Profilaxis habitual con antibióticos después del parto vaginal normal para la reducción de la morbilidad infecciosa materna
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012137.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 noviembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors were involved in development of the protocol.
M Bonet and CE Chibueze assessed relevant trials, extracted, entered and checked data from trials into RevMan.
M Bonet and CE Chibueze interpreted the data and drafted the review.
All authors read and approved the final version of the review for publication.
Sources of support
Internal sources
-
The Grant of National Center for Child Health and Development, 27B‐10, 26A‐5, Japan.
External sources
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), the Department of Reproductive Health and Research (RHR), World Health Organization, Geneva, Switzerland.
-
Japan Agency for Medical Research and Development, Japan.
The National Research Center for Child Health and Development, Japan receives government funding (AMED No.27300101) from the Clinical Research Program for Child Health and Development, AMED, Japan to support the Cochrane Pregnancy and Childbirth Satellite in Japan.
Declarations of interest
Mercedes Bonet: none known.
Erika Ota: none known.
Chioma E Chibueze's work was financially supported by the The Grant of National Center for Child Health and Development, Japan 27B‐10, 26A‐5.
Olufemi T Oladapo: none known.
Acknowledgements
As part of the prepublication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
This work was supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization.
Chioma E Chibueze's work was financially supported by the The Grant of National Center for Child Health and Development, Japan 27B‐10, 26A‐5.
The World Health Organization and Erika Ota and Chioma E Chibueze retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 13 | Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity | Review | Mercedes Bonet, Erika Ota, Chioma E Chibueze, Olufemi T Oladapo | |
| 2016 May 07 | Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity | Protocol | Mercedes Bonet, Erika Ota, Chioma E Chibueze, Olufemi T Oladapo | |
Differences between protocol and review
The name of the outcome 'puerperal infection' was revised to endometritis.
There are no further differences between the published protocol (Bonet 2016) and this full review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amoxicillin [administration & dosage];
- Anti-Bacterial Agents [administration & dosage];
- *Antibiotic Prophylaxis;
- Chloramphenicol [administration & dosage];
- Clavulanic Acid [administration & dosage];
- *Delivery, Obstetric;
- Endometritis [epidemiology, *prevention & control];
- Episiotomy [adverse effects];
- Non-Randomized Controlled Trials as Topic;
- Puerperal Infection [epidemiology, *prevention & control];
- Randomized Controlled Trials as Topic;
- Sulfamethoxypyridazine [administration & dosage];
- Surgical Wound Infection [epidemiology, prevention & control];
- Urinary Tract Infections [epidemiology, prevention & control];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
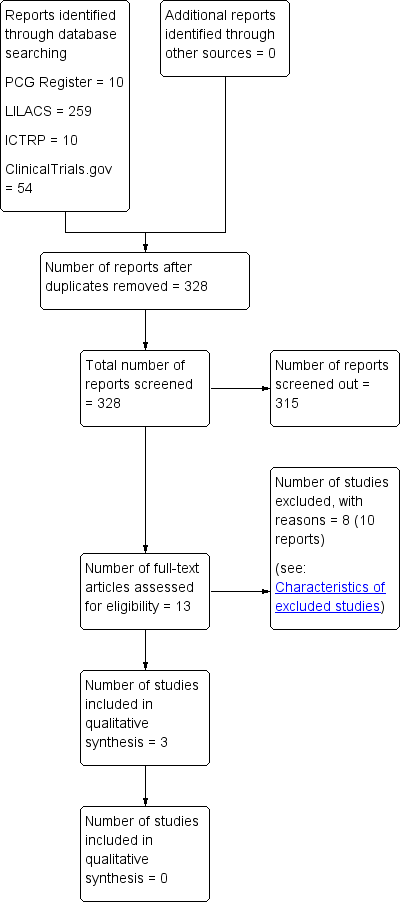
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 1 Endometritis.
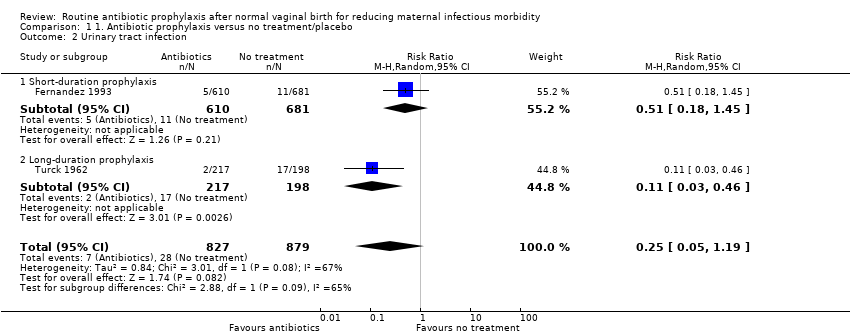
Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 2 Urinary tract infection.

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 3 Wound infection (episiotomy dehiscence).

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 4 Adverse effects of antibiotics (skin rash).

Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 5 Length of maternal hospital stay ‐ days.
| Study | Experimental | Control |
| Fernandez 1993 | 3600 USD | 9000 USD |
Comparison 1 1. Antibiotic prophylaxis versus no treatment/placebo, Outcome 6 Cost of care (cost of antibiotic prophylaxis, prolonged hospitalisation and treatment of endometritis).
| Routine antibiotic prophylaxis compared to no treatment/placebo for preventing infections in women who had normal vaginal births | ||||||
| Patient or population: Women who had normal vaginal births | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment/placebo | Risk with routine antibiotic prophylaxis | |||||
| Incidence of severe maternal infectious morbidity (including septicaemia, septic shock, laparotomy/hysterectomy for infection, maternal intensive care unit admission, or organ failure) | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| Endometritis | Study population | RR 0.28 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 23 per 1000 | 7 per 1000 | |||||
| Urinary tract infection | Study population | RR 0.25 | 1706 | ⊕⊕⊝⊝ | ‐ | |
| 32 per 1000 | 8 per 1000 | |||||
| Wound infection (episiotomy dehiscence) | Study population | RR 0.78 | 1364 | ⊕⊝⊝⊝ | ‐ | |
| 7 per 1000 | 10 per 1000 | |||||
| Adverse effects of antibiotics (skin rash) | Study population | RR 3.03 | 1706 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Length of maternal hospital stay | The mean length of maternal hospital stay (including maternal re‐admission to hospital) was 5.12 days | MD 0.15 lower | not estimable | 1291 | ⊕⊝⊝⊝ | ‐ |
| Individual antimicrobial resistance | not estimable | not estimable | not estimable | 0 | ‐ | No trial reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Two studies with very serious design limitations (‐2). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 2 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.09, 0.83] |
| 2 Urinary tract infection Show forest plot | 2 | 1706 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.19] |
| 2.1 Short‐duration prophylaxis | 1 | 1291 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.45] |
| 2.2 Long‐duration prophylaxis | 1 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.03, 0.46] |
| 3 Wound infection (episiotomy dehiscence) Show forest plot | 2 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.96] |
| 4 Adverse effects of antibiotics (skin rash) Show forest plot | 2 | 1706 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.32, 28.95] |
| 5 Length of maternal hospital stay ‐ days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Cost of care (cost of antibiotic prophylaxis, prolonged hospitalisation and treatment of endometritis) Show forest plot | Other data | No numeric data | ||

