
Dealing with missing data
We planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when we identify a study as an abstract only). However, we identified full‐text reports of all included studies.
Assessment of heterogeneity
We planned to use the I² statistic to measure heterogeneity among the studies in each analysis. If we had identified substantial heterogeneity, we planned to report this and to explore possible causes by conducting prespecified subgroup analyses.
Assessment of reporting biases
We were not able to pool more than 10 studies, so we could not create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We planned to use a random‐effects model and to perform a sensitivity analysis using a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using the outcomes listed in this review. We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used the GRADEpro Guideline Development Tool (GRADEpro GDT). We used footnotes to justify all decisions to downgrade or upgrade the quality of the evidence, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analysesa for primary outcomes.
-
Age of the asthma population (children < 12 years of age, 12 to 18 years of age, adults > 18 years of age).
-
Focus of the intervention (i.e. population randomised to the intervention: healthcare providers vs patients/parents).
-
Duration/extensiveness of intervention (e.g. one‐off or simple intervention vs ongoing SDM sessions).
aChildren, adolescents, and adults may have quite different needs and preferences with respect to SDM, so interventions may have different focuses and effects across age groups. We expected study effects to vary regarding focus and extent of the intervention, and we tried to assess this in the other two subgroup analyses. However, a subgroup analysis can look at only one of these effect modifiers at a time and does not imply causation; therefore, we planned to interpret the results cautiously. We presented these and other possible effect modifiers in Table 1.
| Study ID | Country | Population | Age (years) | Design | Intervention | Aimed at | Control |
| USA | 74 physicians; 637 children | 1 to 12 | Cluster RCT | SDM seminars | HCPs | Usual care | |
| USA | 60 families | 6 to 12 | Individual RCT | SDM portal | HCPs and patients/parents | Usual care + decision support | |
| Holland | 33 children | 6 to 12 | Cluster RCT | SDM online tool | HCPs and patients/parents | Enhanced usual care | |
| USA | 612 adults | 18 to 65 | Individual RCT | SDM structured sessions | HCPs | 1. Guideline‐led decision‐making 2. Usual care |
HCP: healthcare provider; RCT: randomised controlled trial; SDM: shared decision‐making.
We planned to use the formal test for subgroup interactions provided in RevMan (RevMan 2014).
Sensitivity analysis
We planned to perform the following sensitivity analyses by removing the following from the primary analyses.
-
Unpublished data.
-
Studies at high risk in any selection bias domain.
Results
Description of studies
Results of the search
We identified 152 records in main database searches (including a search of clinicaltrials.gov), 21 from the WHO trials portal, four from reference lists of included studies, and one through author correspondence. We found that four were duplicates, and we screened the remaining 174 records. We excluded 137 records that did not meet review inclusion criteria by looking at titles and abstracts, and we obtained full texts for the 37 remaining records. After reviewing full texts, we deemed that 21 records were ineligible for inclusion in the review: 16 because they did not meet the inclusion criteria and five because they were ongoing studies (related to four studies: Federman 2015; Hoskins 2013; NCT02516449; Tapp 2011). We collated the 16 excluded records into 11 unique studies, which we have described under Excluded studies. We collated the other 17 records into four unique studies and included them in the review (Figure 1).
We conducted a further search on 27 June 2017 before preparation of this publication. One study investigating the use of decision aids may meet the inclusion criteria for this review, and we will fully assess this trial for inclusion when we update the review (Studies awaiting classification).
Included studies
Four studies, including a total of 1342 participants, met the inclusion criteria for this review (Clark 1998; Fiks 2015; van Bragt 2015; Wilson 2010). We have presented a summary of study characteristics in Table 1. We have provided more information about each study's design, setting, inclusion criteria, population and intervention, and risk of bias assessments in the Characteristics of included studies tables.
Study design and setting
Wilson individually randomised 612 adults with asthma across five US clinical Kaiser Permanante (KP; a large not‐for‐profit integrated managed care consortium) sites (Wilson 2010). The three remaining studies involved children and their families. Clark cluster‐randomised 74 US general practice paediatricians, with 637 children enrolled under their care, in Michigan and New York State (Clark 1998). Fiks individually randomised 60 families of children with asthma across three primary care practices in Philadelphia (Fiks 2015). Finally, van Bragt randomised five outpatient clinics in the Netherlands, enrolling a total of 33 children with asthma (van Bragt 2015).
Population characteristics
Forty‐three per cent (266/612) of participants in the only adult study were male, and investigators reported a mean age of 45.1 to 46.9 years across the three intervention arms (Wilson 2010). Approximately 60% of participants were Caucasian, 15% Africian American, and 10% Asian, with the remaining participants from Hispanic, Pacific Islander, and American Indian ethnic groups. Approximately 70% of participants reported a household income greater than $40,000 per year, and more than 95% had completed at least high school level education. Eighty‐four per cent of participants were reported to have poorly or very poorly controlled asthma at baseline, with forced expiratory volume in one second (FEV1) < 80% predicted in 70% of participants. Approximately 16% were current smokers.
The Clark study reported that 60% (44/74) of included paediatricians were male, as were 70% (471/637) of enrolled children (Clark 1998). Researchers provided data on an average of 10 children per paediatrician (range 1 to 33). Seven per cent of enrolled children were younger than two years of age, 59% were between two and seven years, and 34% were 8 to 12 years old. Fifteen per cent of enrolled children were Latino/Hispanic, and 15% were Africian American. Study authors provided no details about the ethnicity of the remaining 70%. Approximately 20% of participating families reported a household income less than $20,000 per year, and 16% were below the poverty level of $15,000 annual household income. Almost 90% of parents had at least a high school level education. Investigators did not report baseline asthma severity.
Fiks did not report the gender of the 60 paediatric participants in this trial (Fiks 2015). Children had a mean age of 8.3 years, 47% were black/Africian American, and 42% were white, with the remainder described as Asian, Hispanic, or other. Seventy‐one per cent of parents had at least some college level education, and 75% were in paid employment. Data show that baseline asthma severity was mild in 53% of children, moderate in 42%, and severe in 5%.
Finally, 62% (18/29) of the children included in the last study were male, and their mean age was approximately 8.5 years (van Bragt 2015). Ninety‐seven per cent of children were Caucisian. Eighty‐seven per cent of families in the intervention arm were reported to be from a high socioeconomic group, as were 64% in the control group. Mean FEV1% predicted was > 100% in both groups at baseline. Data indicate that asthma was uncontrolled (ACQ score ≥ 1) at baseline in 3/15 (20%) in the intervention group and in 6/14 (43%) in the control group.
Inclusion and exclusion criteria
Wilson specifically recruited adults whose asthma was not well controlled and were therefore likely to have inadequate adherence to their asthma regimen (Wilson 2010). Eligible patients were between 18 and 70 years of age. Poorly controlled asthma was evident in medical records by overuse of reliever medication or a recent emergency department (ED) visit or hospitalisation for asthma. Participants were excluded if they had intermittent asthma or a primary diagnosis of COPD, or were using regular OCSs. Participants were also excluded if they were already enrolled in an asthma management programme.
Clark enrolled children aged 1 to 12 years through participating paediatric general practitioners (Clark 1998). Eligible children must have had physician‐diagnosed asthma and no other chronic disorders with pulmonary complications, and must have had at least one emergency medical visit for asthma during the past year.
Fiks recruited children aged 6 to 12 years with persistent asthma and an English‐speaking parent or guardian who had consistent access to a computer and the Internet (Fiks 2015). Children were excluded if their asthma was not a primary or current health concern for their parent or guardian, or if they were not taking a "controller medication".
van Bragt recruited children aged 6 to 12 years with physician‐diagnosed asthma who had used asthma medication (bronchodilators and/or inhaled corticosteroids (ICSs)) for at least six weeks over the preceding year (van Bragt 2015). Children were excluded if they had comorbid conditions that would significantly impact their health‐related quality of life, were not receiving mainstream education, or had insufficient Dutch language skills.
Interventions and comparisons
Wilson 2010
Group 1. Shared decision‐making (SDM)
Participants received two face‐to‐face sessions and three phone calls over nine months. Sessions involved eliciting the patient's asthma history, classifying the level of control, and providing asthma education. In the SDM model, this was followed by negotiation of a treatment plan that took into account the participant's goals and preferences. Researchers shared with participants a full list of appropriate guideline‐based treatment options for all levels of asthma severity before arriving at a treatment plan that best accommodated the participant's and the care manager's goals.
Investigators provided a written asthma management and action plan at the end of the first session and adapted it as required in subsequent sessions.
Group 2. Clinical decision‐making (CDM)
As above for SDM, but instead of a negotiated treatment plan, the care manager prescribed an appropriate regimen based on the patient's level of asthma control and explained this decision to the patient.
Group 3. Usual care
Usual care at KP is based on a guideline‐based stepped‐care approach to pharmacotherapy with the goal of long‐term asthma control.
Intervention fidelity
Sixteen nurses, respiratory therapists, pharmacists, nurse practitioners, and physician assistants were recruited to deliver the intervention. Most were already trained asthma care managers. Researchers scored audiotapes of both sessions for 10% of participants against a checklist to ensure fidelity to the study protocol. They also asked participants to report their perceived role in the treatment decision after session one. The SDM model was based on "four key defining features described by Charles and colleagues" (Charles 1997; Charles 1999).
Clark 1998
Group 1. Interactive seminar programme
General practice paediatricians in this group received two interactive face‐to‐face seminars, each lasting approximately 2.5 hours, over a two‐ to three‐week period. Seminars were based on the theory of self‐regulation, "guiding physicians to examine their own behaviour and to identify ways that they could develop a better partnership with their patients". This included a focus on deriving information for making therapeutic decisions, creating a supportive atmosphere, reinforcing self‐management, giving a view of the long‐term therapeutic plan, and building patients’ confidence in controlling symptoms and using medicines. Seminars included brief lectures from respected asthma specialists, a video example, case studies, and a self‐assessment protocol for physicians.
Group 2. Control
General practice paediatricians in this group continued their usual asthma care practices.
Intervention fidelity
Physicians were asked to rate their own performance through a survey. Questions were related not only to prescribing practices but also to procedures such as encouraging self‐management, providing patient teaching, and exhibiting supportive communication and behaviour. Investigators collected similar data from patients and their parents and correlated this information with physicians' reports, noting a good level of agreement. The trial did not include an explicit assessment of intervention fidelity and did not attempt to record or observe physicians interacting with patients and parents.
Fiks 2015
Group 1. MyAsthma shared decision‐making portal
Participants in this group used "MyAsthma", a shared decision‐making portal linked to their electronic health record. Clinicians and families had developed MyAsthma with the aim of promoting SDM. The main features of this online portal included eliciting parents' concerns and asthma treatment goals; tracking symptoms and side effects; providing educational content; and granting access to participants' individual asthma care plans. Families were prompted to complete a monthly survey, the results of which were used to provide guideline‐based decision support for parents and clinicians.
Group 2. Control
Participants in this group did not have access to the MyAsthma portal, but their clinician had access to the decision support system designed to promote guideline‐based asthma care.
Intervention fidelity
Study staff provided "brief training" to families randomised to receive the MyAsthma intervention and sent monthly emails to remind them to complete portal surveys, on which subsequent decision support was based. Acceptibility of the intervention was recorded through surveys at baseline, at three months, and at six months; these surveys included questions about satisfaction with asthma care. The proportion of participants completing the monthly portal survey was used as a measure of feasibility.
van Bragt 2015
Group 1. PELICAN online tool
Children in this group used a self‐administered online health‐related quality of life instrument, specifically developed for children aged 6 to 11 years. Children were invited to respond to a series of questions using a 5‐point Likert scale and to choose from a list of specific asthma problems the ones that may bother them in their daily life. Children completed the PELICAN tool before each study visit, and investigators used their answers to guide asthma management, based on SDM between child, parent, and nurse. After the first session, researchers produced a written action plan that would be reviewed at subsequent sessions.
Group 2. Enhanced usual care
Children in this group were assessed every three months. Specific issues addressed included symptoms, medication use, and exposure to asthma triggers, according to the guidelines of the Dutch College of General Practitoners. Consultations provided by the child's usual general practitioner or nurse typically lasted 10 minutes.
Intervention fidelity
Study authors did not describe the procedure used to train children to use the online tool. Nurses delivering the face‐to‐face shared decision‐making consultation were trained in the process during a two‐hour meeting before the study began and were monitored for a fixed number of "feedback/observation moments". Telephone support was provided for specific questions.
Outcomes
Clark 1998: physician survey (items related to using clinical practice methods/medicines, encouraging self‐management, and providing patient teaching and communications); parent interview form (questions related to symptom status of the child, medicines prescribed, and use of healthcare services for asthma (ED visits, hospitalisations, physician office visits);as well as parents’ observations and opinions of physicians’ teaching and communication behaviours and other aspects of the clinician–patient interaction). Data were collected from physicians at baseline, at five months ("mid‐point"), and at one year after the mid‐point. Investigators tracked patient visits over 22 months and collected data from patients on average two months after their visit.
Fiks 2015: feasibility (assessed as percentage of participants in the intervention group completing the monthly portal survey); acceptability of asthma care (measured at six months on an 11‐point Likert scale); clinical outcomes (numbers of asthma ED visits, hospitalisations, and specialist and general practitioner visits over the six‐month study); number of prescriptions assessed through electronic health records; number of days of missed school (child) or work (parent) over past month; Parent Patient Activation Measure (tool that can be used to assess the knowledge, skills, and confidence needed to manage a child's health care; regarded as a measure of satisfaction (higher score = higher activation)); Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF); and asthma control test. Families completed surveys at enrolment and at three and six months.
van Bragt 2015: primary outcome: quality of life (Pediatric Asthma Quality of Life Questionnaire (PAQLQ)); secondary outcomes: asthma control (ACQ); symptoms and medication via a diary; cost‐effectiveness; caregiver quality of life (Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ)); process outcomes. Investigators collected data at three, six, and nine months after the baseline assessment.
Wilson 2010: primary outcomes: adherence to controller medications; better asthma‐related quality of life; and improved healthcare utilisation; secondary outcomes: short‐acting beta‐agonist (SABA) use; lung function; and asthma control. Investigators collected data at 12 and 24 months post randomisation.
Excluded studies
We excluded 11 studies after viewing full texts: Nine studies tested an intervention that was not focused on improving shared decision‐making (Ford 1996; Gorelick 2006; Moffat 2008; NCT00170248; NCT00214669; Smith 2008; Sockrider 2001; Tapp 2014; Tieffenberg 2000). One was not an RCT (NCT01522144). Another study recruited a mixed respiratory population (Early 2015). In addition, we have listed four relevant studies as ongoing (Federman 2015; Hoskins 2013; NCT02516449; Tapp 2011).
Risk of bias in included studies
We have provided a summary of our risk of bias judgements in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered one study to be at low risk of bias because trial authors described computerised methods of generating the random sequence and concealing allocation (Wilson 2010). Another study used minimisation software to generate the random sequence but did not describe allocation concealment, so we rated risks of bias as low and unclear, respectively (van Bragt 2015). We rated another study as having unclear and low risks of bias because it did not describe random sequence generation but used sealed envelopes to conceal allocation (Fiks 2015). We rated the remaining study as having unclear risk of bias for random sequence generation and high risk of bias for allocation concealment because the method of selecting participants for inclusion was not well concealed, and this may have introduced a selection bias (Clark 1998).
Blinding
We considered three studies to be at high risk of bias for both blinding domains because patients, physicians, or both were aware of group allocation, and this may have affected how they behaved and responded during and after the intervention (Fiks 2015; van Bragt 2015; Wilson 2010). The other included study blinded patients and parents to physicians' involvement in the study, so outcomes measured by patients and parents would be at low risk of detection bias, but outcomes rated by physicians would be at higher risk (Clark 1998). We assessed separately the likelihood that each outcome would be subject to performance and detection biases when GRADE ratings were applied.
Incomplete outcome data
Two studies were at low risk of attrition bias because a similar and low proportion of participants from either group could not be included in the final analyses (Fiks 2015; Wilson 2010). We considered the other two studies to be at high risk of attrition bias because overall dropout was high and numbers randomised and completed in each group were not reported fully, or because all dropouts came from the control group (Clark 1998; van Bragt 2015).
Selective reporting
We rated one study as having low risk of reporting bias because it was prospectively registered and researchers reported all specified outcomes as planned (van Bragt 2015). We rated three studies as having high risk of reporting bias; two were prospectively registered and the full report did not include data for all planned outcomes or time points, and one reported some outcomes narratively or in a way that meant data could not be pooled in a meta‐analysis (Clark 1998; Fiks 2015; Wilson 2010).
Other potential sources of bias
We did not note any additional sources of bias in two studies (Clark 1998; Wilson 2010). In another study, study authors noted: "The study population was a convenience sample based largely on clinician recommendation and was not designed to be representative of all children with asthma in the care network", but it is unclear whether this introduces bias (Fiks 2015). We rated another study as having high risk of bias because the 33 children recruited were significantly fewer than the 170 planned, potentially leading to underpowered analyses. In addition, groups were not balanced at baseline for asthma control or for socioeconomic status (van Bragt 2015).
Effects of interventions
We did not consider interventions, comparisons, or outcomes reported in the included studies to be sufficiently similar for pooling to make sense. We present a narrative description of the outcomes of interest for each included study, structured according to our prespecified primary and secondary outcomes. When possible, we present findings from individual studies on forest plots to provide a visual representation of the effect estimate.
Primary outcomes
Asthma‐related quality of life
Three studies reported asthma quality of life.
Fiks reported three subscales of the ITG‐ASF (higher score = poorer quality of life) as change from baseline for 53 participants but did not report a measure of variance (Fiks 2015). We back‐calculated standard deviations (SDs) from reported P values for differences between arms. Confidence intervals include no differences for each of the subscales. We presented results in Analysis 1.2. (very low‐quality evidence).
Wilson (a three‐arm trial) also reported on the endpoint quality of life, using the symptoms domain of the mini‐AQLQ (Wilson 2010). We have presented SDM versus usual care comparisons in Analysis 1.3. We back‐calculated SDs from the P value given for the difference (P = 0.0003). Although the mean difference falls below the minimal clinically important difference (MCID) of 0.5 for this scale, responder analysis demonstrates that significantly more people experienced an improvement of at least 0.5 units (odds ratio (OR) 1.90, 95% confidence interval (CI) 1.24 to 2.91; participants = 371; studies = 1; Analysis 1.1). We have moderate confidence in these results.
Of note, Wilson reported that the mean difference in mini‐AQLQ symptom score for SMD versus CDM was 0.1 and described this finding as non‐significant. A responder analysis for this comparison revealed that the number of people in the CDM group with improvement greater than 0.5 units was 110/180. If this is used as the control group, the effect is smaller and the lower confidence interval shows no difference (OR 1.51, 95% CI 0.97 to 2.34; data not presented).
van Bragt reported child and parent scores on the AQLQ as medians and interquartile ratios (IQRs) (van Bragt 2015). We noted baseline imbalances, and although investigators stated in the methods section that they would adjust for this, it is unclear whether this was done, as data were not normally distributed. Scores were slightly higher in the SDM group than in the control group, and the number of participants was small (6.78 vs 6.5 children (n = 29); 6.96 vs 6.85 parents (n = 25); IQRs between 0.31 and 0.96).
Patient/parent satisfaction
Clark reported parental views on the "demeanour and communications skills of the paediatrician", adjusted for clustering, using a number of different measures, but these investigators presented results without a measure of variance, so we have not presented them graphically (Clark 1998). Study authors followed up a total of 472 parents of enrolled children for this outcome. Parents in the intervention group were significantly more likely to report that the paediatrician was reassuring and encouraging; described as a goal that the child could be fully active; looked into how the family managed asthma on a day‐to‐day basis; and gave parents information to relieve their specific worries and concerns about asthma (Table 2).
| Was/did the clinician: | SDM | Control | P value (GEEa) |
| Reassuring and encouragingb | 4.63 | 4.42 | 0.006 |
| Look into how family managed | 3.98 | 3.69 | 0.02 |
| Describe how child should be fully | 71.% | 59% | 0.007 |
| Describe at least 1 of 3 goals: | 75% | 64% | 0.07 |
| Give information to relieve specific | 4.1 | 3.9 | 0.007 |
| Enable family to know how to make | 4.3 | 4.2 | 0.07 |
aGEE method to assess "Time2" (follow‐up) scores with baseline scores and group assignment as covariates in regression models.
bA Likert‐type response scale was used, where 1 = strongly disagree and 6 = strongly agree.
cQuestion asked at "Time2" (follow‐up) only.
NB: A total of 472 parents were followed up; numbers in each group are not given.
Fiks reported the number of parents who completed the portal survey for each of the six months of the study and considered this to be a measure of acceptability of the intervention (Fiks 2015). It should be noted that parents of children in the control group did not have access to the portal, and therefore this outcome was measured only in the SDM group. Of the 30 families randomised to the intervention group, 17 (57%) completed the survey five or more times, which was defined as frequent use, and 77% completed the survey more than once. It was also noted that parents of children with more severe asthma were more likely to be frequent users of the portal (75% vs 47% with mild persistent asthma). Twenty‐two out of 24 parents reported that the MyAsthma intervention made it easier to care for their child with asthma, and 10 of 24 parents reported that the portal made it easier to communicate with their child’s healthcare providers. Six parents reported that the portal increased their awareness of the importance of asthma management.
This same study reported "parental activation" using the Parent Patient Activation Measure. This tool assesses the knowledge, skills, and confidence needed to manage a child's health care and could be regarded as a measure of satisfaction (higher score = higher activation). Data showed no significant differences between study arms; change scores were reported as 2.3 and 2.4 in SDM and control groups, respectively (P = 0.9).
Medication adherence
Fiks reported the mean number of "controller" medication prescriptions over 26 weeks as 1.1 in the SDM group (n = 26) and 0.7 in the control group (n = 27) (Fiks 2015).
Wilson reported medication adherence for all medications and for inhaled corticosteroid (ICS) alone as continuous medication acquisition (CMA) (Wilson 2010). This is calculated as the total days’ supply acquired in a given year divided by 365 days. Results suggest that SDM increases CMA when compared with usual care (Analysis 1.4; all medication: mean difference (MD) 0.21, 95% CI 0.11 to 0.31; ICS alone: MD 0.22, 95% CI 0.11 to 0.33; participants = 371; moderate‐quality evidence). Our confidence in this finding was reduced by the potentially indirect nature of using CMA to measure adherence. The CMA mean difference between SDM and CDM in the Wilson study was 0.029 for all medication and 0.017 for ICS alone; these mean differences are smaller than those for SDM versus usual care but are also reported as statistically significant (Wilson 2010). Of note, trialists also collected CMA data at two years and reported that between‐group differences were no longer significant.
CMA findings are supported by an additional metric of the beclomethasone dipropionate (BDP) equivalent of canisters acquired, which shows an effect in favour of SDM at one year and at two years, although a smaller difference after two years (data not shown).
Secondary outcomes
Exacerbations of asthma (leading to a course of oral corticosteroids or unscheduled visit to a healthcare professional)
Clark reported mean numbers of ED visits and hospitalisations per child and showed no clear between‐group differences (mean number of ED visits: SDM = 0.65, usual care = 0.67; hospitalisations: SDM = 0.081, usual care = 0.076; both P values were adjusted for clustering and were reported as non‐significant) (Clark 1998).
Fiks reported the mean number of oral corticosteroid (OCS) prescriptions over 26 weeks, without variance, as 0.4 in the SDM group (n = 26) and 1 in the control group (n = 27) (Fiks 2015). This study also reported the numbers of children with exacerbations requiring hospital admission, an ED visit, a specialist visit, and a general practitioner visit. We have presented these data in Analysis 1.5; all four point estimates favour shared decision‐making, but confidence intervals are wide, and our confidence in these findings is low. Finally, study authors reported the change in the number of asthma exacerbations, captured by the "Asthma Control Tool" (a validated instrument in children), as ‐3.3 in the SDM group and ‐1.3 in the control group (25‐point scale; P = 0.02).
Wilson reported rates of asthma‐related visits in this three‐arm study (Wilson 2010). During year 1, both SDM and CDM groups had significantly lower visit rates (1.0/y and 1.1/y) than the usual care group (1.4/y; P = 0.0161 and 0.0147, respectively).
Asthma control
Fiks reported change in "asthma symptoms while at best" on the "Asthma Control Tool" as ‐2.8 in the SDM group and ‐0.6 in the control group (P = 0.10), with a lower score indicating less severe symptoms (Fiks 2015).
van Bragt assessed asthma control using the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT) and presented results as medians and IQRs. Baseline imbalances were notable (ACQ in favour of intervention and ACT in favour of control), and, as data were not normally distributed, it is unclear whether scores were adjusted accordingly (van Bragt 2015). This same trial dichotomised participants into well controlled and not well controlled (well controlled seen as < 1 on the ACQ and > 22 on the ACT). Study authors detected no between‐group differences, but confidence intervals were wide and the number classified as 'well controlled' at baseline was unbalanced (Analysis 1.6; low‐quality evidence).
Wilson reported change from baseline on the Asthma Therapy Assessment Questionnaire (ATAQ) but did not give any measure of variance (Wilson 2010). Changes were as follows: ‐0.8 in the SDM group, ‐0.54 in the CDM group, and ‐0.46 in the usual care group, with lower scores indicating better control. This same study used the ATAQ to report the number of people with 'no asthma problems' (ATAQ score = 0). We have presented SDM versus usual care in Analysis 1.6 (moderate‐quality evidence); the odds ratio for the SDM versus CDM comparison shows a smaller but still significant effect in favour of SDM: 1.6 (1.1 to 2.4, P = 0.0239).
Acceptability/feasibility from the perspective of healthcare professionals
We did not find any data about this.
Adverse events (all)
None of the included studies measured or reported adverse events.
Subgroup and sensitivity analyses
It was not possible to conduct any of the planned subgroup analyses (age; who the intervention was aimed at; extensiveness of intervention), as we did not perform any meta‐analyses. We have presented a summary of study characteristics in Table 1.
Similarly, it was not possible to test the robustness of study results by performing sensitivity analyses while excluding unpublished data and studies at high risk of selection bias.
Discussion
Summary of main results
This review includes four studies of shared decision‐making (SDM), allocating a total of 1342 participants to either SDM interventions or control. Study design, populations, interventions, comparisons, and outcomes are substantially different between the four studies. Three studies recruited children with asthma and their care‐givers (Clark 1998; Fiks 2015; van Bragt 2015). One study recruited adults (Wilson 2010). Asthma severity ranged from mild to severe. Three studies took place in the United States (Clark 1998; Fiks 2015; Wilson 2010). One was conducted in the Netherlands (van Bragt 2015). Trial duration was between six and 24 months, and outcomes were measured at a range of time points from six months to two years.
All studies were conducted in a primary care or outpatient setting, and the intervention was delivered in various ways, either to participants directly or to healthcare professionals. Two studies in children used an online portal to elicit key asthma management concerns and goals; this was followed by face‐to‐face discussions with a healthcare professional based on shared decision principles (Fiks 2015; van Bragt 2015). Clark provided seminars aimed at developing skills in SDM among paediatric general practitioners, who in turn enrolled their patients into the study (Clark 1998). Wilson provided to participants a mixture of face‐to‐face discussions and telephone calls with personnel trained in SDM or in clinical decision‐making (CDM) (Wilson 2010). The duration and content of interventions varied, but SDM was a key component of the intervention provided in all included studies. Owing to the nature of the intervention, it was not possible to blind participants or trial personnel to group allocation. Review authors considered the impact of the lack of blinding on an outcome‐specific basis when assigning GRADE ratings.
Meta‐analysis of results was not possible owing to the small number of heterogenous trials included. Three studies used different tools to assess asthma‐related quality of life and reported inconsistent results. Fiks conducted a study in children that compared an SDM online portal versus guideline‐based care presented in subscales of the Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF) and did not demonstrate between‐group differences, although confidence intervals were wide (Fiks 2015). Similarly, van Bragt conducted a study in children using an online tool and found little difference between SDM and control groups (van Bragt 2015). Wilson completed a study in adults involving face‐to‐face and telephone consultations and identified benefit of SDM over usual care, using the mini‐Asthma Quality of Life Questionnaire (AQLQ) symptom scale (Wilson 2010). This benefit was confirmed by a responder analysis.
Two studies reported patient/parent satisfaction, or proxy measures. In a cluster‐randomised trial in which SDM training was provided to physicians, Clark reported that parents of children in the intervention group were significantly more likely to report satisfaction with the paediatrician (Clark 1998). Fiks reported "parental activation" using the Parent Patient Activation Measure but noted no significant differences between study arms (Fiks 2015).
Two studies reported medication adherence. Fiks indicated that the mean number of controller medication prescriptions over 26 weeks was greater in the SDM group (Fiks 2015). Wilson reported medication adherence for all medications and for inhaled corticosteroids (ICSs) alone as continuous medication acquisition (CMA) (Wilson 2010). Results suggest that SDM increases CMA when compared with usual care, but that differences are lessened over time.
Of our secondary outcomes, study authors reported only exacerbations and asthma control. Three studies reported exacerbations.Mean numbers of emergency department (ED) visits and hospitalisations per child reported by Clark show no clear between‐group differences (Clark 1998). Fiks indicated that the mean number of oral corticosteroid (OCS) prescriptions over 26 weeks was reduced in the SDM group compared with the control group (Fiks 2015). This study also reported the number of children with exacerbations requiring an unscheduled visit or hospital admission; point estimates favoured SDM, but confidence intervals were wide. Wilson reported rates of asthma‐related visits and indicated that the SDM group had significantly lower visit rates than the usual care group (Wilson 2010). Three studies reported asthma control. Changes in "asthma symptoms while at best" on the "Asthma Control Tool" as reported by Fiks were noted to be lower in the SDM group than in the control group (Fiks 2015). van Bragt assessed asthma control using the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT) and dichotomised participants into two groups: well controlled and not well controlled (van Bragt 2015). Researchers reported no between‐group differences, but confidence intervals were wide. One study used the Asthma Therapy Assessment Questionnaire (ATAQ) to report the number of people with 'no asthma problems' (ATAQ score = 0) and described benefit of SDM over control (Wilson 2010).
Overall completeness and applicability of evidence
Only four studies met the inclusion criteria for this review, thus the body of evidence available from randomised controlled trials (RCTs) is limited at this time. Substantial differences in study design, populations, interventions, comparisons, and outcomes prevent overall conclusions. Although we identified several randomised trials in asthma that included an element of SDM, we considered this to be only one element of a broader intervention and thus excluded these studies (see Characteristics of excluded studies). This may have resulted in loss of useful information, but we judged it would not have been possible to confidently ascribe any clinical benefit to SDM in the context of a much broader intervention. The small number of trials identified also meant that no subgroup analysis could be performed as planned on the basis of content, intensiveness, or duration of the intervention; these are all likely to be important effect modifiers.
Whether or not the intervention was delivered with a high level of fidelity is also an important consideration when outcomes of SDM interventions are assessed. All four studies attempted to capture fidelity or intervention adherence using different approaches. Investigators in two studies reported observing or recording trial staff to ensure that the intervention was delivered as planned (van Bragt 2015; Wilson 2010). Investigators in another trial asked physicians, who were the primary recipients of the intervention, to rate their own performance, which was reported as having a high level of correlation with their patients' reports (Clark 1998). However, this trial report did not describe attempting to observe or record physicians while interacting with patients. Families recruited in another study received "brief training" by study staff on use of the online portal and recorded acceptability through surveys that included questions about satisfaction with asthma care (Fiks 2015). The proportion of participants completing the monthly portal survey was used as a measure of feasibility, and trialists reported that 77% of parents completed the survey at least twice, out of a possible six times. Fifty‐seven per cent completed the survey five or more times.
Although adverse events might not be anticipated in trials of SDM, none of the included studies set out to systematically measure and report this outcome; this is another limitation of the evidence presented. Another important gap is the fact that none of the included studies focused on adolescents. Adolescents are at higher risk of poor asthma outcomes, including death, when compared with younger children (Akinbami 2002; Akinbami 2006). Asthma management during adolescence may require particularly high levels of trust and good communication between care providers and patients; therefore SDM interventions have the potential for substantial impact (de Benedictis 2007).
Three out of the four included studies were conducted in the United States, and the fourth in another high‐income setting (the Netherlands). This may limit applicability of findings to other healthcare systems facing greater resource constraints and with different cultural approaches to the relationship between healthcare professionals and patients. Cost‐effectiveness is also not addressed in this review nor in the included studies. Evidence suggests that SDM interventions may not be cost‐neutral, so studies including an economic evaluation would be a useful addition to the evidence base (Veroff 2013).
We also noted that baseline asthma severity and control varied between studies (e.g. most participants in the Wilson study had poorly controlled asthma, whereas mean ACQ score in the van Bragt trial was < 1, suggesting overall good asthma control; Fiks reported that a large majority of participants had mild or moderate asthma) (Fiks 2015; van Bragt 2015; Wilson 2010). A possible direction for future research would be to investigate whether people with more or less severe asthma benefit more or less from SDM than those given usual care. The limited number of studies in this review means that we cannot currently comment on this. A further consideration is that those who agree to participate in SDM trials and those who adhere to the trial protocol once recruited may differ substantially from those not recruited. This may limit generalisability of findings from such trials to the wider asthma population.
Finally, choice of control group and treatment setting may have an impact on whether an SDM intervention leads to improvement in asthma outcomes. Usual care practices vary widely between settings; some may include elements of SDM routinely, which would likely limit differences seen between intervention and control groups. A thorough description of routine practices is important for an understanding of local applicability of findings from individual trials.
Quality of the evidence
We were not able to apply GRADE to all outcomes as planned because we had no pooled data for some analyses, including patient/parent satisfaction; acceptability from the perspective of the healthcare professional; and adverse events. When we were able to make a judgement, our confidence ranged from very low to moderate. We downgraded subjective outcomes (quality of life and asthma control) owing to inherent risk of bias introduced by unblinded trials, although it is difficult to conceive a trial of SDM in which effective participant and personnel blinding would be possible. We did not consider the open‐label design of trials to pose such a threat to outcomes such as medication adherence and exacerbations.
We had concerns about indirectness in trials that reported subscale scores from a quality of life questionnaire, rather than total scores, and we downgraded evidence for this reason. We also downgraded medication adherence evidence, as we judged continuous medication acquisition to be a proxy measure of adherence that may overestimate true adherence. We noted that imprecision was a problem for several outcomes, including quality of life, exacerbations, and asthma control, with confidence intervals including the possibility of both harm and benefit from the intervention.
We did not detect statistical heterogeneity because we did not pool studies owing to differences in study design, outcomes reported, or both (i.e. high clinical heterogeneity); therefore we ran no tests for heterogeneity. We have reported findings narratively when relevant. We did not suspect publication bias but did not include sufficient studies to produce a funnel plot.
Potential biases in the review process
We carried out the review according to methods provided in the published protocol and detailed deviations from the protocol in the Differences between protocol and review section (Kew 2016). As planned, two review authors independently screened search results and resolved discrepancies by discussion. We did not restrict the search by date or by language. At least two review authors extracted all study characteristics and numerical data and resolved discrepancies through discussion. The same was true for risk of bias ratings and GRADE ratings, for which a third person was consulted as required to resolve disagreements. Two additional review authors joined the team to complete the update (RN and KA). Insufficient data prevented completion of planned meta‐analyses and subgroup and sensitivity analyses.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review that synthesises evidence from RCTs on SDM in asthma. Several other systematic reviews have explored the association between SDM and health outcomes and behaviours across a range of medical conditions. A consistent theme across these reviews, in keeping with the present review, is the difficulty of meaningfully combining evidence from the wide range of trials taking place in this field.
A recent review, including 39 studies, most of which were observational, found that although affective‐cognitive outcomes may be favourable if participants perceive that SDM has occurred, evidence linking empirical measures of SDM to health and behavioural outcomes is lacking (Shay 2015). Joosten and colleagues identified 11 RCTs of SDM involving adults across various medical conditions (Joosten 2008). Although these review authors concluded that SDM may be beneficial, especially in the context of chronic illness, they noted that evidence from RCTs regarding impact on health outcomes is lacking.
A 2015 review of SDM in paediatrics identified 61 studies, most of which were observational in design, and focused on satisfaction, decisional conflict, and knowledge, rather than health outcomes. Only 15 studies could be meta‐analysed, and review authors concluded that SDM interventions in paediatrics remain poorly defined, but limited available evidence suggests that SDM may reduce decisional conflict,and improve parent knowledge (Wyatt 2015). Durand and colleagues in their systematic review specifically addressed whether SDM interventions can reduce health inequalities (Durand 2014). Review authors concluded following a narrative synthesis of evidence that SDM interventions may be more beneficial for those from disadvantaged groups, but confidence in their findings was reduced by between‐study heterogeneity.
Légaré and colleagues synthesised evidence related to effectiveness of interventions aimed at patients or healthcare professionals to improve SDM (Légaré 2014). They identified 39 studies, 38 of which were RCTs. Despite the large number of studies included, review authors were not able to conclude whether interventions to improve adoption of SDM are effective, although they suggest that targeting both patients and healthcare professionals is likely to be more effective than targeting just one or the other.
Reviews investigating the role of SDM in other specific conditions have demonstrated benefit; for example, one trial addressed SDM in the context of antibiotic prescribing for acute respiratory illness, and another investigated SDM for people with dementia (Coxeter 2015; Daly 2016).

Study flow diagram.
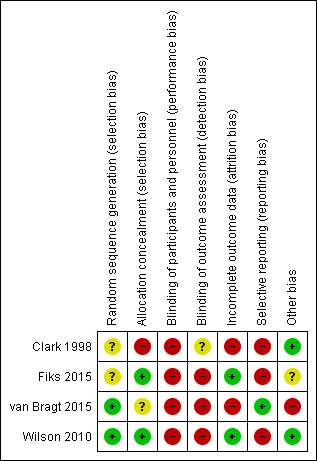
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Shared decision‐making versus usual care, Outcome 1 Quality of life improvement (AQLQ responders).

Comparison 1 Shared decision‐making versus usual care, Outcome 2 Quality of life scores (ITG‐ASF).

Comparison 1 Shared decision‐making versus usual care, Outcome 3 Quality of life scores (mini‐AQLQ).
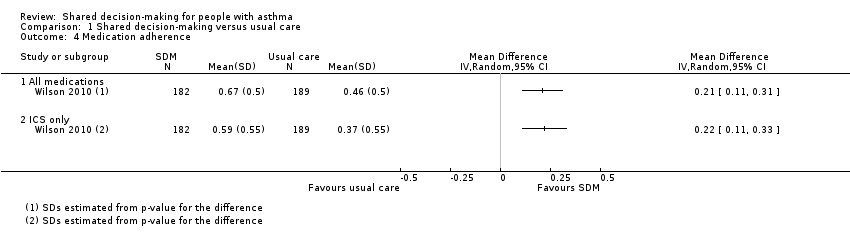
Comparison 1 Shared decision‐making versus usual care, Outcome 4 Medication adherence.

Comparison 1 Shared decision‐making versus usual care, Outcome 5 Exacerbations of asthma.
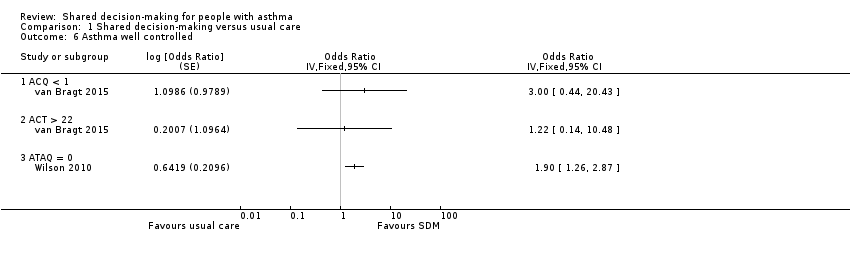
Comparison 1 Shared decision‐making versus usual care, Outcome 6 Asthma well controlled.
| Shared decision‐making compared with usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | ||
| Risk with usual care | Risk with shared decision‐making | ||||||
| Asthma‐related quality of life (follow‐up: 6 to 24 months) | AQLQ responders | 556 per 1000 | 704 per 1000 | OR 1.90 | 371 | ⊕⊕⊕⊝ | Participants achieving > 0.5‐point improvement (MCID for this scale) |
| ITG‐ASF daytime symptom scale | Mean ITG‐ASF daytime symptom score was 12 | MD 4 higher | ‐ | 53 | ⊕⊝⊝⊝ | Higher score = Better quality of life The same study also reported mean night‐time symptom scale and functional limitation scale (see Analysis 1.2). | |
| Mini‐AQLQ | Mini‐AQLQ score was 5.5 | MD 0.4 higher | ‐ | 371 | ⊕⊕⊝⊝ | Higher score = Better quality of life. MCID 0.5 | |
| Parent/patient satisfaction | Presentation on forest plot not possible; summarised narratively in text and Table 2 | ‐ | ‐ | ‐ | |||
| Medication adherence (follow‐up: 12 to 24 months) | ICS only | The ICS adherence was 0.59 | MD 0.22 higher | ‐ | 371 | ⊕⊕⊕⊝ | Adherence calculated using continuous medication acquisition (CMA) from pharmacy data. Maximum score 1. The same study reported all‐medication adherence (see Analysis 1.4). |
| Exacerbations of asthma (follow‐up: 6 months) | Requiring ED visit | 222 per 1,000 | 77 per 1,000 | OR 0.29 | 53 | ⊕⊕⊝⊝ | The same study reported exacerbations requiring hospital admission, "specialist visits", and GP visits (see Analysis 1.5). |
| Asthma control (follow‐up: 12 to 24 months) | Asthma well controlled; ATAQ = 0 | No control group risk presented | Not estimable | OR 1.90 | 371 (1 RCT) | ⊕⊕⊕⊝ | Lower score = Better asthma control A different small study reported asthma control on ACT and ACQ (see Analysis 1.6). |
| Adverse events (all) | Included trials did not measure or report any adverse events. | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence. | |||||||
| aRisk of performance and detected bias. Downgraded once. bOne study. Confidence intervals include possible harm and benefit of intervention. Downgraded once. cOnly quality of life subscales reported. Downgraded once for indirectness. dAlthough the mean difference for this scale lies below the MCID, the responder analysis suggests that significantly more people achieved the MCID change with the intervention. No downgrade. eAdherence calculated using continuous medication acquisition from pharmacy data. This is a proxy measure and may overestimate true adherence. Downgraded once. fOne study. Confidence intervals very wide and include possible harm and benefit of intervention. Downgraded twice. | |||||||
| Study ID | Country | Population | Age (years) | Design | Intervention | Aimed at | Control |
| USA | 74 physicians; 637 children | 1 to 12 | Cluster RCT | SDM seminars | HCPs | Usual care | |
| USA | 60 families | 6 to 12 | Individual RCT | SDM portal | HCPs and patients/parents | Usual care + decision support | |
| Holland | 33 children | 6 to 12 | Cluster RCT | SDM online tool | HCPs and patients/parents | Enhanced usual care | |
| USA | 612 adults | 18 to 65 | Individual RCT | SDM structured sessions | HCPs | 1. Guideline‐led decision‐making 2. Usual care | |
| HCP: healthcare provider; RCT: randomised controlled trial; SDM: shared decision‐making. | |||||||
| Was/did the clinician: | SDM | Control | P value (GEEa) |
| Reassuring and encouragingb | 4.63 | 4.42 | 0.006 |
| Look into how family managed | 3.98 | 3.69 | 0.02 |
| Describe how child should be fully | 71.% | 59% | 0.007 |
| Describe at least 1 of 3 goals: | 75% | 64% | 0.07 |
| Give information to relieve specific | 4.1 | 3.9 | 0.007 |
| Enable family to know how to make | 4.3 | 4.2 | 0.07 |
| aGEE method to assess "Time2" (follow‐up) scores with baseline scores and group assignment as covariates in regression models. NB: A total of 472 parents were followed up; numbers in each group are not given. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life improvement (AQLQ responders) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Quality of life scores (ITG‐ASF) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 ITG‐ASF night‐time symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ITG‐ASF daytime symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 ITG‐ASF functional limitation scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life scores (mini‐AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Medication adherence Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 All medications | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 ICS only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations of asthma Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Requiring hospital admission | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Requiring ED visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Requiring specialist visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Requiring GP visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma well controlled Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 6.1 ACQ < 1 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 ACT > 22 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 ATAQ = 0 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

