نقش استروئیدهای خوراکی آغاز شده توسط بیمار و والدین برای حملات آسم
چکیده
پیشینه
آسم یک بیماری التهابی مزمن راههای هوایی است که به طور تخمینی حدود 334 میلیون نفر را در سراسر جهان مبتلا کرده است. بیماران حین حملات شدید، ممکن است نیاز به مراجعه به مراکز پزشکی یا بخش اورژانس بیمارستان برای درمان با کورتیکواستروئیدهای سیستمیک داشته باشند که میتوانند به صورت داخل وریدی یا خوراکی مصرف شوند. برخی افراد مبتلا به آسم در زمان رخداد یک حمله، کورتیکواستروئیدهای خوراکی (oral corticosteroids; OCS) را به صورت خود‐تجویزی (یعنی آغاز شده توسط بیمار) یا برای استفاده در کودک مبتلا به آسم خود (یعنی آغاز شده توسط والدین)، مصرف میکنند. این روش درمانی، به طور فزایندهای در حال شایع شدن است.
اهداف
ارزیابی اثربخشی و ایمنی استروئیدهای خوراکی آغاز شده توسط بیمار یا والدین برای بزرگسالان و کودکان مبتلا به حملات آسم.
روشهای جستوجو
ما کارآزماییها را از طریق پایگاه ثبت تخصصی گروه راههای هوایی در کاکرین (Cochrane Airways' Specialised Register; CASR) شناسایی کردیم، همچنین پایگاه ثبت کارآزماییهای بالینی در حال انجام مؤسسات ملی سلامت ایالات متحده ClinicalTrials.gov؛ (www.clinicaltrials.gov) و پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (apps.who.int/trialsearch) را جستوجو کردیم. ما CASR را از آغاز تا 18 می 2016 و پایگاههای ثبت کارآزمایی را از زمان آغاز تا 24 آگوست 2016 جستوجو کرده و هیچ محدودیتی از نظر زبان انتشار اعمال نکردیم.
معیارهای انتخاب
ما به دنبال کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) گزارش شده به شکل متن کامل، منتشر شده به صورت فقط چکیده و دادههای منتشر نشده بودیم؛ کارآزماییهای متقاطع را از مرور خارج کردیم.
به دنبال مطالعاتی بودیم که بزرگسالان (18 سال یا بالاتر) یا کودکان در سن مدرسه (5 سال یا بالاتر) مبتلا به آسم را برای دریافت این موارد تصادفیسازی کرده باشند: الف) هرگونه OCS آغاز شده توسط بیمار/والدین یا ب) دارونما (placebo)، مراقبت معمول، درمان فعال جایگزین یا یک برنامه درمانی آسم که به طور یکسانی شخصیسازی شده باشد، بدون جزء OCS آغاز شده توسط بیمار یا والدین.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم نتایج جستوجو را برای شناسایی هر مطالعهای که دارای معیارهای ورود از پیش تعیین شده باشد، غربالگری کردند.
پیامدهای اولیه از پیش تعیین شده شامل بستری در بیمارستان برای آسم، نشانههای آسم طی پیگیری و حوادث جانبی جدی بود.
نتایج اصلی
علیرغم جستوجوهای جامع در بانکهای اطلاعاتی الکترونیکی و پایگاههای ثبت کارآزمایی بالینی، هیچ مطالعهای را شناسایی نکردیم که دارای معیارهای ورود برای این مرور باشد. پنج مطالعه بالقوه مرتبط، به دو دلیل خارج شدند: مداخلات دارای معیارهای ورود برای این مرور نبودند (سه مطالعه) و مطالعات دارای طراحی متقاطع بودند (دو مطالعه). دو مطالعه خارج شده، سوال بالینی مرتبطی را پرسیده بودند. با این حال، این مطالعات به خاطر طراحی متقاطع و بنا بر پروتکل، حذف شدند. ما با نویسندگان این کارآزماییهای متقاطع تماس گرفتیم که قادر به ارائه دادههای مربوط به دوره نخست درمان (یعنی پیش از متقاطع کردن) نبودند.
نتیجهگیریهای نویسندگان
اکنون هیچ شواهدی از کارآزماییهای تصادفیسازی شده (با طراحی غیر‐متقاطع) برای آگاهیبخشی درباره استفاده از کورتیکواستروئیدهای خوراکی آغاز شده توسط بیمار یا والدین در افراد مبتلا به آسم وجود ندارد.
PICO
خلاصه به زبان ساده
درمان با کورتیکواستروئیدهای خوراکی آغاز شده توسط بیماران یا والدین آنها حین یک حمله شدید آسم
پیشینه
آسم یک بیماری التهابی طولانیمدت راههای هوایی است که حدود 334 میلیون نفر را در سراسر جهان متاثر میکند. افراد حین حملات شدید، ممکن است نیاز به ویزیت در یک مرکز پزشکی یا بخش اورژانس بیمارستان برای درمان با کورتیکواستروئیدهای سیستمیک داشته باشند که میتواند مستقیما از راه ورید یا دهان مصرف شود. برای بعضی از افراد مبتلا به آسم، استروئیدهای خوراکی تجویز میشود که میتوانند در زمان رخداد یک حمله، خودشان مصرف کنند (آغاز شده توسط بیمار) یا به کودکشان بدهند (آغاز شده توسط والدین). این روش درمانی، به طور فزایندهای در حال شایع شدن است.
سوال مطالعه مروری
ما به دنبال مطالعات مربوط به مقایسه: الف) استروئیدهای خوراکی آغاز شده توسط بیمار یا والدین با ب) عدم استفاده از استروئیدهای خوراکی آغاز شده توسط بیمار یا والدین (به عنوان مثال، مراجعه به یک مرکز پزشکی یا بخش اورژانس بیمارستان برای درمان بیشتر به وسیله پزشک یا پرستار) بودیم. این مطالعات باید شامل بزرگسالان 18 سال یا بالاتر یا کودکان در سن مدرسه 5 سال یا بالاتر میبود. دو نویسنده مرور، نتایج جستوجو را به صورت مستقل از یکدیگر غربالگری کردند. جستوجوی اولیه در می 2016 انجام گرفت.
نتایج
ما 61 مطالعه را در مجموع غربالگری کردیم، اما هیچ مطالعهای را که منطبق با معیارهای بالا باشد، نیافتیم. پنج مطالعه، به علت طراحی آنها که بر اساس پروتکل مرور ما مجاز نبود، از مرور خارج شدند. دو مورد از این مطالعات، سوال بالینی صحیح را پرسیده بودند، اما به دلیل به کارگیری نوعی از طراحی کارآزمایی که طبق پروتکل مرور ما مجاز نبود، حذف شدند.
نتیجهگیریها
در حال حاضر شواهدی راجع به ایمنی یا اثر مثبت درمان در استفاده از استروئیدهای خوراکی آغاز شده توسط بیمار یا والدین در بیماران مبتلا به آسم وجود ندارد. این امر نگران کننده است زیرا این روش درمانی در حال شایعتر شدن است.
Authors' conclusions
Summary of findings
| Patient‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: adults aged 18 years or older with asthma Settings: outpatient Intervention: patient‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days lost of study/work | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||
| Parent‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: children aged 5 years or older with asthma Settings: outpatient Intervention: parent‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days off school | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||
Background
Description of the condition
Asthma is a chronic condition of the airways affecting an estimated 334 million people worldwide (Global Asthma Report 2014). Direct treatment costs and indirect costs associated with lost productivity are substantial and are among the highest for non‐communicable diseases (Global Asthma Report 2014). Asthma triggers may be allergic or non‐allergic, resulting in airway inflammation (including an eosinophilic and/or neutrophilic component), hyper‐responsiveness and airflow obstruction. During a worsening of asthma symptoms (i.e. an exacerbation), which include tightness of the chest, wheeze and breathlessness, patients will typically exhibit an acute narrowing of the airway and reduced lung function (BTS/SIGN 2016). Impairment of lung function can be reversed with treatment and may return to normal. From a patient perspective, the goals of asthma treatment are to prevent exacerbations, achieve control of daytime and nocturnal symptoms, and permit normal exercise and functional capacity (GINA 2016). Treatment of asthma should be guided by a personalised asthma action plan (GINA 2016), and includes the avoidance of potential triggers, the use of inhaled corticosteroids (ICS) or leukotriene receptor antagonists or both to reduce airway inflammation, and the use of inhaled long‐acting beta2‐agonists (LABA), short‐acting beta2‐agonists (SABA) and anti‐cholinergic bronchodilators (i.e. long‐acting muscarinic antagonists (LAMAs) to relieve airflow limitation (NICE 2007; NICE 2013; BTS/SIGN 2016; GINA 2016). During severe exacerbations, patients may need to attend a medical centre or hospital emergency department for treatment with systemic corticosteroids, which can be administered intravenously or orally (BTS/SIGN 2016; GINA 2016). Some people with asthma are prescribed oral corticosteroids (OCS) for self‐administration (i.e. patient‐initiated) or to administer to their child with asthma (i.e. parent‐initiated), in the event of an exacerbation (Vuillermin 2007).
Description of the intervention
Prophylactic treatment with corticosteroids is commonly used in patients with asthma to reduce and control airway inflammation (BTS/SIGN 2016; GINA 2016), thus serving to improve asthma control and reduce future risks. ICS are used in preference to systemic corticosteroids because the inhaled dose is delivered directly to the respiratory tract (i.e. drug target), lowering the propensity for systemic side effects. Adverse effects associated with the long‐term use of systemic steroids include: effects on bone density (e.g. osteoporosis and increased risk of femur neck fractures), growth retardation in children, a tendency to hyperglycaemia, and suppression of the response to infection or injury (Rang 2015). Recurrent short courses of prednisolone may also be associated with adverse events, in particular, with a reduction of bone mineral accrual as reported among participants in the Childhood Asthma Management Program (CAMP) trial (CAMP Research Group 2000; Kelly 2008). However, evidence supports the short‐term use of systemic corticosteroids during acute asthma exacerbations (Rowe 2007; Fernandes 2014). Patients remain particularly prone to repeat exacerbations in the period immediately after an asthma exacerbation and the use of systemic steroids can reduce the risk of a relapse and the need for reliever inhalers, without major adverse effects (Rowe 2007). Prescription of a 'rescue pack' (containing a course of OCS) to a patient or their career permits self‐administered treatment in the event of an exacerbation, as guided by a patient's personalised asthma action plan (BTS/SIGN 2016).
How the intervention might work
Patient‐initiated OCS may feature as part of a written asthma action plan (GINA 2016), which should state when and how to initiate treatment with OCS, and when to access medical care if symptoms fail to respond to treatment. Compared with OCS administered by an emergency department physician, patient‐ or parent‐initiated treatment permits early administration of systemic corticosteroids following the onset of an acute exacerbation. The benefits of OCS have been demonstrated within three hours of administration, and delayed dosing of OCS is less effective at resolving acute asthma (Streetman 2002). Indeed, there is some evidence from studies in children that early administration of systemic steroids during an exacerbation can reduce asthma symptoms and the number of days of missed school, compared with physician‐initiated steroids (Vuillermin 2010). Furthermore, recurrent severe exacerbations are associated with accelerated lung function decline, suggesting that aggressive treatment of intermittent airway inflammation may be important to prevent airway remodelling (Bai 2007).
Why it is important to do this review
The use of patient‐initiated oral steroids is common practice in chronic obstructive pulmonary disease (COPD) (DoH 2010) and the appropriate use of rescue packs is currently included in a National Institute for Health and Care Excellence (NICE) quality statement for managing COPD (NICE 2010). In line with their use in COPD, the use of patient‐ and parent‐initiated OCS for asthma appears to be increasingly common in clinical practice (Vuillermin 2007; BTS/SIGN 2016). For example, in an Australian survey of 252 doctors involved in the care of children with asthma, 85% of doctors reported recommending parent‐initiated OCS to parents of children with asthma (Vuillermin 2007). Additionally, British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines on personalised action plan content recommend coverage on starting oral steroids, "which may include provision of an emergency course of steroid tablets" (BTS/SIGN 2016). However, to date there is limited evidence for the use of patient‐ or parent‐initiated OCS for treating asthma exacerbations (NACA 2015). An earlier Cochrane review evaluated the evidence around parent‐initiated oral corticosteroid therapy for intermittent wheezing illnesses in children (Vuillermin 2006). We will not consider pre‐school wheeze in the present review.
It is important to consider the potential benefits of earlier treatment with OCS against potential harms, which include the safety issues around delaying access to medical care when OCS are taken at home. Furthermore, the provision of rescue packs to patients with asthma or to their carers will likely increase overall administration of oral corticosteroids; this has implications for the incidence of steroid‐associated side‐effects, particularly in children. Taken together, this information highlights the importance of synthesising the evidence to establish whether this intervention is safe and effective in people with asthma.
Objectives
To evaluate the effectiveness and safety of patient‐ or parent‐initiated oral steroids for adults and children with asthma exacerbations.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), reported as full‐text, those published as abstract only, and unpublished data. We excluded cross‐over trials because the effects of corticosteroids can persist for a number of weeks or months (Haahtela 1994) and studies did not employ a sufficient washout period between treatment periods to eliminate cross‐over effects.
Types of participants
We planned to include adults (aged ≥ 18 years) and children of school age or older (i.e. aged ≥ 5 years) with a diagnosis of asthma. Adults and children were to be considered in separate comparisons. Preschool wheeze was not considered by this review and as such we excluded studies of preschool children. The diagnosis of asthma was required to be determined by a clinician according to validated national or international guidelines. We excluded participants with any respiratory comorbidities (e.g. bronchiectasis, chronic obstructive pulmonary disease). If a study contained both adults and children, we contacted the study authors to check if disaggregated data were available; if we were unable to source these data we used the average age (≥ 18 years) of study participants to determine suitability for inclusion. If the average age of study participants was less than 18 years, we planned to perform a sensitivity analysis to examine the effect of including or excluding these studies. Finally, if a study included children of both school and preschool age, we excluded the study if the average age was less than five years old.
Types of interventions
We planned to include studies comparing any patient‐ or parent‐initiated oral corticosteroid (OCS), with either placebo, normal care, an alternative active treatment plan (e.g. doubling the dose of inhaled steroids) or an identical personalised asthma exacerbation management plan without patient‐ or parent‐initiated OCS. OCS (any dose or duration) could be combined with other measures for the management of an exacerbation (e.g. personalised asthma action plan, increased use of reliever inhaler) provided that the measure was not part of the randomised treatment. We planned to perform separate comparisons for each type of comparator (e.g. patient‐initiated steroids versus placebo; patient‐initiated steroids versus normal care, etc); separate comparisons would also be performed for adults and children. We defined normal care as any measure that the patient would usually take to manage an exacerbation (e.g. increase reliever inhaler use, seek medical advice, etc). We also planned to include studies where the comparator group comprised a combination of the above (e.g. personalised asthma action plan plus placebo).
Types of outcome measures
Primary outcomes
-
Hospital admissions for asthma.
-
Asthma symptoms at follow‐up (measured on a validated scale (e.g. Asthma Control Questionnaire (ACQ))).
-
Serious adverse events.
We selected the primary outcomes to represent an important measure of resource use, a patient‐reported outcome, and safety.
Secondary outcomes
-
Unscheduled visit to a healthcare provider (e.g. accident and emergency, general practitioner).
-
New exacerbation in follow‐up period (asthma control).
-
Health‐related quality of life (using a validated scale).
-
Reliever medication use.
-
Days of school (children) or study/work (adults) lost.
-
Time to full resolution of symptoms.
-
Adverse events.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review. If a study used more than one scale to report the same outcome, or if different scales were used across studies, we planned to analyse the different scales together using the standardised mean difference.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR) (searched 18 May 2016), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library; MEDLINE Ovid; Embase Ovid; CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; AMED Ovid (Allied and Complementary Medicine; and PsycINFO Ovid; and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2.
We also conducted a search of the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 24 August 2016) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 24 August 2016).
When searching all databases we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We planned to search for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and to report the date this was done.
Data collection and analysis
Selection of studies
Two review authors (MBG, MG) independently screened titles and abstracts of all the potentially‐relevant studies that we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and two review authors (MBG, MG) independently screened the full‐text articles to confirm studies for inclusion, or to identify and record reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (MM). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We planned to use a data collection form for study characteristics and outcome data, which would be piloted on at least one study in the review. Two review authors (MBG, DE) planned to extract study characteristics from included studies. We planned to extract the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (MBG, DE) planned to independently extract outcome data from the included studies. We planned to note in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We planned to resolve disagreements by consensus or by involving a third review author (MM). One review author (DE) was responsible for transferring data into the Review Manager (RevMan 2014) file. We planned to double‐check that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (DE) was responsible for spot‐checking study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (MBG, DE) planned to independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any disagreements by discussion or by involving another review author (MM). We planned to assess the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We planned to grade each potential source of bias as high, low or unclear risk, and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We planned to summarise the 'Risk of bias' judgements across different studies for each of the domains listed, and to consider blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias relates to unpublished data or correspondence with a trialist, we planned to note this in the 'Risk of bias' table.
When considering treatment effects, we would take into account the risk of bias for the studies that contribute to that outcome.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol (Ganaie 2016) and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We planned to analyse dichotomous data as odds ratios and continuous data as mean difference or standardised mean difference. We planned to enter data presented as a scale with a consistent direction of effect.
We planned to undertake meta‐analyses only where this was meaningful (i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We planned to narratively describe skewed data reported as medians and interquartile ranges.
Where multiple trial arms were reported in a single trial, we planned to include only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we planned to halve the control group to avoid double‐counting.
Unit of analysis issues
For dichotomous outcomes, we planned to report participants, rather than events, as the unit of analysis. For example, for the secondary outcome 'unscheduled visit to a healthcare provider' we would record the number of participants with an unscheduled visit, rather than the number of unscheduled visits per participant.
Dealing with missing data
We planned to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Assessment of heterogeneity
We planned to use the I2 statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity we would report it and explore possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 studies, we planned to create and examine a funnel plot to explore possible small study and publication biases.
Data synthesis
We planned to use a random‐effects model and perform a sensitivity analysis with a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table for each comparison using the following outcomes: hospital admissions for asthma; asthma symptoms at follow‐up; serious adverse events; unscheduled visit to a healthcare provider; asthma control at follow‐up; days of school or study/work lost; and adverse events. We planned to use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2011). We planned to use methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT). We planned to justify all decisions to downgrade or upgrade the quality of studies using footnotes and to make comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Baseline asthma severity (stratified by background medication).
We planned to use the following outcomes in subgroup analyses.
-
Hospital admissions for asthma.
-
Asthma symptoms at follow‐up.
-
Serious adverse events.
We planned to use the formal test for subgroup interactions in Review Manager (RevMan 2014).
Sensitivity analysis
If necessary, we planned to carry out the following sensitivity analyses to explore the effect of including/excluding:
-
studies that included both adults and children/adolescents, where the average age of participants was < 18 years;
-
unpublished data (i.e. no peer‐reviewed full‐text paper available);
-
studies at high risk of performance or detection bias;
-
studies at high risk of any other bias;
-
studies with missing data.
Results
Description of studies
Results of the search
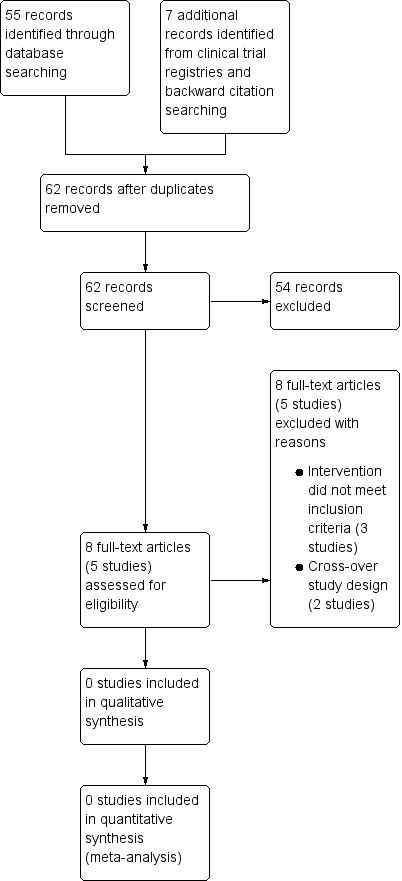
A search of the Cochrane Airways' Specialised Register (CASR) returned 55 references and the search of the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform yielded a further 7 records. Two review authors screened the 62 references/records using Covidence (Covidence 2016) and 54 records were discarded. We selected eight references (five studies) as potential candidates for inclusion in this review and sourced the corresponding full‐text articles. Two review authors screened the full‐text articles independently and all five studies were excluded with reasons. The PRISMA flow diagram is presented in Figure 1.
Study flow diagram.
Included studies
We included no studies.
Excluded studies
We excluded five studies with reasons (see Characteristics of excluded studies table). Three studies were excluded because the intervention did not meet the criteria for inclusion in this review (oral corticosteroids (OCS) must be part of the randomised treatment and could be combined with other measures for the management of an exacerbation such as a personalised asthma action plan, provided that the co‐intervention was not part of the randomised treatment). Specifically, the OCS were not part of the randomised treatment in the study by Boushey 2005. Participants in the study reported by Milenović 2007 could initiate oral corticosteroids as per a personalised asthma action plan (i.e. co‐intervention), which was part of the randomised treatment and was not available to participants in the control group. In van Der Meer 2009, the oral corticosteroids were optional at step seven of an internet‐based asthma plan (i.e. co‐intervention), which was part of the randomised treatment. Furthermore, the oral corticosteroids were taken following contact with an asthma nurse, and the usual care group received no plan. Two studies were excluded because of their cross‐over design (Grant 1995; Vuillermin 2010); we contacted the authors of the respective studies, who were unable to provide data for the first treatment period (i.e. prior to cross‐over).
Risk of bias in included studies
Not applicable.
Effects of interventions
See: Summary of findings for the main comparison Summary of findings: patient‐initiated steroids; Summary of findings 2 Summary of findings: parent‐initiated steroids
Not applicable.
Discussion
Summary of main results
After screening the results of extensive searches, we identified no relevant randomised controlled trials to include in this review. Five potentially relevant studies were excluded based on our pre‐specified criteria. Two of the excluded studies asked the relevant clinical question (Grant 1995; Vuillermin 2010). However, these studies were excluded due to their cross‐over design, as per the protocol. The authors were contacted but were unable to provide data for the first treatment period only (i.e. prior to cross‐over).
Overall completeness and applicability of evidence
We identified no relevant studies to include in this review.
Quality of the evidence
Despite performing a comprehensive search and a duplicate (independent) screening and reviewing process, we identified no relevant studies to include in this review.
Potential biases in the review process
This review process could potentially be subject to a risk of bias in two areas: searching and drawing conclusions. However, Cochrane Airways' Information Specialist designed and conducted the main electronic search and two clinicians in the author team (MG, MBG) with expert knowledge in the area independently sifted and reviewed the search results. Consistent with Cochrane methodology, we excluded no trials on the basis of language, publication status, or the outcomes reported, so we are confident that we identified all potentially relevant randomised evidence. Our conclusions are consistent with the lack of included studies.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no existing systematic reviews on this topic. The two randomised controlled trials that asked the relevant clinical question, but were excluded from the present review due to their cross‐over design, reported contrasting findings. Grant and colleagues examined the effectiveness of a single oral dose of prednisone administered by a parent to a child early in an asthma attack (Grant 1995). Contrary to expectation, the authors found that participants in the parent‐initiated oral prednisone group had significantly more asthma exacerbations resulting in outpatient visits than when they were in the placebo group (Grant 1995). The authors speculated that the results may be specific for a population of children with suboptimal use of beta‐agonist therapy, and stressed that further studies are required in different populations of asthmatic children (Grant 1995). In contrast, Vuillermin and colleagues found that among children of school age, a short course of parent‐initiated oral prednisolone during an asthma exacerbation may result in a reduction in asthma symptoms, health resource use, and days off school (Vuillermin 2010). However, the authors cautioned that the modest benefits of this strategy should be balanced against potential side effects of repeated oral corticosteroid use (Vuillermin 2010).

Study flow diagram.
| Patient‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: adults aged 18 years or older with asthma Settings: outpatient Intervention: patient‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days lost of study/work | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||
| Parent‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: children aged 5 years or older with asthma Settings: outpatient Intervention: parent‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days off school | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||

