Malla o injertos transvaginales comparados con reparación con tejido autólogo para el prolapso vaginal
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT for stage 2 POPQ prolapse PC‐generated randomisation 2‐year follow‐up No CONSORT statement Blinding not stated Authors state power of 85% need sample size of 20 in each arm | |
| Participants | 40 randomised in abstract, however 44 were randomised, 4 of whom failed to return postoperatively and were excluded Inclusion criteria: stage 2 POPQ cystocele with no plans of pregnancy in 12 months Exclusion criteria: contemplating pregnancy, women with paravaginal defects, needing continence surgery, prior colposuspension or vaginal surgery, immunocompromised, or diabetics | |
| Interventions | A (n = 23): anterior colporrhaphy AC 0 polyglactin (Vicryl) suture B (n = 21): self styled armless soft polypropylene (Gynemesh) mesh without AC | |
| Outcomes | Assessed at 6 weeks, 3 months, then every 6 months to 2 years postop Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number tables |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes to ensure allocation concealment; as not consecutive sealed, opaque envelopes unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers blinded except when mesh exposure occurred |
| Incomplete outcome data (attrition bias) | Low risk | At 1‐year, group A 20/23, group B 20/21 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funding not stated; authors no COI |
| Methods | Single‐centre RCT Inclusion grade 3 or 4 cysto‐urethrocele (BW halfway system) No exclusion No power Randomisation and concealment, blinding not stated 6/12 follow‐up | |
| Participants | No CONSORT N = 108 Inclusion: women with grade 3 or 4 cysto‐urethrocele (BW halfway system) There were no significant differences between the groups regarding preoperative storage symptoms, urodynamics, and degree of prolapse | |
| Interventions | A (54): anterior colporrhaphy alone B (54): anterior colporrhaphy with tension‐free polypropylene (Gynemesh PS) overlay | |
| Outcomes | Assessed at 6 months' postop Reports the following review outcomes:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | At 6 months, group A 43/54, group B 46/54; greater than 15% loss to follow‐up at 6 months |
| Selective reporting (reporting bias) | Unclear risk | Did not report any of the primary outcomes of this review; only has 6 months' follow‐up |
| Other bias | Unclear risk | No statement about funding |
| Methods | Single‐centre RCT comparing vaginal fascial repair with or without polyglactin mesh and with polydioxanone or polyglactin sutures, 2 x 2 factorial design PC randomisation, "secure" remote concealment Blinded women, ward staff, and follow‐up assessor Follow‐up 3 months with exam, 6 months with non‐validated questionnaire, 2 years with validated questionnaire | |
| Participants | 73 randomised, 7 ineligible after randomisation, 66 in trial Lost to follow‐up: 8 at 3 months; 4 at 6 months; 12 at 2 years Inclusion: grade 2 or more prolapse (unclear examination technique), anterior or posterior prolapse, or both Concomitant procedures: vaginal hysterectomy 14; cervical amputation (Manchester) 18; tension‐free vaginal tape 13 | |
| Interventions | Comparing vaginal fascial repair with or without polyglactin mesh and with polydioxanone or polyglactin sutures, 2 x 2 factorial design A (32): fascial repair plus polyglactin mesh overlay B (34): fascial repair without mesh C (33): repair of fascia with polydioxanone sutures D (33): repair of fascia with polyglactin sutures | |
| Outcomes | Assessed at 3 months', 6 months', and 2 years' postop Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Secure method of concealment of randomisation (remote computer allocation) |
| Blinding of participants and personnel (performance bias) | Low risk | Not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Participant‐completed questionnaires, data entry blinded to randomisation |
| Incomplete outcome data (attrition bias) | High risk | Equal non‐response between the groups at 2 years, medical records seen for all non‐responders |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Unfunded study |
| Methods | Multi‐centre RCT: 53 centres, 58 surgeons 90% powered to detect 20% difference between groups with 1% type 1 error, central randomisation PC Participant blinded Reviews conducted 2 and 12 months by surgeon 1/3, non‐surgeon 2/3 Completed pre‐ and 1‐year UDI and PISQ‐12 | |
| Participants | 1685 screened; 389 randomised Underwent surgery: A 182, B 191 Lost to follow‐up A 7, B 14 (1 year: A 182, B 186) Inclusion: > 18 yrs, ≥ stage 2 symptomatic cystocele POPQ Exclusion: previous cancer of any pelvic organ, systemic glucocorticoid treatment, insulin‐treated diabetes, an inability to participate or to provide consent, or need concomitant surgery | |
| Interventions | A (182): anterior colporrhaphy slow absorption monofilament thread, sham skin markings, excessive trimming vagina discouraged B (191): Gynecare transvaginal anterior mesh (Prolift), absorbable sutures, excessive vaginal trimming discouraged, catheter care discretion surgeon | |
| Outcomes | Assessed at 1‐year postop Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Secure concealment with remote computer |
| Blinding of participants and personnel (performance bias) | Low risk | Women blinded (sham skin markings) |
| Blinding of outcome assessment (detection bias) | High risk | Reviewers surgeon 1/3, non‐surgeon 2/3 Woman‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | 1‐year AC 174/182; mesh 186/191 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Funded by Karolinska Institute and Ethicon; conflict of interest statements of members of Nordic transvaginal mesh group who were reviewers of surgery were not reported |
| Methods | Single‐centre RCT CONSORT: no Randomisation: computer generated Allocation concealment: N/S Women, surgeons, and reviewers not blinded 12 months' follow‐up | |
| Participants | Inclusion criteria: women who were recommended vaginal surgery for anterior and posterior compartment with ≥ grade 2 prolapse Exclusion criteria: only requiring anterior or posterior compartment surgery, apical prolapse beyond the hymen, or those requiring abdominal mesh surgery Randomised: 139 (A 70, B 69); 10 women breached study protocol, and 11 more recruited. All were analysed Lost to follow‐up: A 6, B 9 Analysed 12 months: A 63, B 61 | |
| Interventions | A (70): traditional anterior and posterior fascial plication using polydioxanone sutures B (69): anterior and posterior repair with Gynemesh PS augmentation | |
| Outcomes | Assessed at 6 months and 1 year postop Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | Native tissue 63/70; mesh 63/69 1 year |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Funding not stated: authors' conflict of interest financial agreement with Ethicon manufacturer of product evaluated in study |
| Methods | Multi‐centre (4) RCT for stage 3 to 4 POPQ (any compartment) Brazil Computerised randomisation Sample size n = 90 in each group, 90% power and allowing 20% loss of follow‐up No ITT analysis Women unblinded Reviewers blinded | |
| Participants | Inclusion criteria: grade 3 to 4 POP (any POPQ measurement > +1) No exclusion criteria 199 screened, 184 randomised Native tissue n = 90 randomised, n = 81 completed 1 year Mesh n = 94 randomised, n = 88 1 year | |
| Interventions | Gp A: site‐specific native tissue: site‐specific anterior and/or posterior 1.0 non‐absorbable suture (polypropylene), apical 1.0 non‐absorbable sacrospinous right; uterine prolapse hysterectomy in both groups Gp B: mesh group: polypropylene macroporous monofilament Prolift mesh Concomitant surgery allowed Prior to study each centre performed at least 3 surgeries Hb 24 hours postop Assessed 1 week 1, 6, 12 months Pain assessed variable rating scale Gp A: 74/90 anterior compartment prolapse ∓ other surgery, posterior alone n = 7, apical alone n = 9 Gp B: mesh group similar breakdown, mid‐urethral slings: 5/90 native tissue, 9/94 mesh; vaginal hysterectomy: 32/90, 29/94 | |
| Outcomes | Assessed at 1‐year postop Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not able to be blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) | Low risk | Native tissue: randomised 90, 1 year 81 completed Mesh: randomised 94, 1 year 88 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | J&J donated product; no financial input study |
| Methods | Multi‐centre (8) Swedish open RCT Computer‐generated block randomisation stratified for each centre Allocation concealment in opaque, sealed envelopes Sample size was based on the assumption that a 15% difference in objective cure rate after 3 years between the implant‐augmented repair and the traditional colporrhaphy with 90% power should be significant at a 5% level. It was estimated that 160 women, 80 in each arm of the study, including a drop‐out of 10%, were needed 3‐year review ITT and CONSORT guidelines reporting not stated | |
| Participants | Inclusion: recurrent (prior surgery on the prolapsing site) POP in anterior or posterior compartment, or both No exclusion criteria 135 randomised Gp A native tissue repair 66, and 3 years 60/66 Gp B porcine dermis repair 65, and 3 years 65/68 | |
| Interventions | Standardised surgery with 2 meeting workshops prior to study Native tissue repair: midline fascial plication interrupted polydioxanone suture, vagina closed polyglactin absorbable suture Porcine: porcine dermal implant (Pelvicol, Bard Sweden) as inlay with no fascial plication: inlay anchored to vaginal wall and fascia 6‐8 polydioxanone sutures, vagina closed polyglactin suture Concomitant mid‐urethral sling, apical support, and levator plication performed as required | |
| Outcomes | Assessed at 3 months and 3 years Reports the following review outcomes:
| |
| Notes | Did not reach sample size as slow to recruit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation list stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes (not stated if consecutive or not) |
| Blinding of participants and personnel (performance bias) | High risk | Nil |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Gp A 60/68 and Gp B 65/68 completed 3‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI; funded by local research institutes |
| Methods | Multi‐centre RCT comparing infracoccygeal sacropexy and sacrospinous suspension for uterine or vaginal vault prolapse No CONSORT statement Power calculation: yes, 77 required in each arm. Recruitment stopped after change in mesh material (multi‐filament mesh replaced by monofilament) No ITT analysis No data on type of randomisation, blinding strategy, or allocation concealment No definition of cure or failure Mean follow‐up 16.8 months (range 1.5 to 32) both arms Prolapse assessment: POPQ Validated questionnaires: PFDI, PFIQ, PISQ‐12, French version | |
| Participants | Inclusion: symptomatic uterine or vaginal vault prolapse (stage 2 or higher) Exclusion: isolated cystocele, stage 1 prolapse, rectal prolapse, and intestinal inflammatory disease 49 randomised 4 lost to follow‐up 45 analysed | |
| Interventions | A (21): infracoccygeal sacropexy (multi‐filament polypropylene tape, posterior IVS) B (24): sacrospinous suspension Concomitant surgery: cystocele repair, posterior repair, hysterectomy, suburethral tape Types of repair and indications for repair were not described | |
| Outcomes | Assessed at "medium term" follow‐up (mean 16.8 months postop, range 1.5 to 32) Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | At 1 year 45/49 completed review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | COI or funding unstated |
| Methods | Multi‐centre (12 French hospitals) RCT 12‐month review Randomisation by drawing lots, stratified by centre, allocation concealment not discussed Intention to treat stated yes, but women already randomised were removed if cystotomy occurred during surgery CONSORT guidelines Sample size of 194 provided 80% power to detect 20% difference with an alpha error 5% and drop‐out rate 10% Assessors not clear | |
| Participants | Inclusion criteria: symptomatic stage 2 anterior wall prolapse, aged 60 years or older Exclusion criteria: steroids, poorly controlled diabetes, prior pelvic radiation, untreated vaginal or urinary infection, ascites, bladder injury during the procedure All used preoperative estrogen therapy 163 included, 162 randomised Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/60 Preop demographics and potential confounders similar in both groups, except colorectal impact was greater group A | |
| Interventions | Gp A: anterior colporrhaphy no mesh (plication of fascia with 2.0 polyglactin absorbable suture), uterosacral colpopexy and hysterectomy as required Gp B: anterior polypropylene macroporous mesh (Ugtex, Sofradim, Covidien) 4‐armed transobturator mesh fixed with 2 x 2.0 permanent polypropylene sutures to uterine isthmus or uterosacral ligaments and 2 x 2.0 polyglactin sutures to inferior edge of pubic rami; vaginal trimming minimised Concomitant surgery mid‐urethral sling, hysterectomy, and any native tissue repair, however no other transvaginal mesh intervention included | |
| Outcomes | Assessed at 1‐year follow‐up Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by drawing lots? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/80 (20% attrition). 2 women who had bladder injury were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Author COI with Sofradim, who provided partial funding and whose product was being evaluated. 2 women who had bladder injury were excluded from analysis; this outcome not reported clearly in both groups |
| Methods | Single‐centre non‐inferiority RCT Computer‐generated random number list Allocation at inclusion with surgeon aware only in operating theatre Envelopes allocation Sample size: 35 in each group, 80% power to detect 5% significant change with 10% drop‐out ITT analysis Assessors blinded Women unblinded | |
| Participants | Any anterior POP point Ba ≥ +1 on POPQ Excluded malignant urogenital disease, prior radiation, acute genitourinary infection, connective tissue disorders, steroid treatments, insulin‐dependent diabetes | |
| Interventions | All procedures under spinal by 3 experienced surgeons 1. AC: plicate fascia purse string 0 polyglactin (Vicryl), vaginal trimming, transvaginal trocar‐guided polypropylene mesh (kits donated by Promedon) Nazca TC (Promedon, Córdoba, Argentina) I prepubic and 2 transobturator macroporous monofilament; vagina closed overlapping fashion 355 accessed, 79 randomised AC 39 completed, 1‐year review n = 39 2. Anterior mesh 40 randomised, 40 completed 1‐year review Concomitant surgery as required | |
| Outcomes | Assessed at 1 year Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation tables |
| Allocation concealment (selection bias) | Unclear risk | Envelopes (opaque?, sealed?) |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded assessors |
| Incomplete outcome data (attrition bias) | Low risk | 79 randomised, and all completed 1‐year review |
| Selective reporting (reporting bias) | Unclear risk | Did not clearly report any of the primary outcomes of this review |
| Other bias | Low risk | Funded by Federal University of Sao Paulo, Brazil; Promedon contributed product free of charge No author COI |
| Methods | Single‐centre RCT Randomisation and allocation concealment described Evaluated 1 year after AC as compared to small intestine submucosa graft Blinded reviewers Sample size of 60 women was required to achieve a significance level of 0.05 and a power of 80%. This was based on the assumptions of a 25% difference in cure rates between the groups with a 10% loss to follow‐up rate | |
| Participants | Inclusion criteria: women with point Ba ≥ ‐1 Exclusion criteria: hypertension, prior radiation, pelvic sepsis, diabetes, and chronic illness Concomitant surgery allowed including vaginal hysterectomy if greater than stage 2 uterine prolapse | |
| Interventions | Gp A (27) AC with interrupted 0 polyglactin (Vicryl) sutures GP B (29) non‐cross‐linked xenograft porcine small intestine submucosa 7 x 10 cm with dissection to suprapubic arch fixed with 0 prolene x3 each side | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Centrally controlled allocation concealment appropriate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded reviewers and participant‐completed validated questionnaires |
| Incomplete outcome data (attrition bias) | High risk | 1 year: Gp A 20/27(74%); Gp B 22/29 (76%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI and no external funding |
| Methods | Single‐centre RCT (computer‐generated, opaque envelopes, adequate concealment) | |
| Participants | 162 signed consent form | |
| Interventions | A (76): "ultra‐lateral" midline plication of anterior endopelvic connective tissue using polyglactin (Vicryl) buttress sutures (as described by Weber 2001), plus additional cadaveric fascia lata patch (Tutoplast) anchored at the lateral limits of the colporrhaphy | |
| Outcomes | Assessed at 1 year Reports the following review outcomes:
| |
| Notes | Unclear participant numbers (disparity with loss to follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, consecutive envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Data largely complete; 2/155 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI or funding statement |
| Methods | Multi‐centre RCT 24‐month follow‐up Randomisation computer generated Allocation concealment without blinding of women or surgeon Not according CONSORT Sample size was calculated by estimating a recurrence rate of 35% with AC and 10% with graft reinforcement. Assuming a 2‐tailed hypothesis test with 5% type 1 error and 80% power, 80 women would be required. We enrolled 94 women assuming a drop‐out rate of 15% | |
| Participants | Randomised: Gp A 47, Gp B 47 2 years: Gp A 33, Gp B 26 Examination: Gp A 27, Gp B 17 Inclusion criteria: point Ba ≥ ‐1 Exclusion criteria: total vaginal length < 6 cm, severe atrophy, isolated paravaginal defect, allergic to bovine material, prior vaginal implant surgery, or ulceration | |
| Interventions | A (n = 46): AC B (n = 44): AC with bovine pericardium collagen matrix graft reinforcement | |
| Outcomes | Assessed at 6 months, 1 year, and 2 years Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes opened in theatre (not consecutive) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if assessors blinded, participant‐completed questionnaire |
| Incomplete outcome data (attrition bias) | High risk | Equal losses in both groups; only 50% completed 2‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Extensive COI reported; study partly funded Synovis Life Technologies, whose bovine pericardium product was being evaluated |
| Methods | Single‐centre RCT India Computer‐generated randomisation Allocation concealment: not stated Blinding of participants and reviewers: not stated Sample size 106 with 80% power to detect 21% difference between the groups with 5% type 1 error | |
| Participants | Inclusion criteria: stage 2 or greater anterior compartment prolapse Exclusion criteria: SUI, dominant post‐vaginal prolapse, suspected malignancy, vaginal infections | |
| Interventions | Group A: AC 2.0 polyglactin (Vicryl); n = 54, 1 year n = 41 Group B: self‐styled 4‐arms monofilament polypropylene mesh (Vypro mesh, J&J); n = 52, 1 year n = 44 | |
| Outcomes | Assessed at 6 months, 1 year Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding of participants and personnel (performance bias) | Unclear risk | No statement |
| Blinding of outcome assessment (detection bias) | Unclear risk | No statement |
| Incomplete outcome data (attrition bias) | High risk | Gp A 41/54, Gp B 44/52 at 1 year (20% attrition) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI statement |
| Methods | Multi‐centre randomised trial Computer‐generated randomisation table Allocation concealment not defined 70% power to detect 20% difference in groups | |
| Participants | Inclusion criteria: central posthysterectomy vault prolapse: POPQ greater or equal stage 2 Exclusion criteria: pelvic malignancy, < 18 years, prior radiotherapy, requiring hysterectomy Allocated: Gp A 83, Gp B (Mesh) 85 1 year: Gp A 72, Gp B 79 Recurrence defined as stage 2 or greater POPQ Not clear who performed assessments | |
| Interventions | Gp A (83) anterior repair. Sacrospinous colpopexy (2x non‐absorbable sutures Nurolon) ± posterior repair (approximation of levator muscles) and moderate excision of redundant vagina GP B (85) total Prolift mesh secured with 2.0 PDS Intervention performed by surgeons with greater than 20 cases experience of each type of surgery | |
| Outcomes | Assessed at 1 year Reports the following review outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 year Gp A 72/83; Gp B 79/85 (89%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded by grant from Czech Ministry of Health, authors no COI |
| Methods | Single‐centre RCT Computer‐generated randomisation and allocation concealment were appropriate with sealed envelopes opened in operating room Reviews by non‐blinded surgeon No concomitant surgery 80% power to detect 20% difference between the groups with 5% type 1 error: 60 randomised | |
| Participants | Inclusion criteria: symptomatic prolapse point Ba ≥ ‐1 Exclusion criteria: defects posterior or apical compartment, prior pelvic surgery, history of collagen or endocrine disorders Allocated: Gp A 31, Gp B 30 1 year: Gp A 26, Gp B 28 | |
| Interventions | A (31): 2.0 interrupted polyglactin (Vicryl) plication B (30): no plication, Pelvicol porcine dermis 4 x 7 cm anchored with 2.0 polyglactin (Vicryl) sutures No concomitant surgery | |
| Outcomes | Assessed at 1 year Reports the following review outcomes:
| |
| Notes | Irregularities exist: methods failure defined as e Ba ≥ ‐1 results > ‐1; in table 2 Gp A range Ba 2 to 8, and states in table 3 that 4 had stage 2 prolapse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed, non‐transparent, consecutive envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Reviewers not blinded, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | 1 year: Gp A 26/31, Gp B 28/30 (88%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI declared; no statement funding |
| Methods | Multi‐centre RCT Double blinded Power calculation included Randomisation computer generated stratified for presence uterine prolapse, allocation concealment, CONSORT guidelines met, no ITT analysis | |
| Participants | 173 excluded variety reasons Gp A 33, Gp B 32 Lost to follow‐up: Gp A 0, Gp B 0 Prior to surgery all demographic details similar between the 2 groups, except Gp B had lower POPDI‐6 score than Gp A Inclusion criteria: ≥ 21 yrs, grade 2 to 4 (POPQ) uterovaginal or vaginal prolapse who agreed to undergo vaginal surgery, available for 12 months' review, and can complete questionnaires Exclusion criteria: multiple medical contraindications, short vagina, uterus > 12 weeks size, desire future fertility, and postpartum | |
| Interventions | Gp A: uterosacral colpopexy with polytetrafluoroethylene sutures or sacrospinous colpopexy (Gortex sutures) and hysterectomy performed if uterus present Gp B: if point C or D on POPQ was ≥ ‐3 apical suspension with total vaginal mesh (Prolift), and if C or D was < ‐3 anterior Prolift was utilised. No T incisions were performed, and hysterectomy performed if uterus present | |
| Outcomes | Assessed at 1, 2, and 3 years Reports the following review outcomes (at 3 years unless otherwise stated):
| |
| Notes | The ethics committee stopped the study prior to completion due to predetermined stopping criteria of mesh erosion rate of > 15% being reached, with 65 of the desired sample size of 90 having undergone interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Consecutive, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) | Low risk | 3 years: Gp A 26/32, Gp B 25/33 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded American Urogynecologic Society Foundation and MedStar research; authors reported no COI |
| Methods | Single‐centre RCT France Computer‐generated, 6‐block randomisation Allocation concealment: not stated Blinding: no women or reviewers Intention to treat: not stated | |
| Participants | Inclusion criteria: stage 3 or greater anterior compartment prolapse Exclusion criteria: pregnancy, family not completed, prior cancer or radiation, poorly controlled diabetes mellitus, polypropylene sensitivity, immunocompromised. Concomitant surgery performed | |
| Interventions | Gp A: AC with bilateral vaginal colposuspension (Ethibond suture) n = 35, at 2 years n = 32 Gp B: polypropylene transobturator mesh (Perigee AMS) n = 33, at 2 years n = 31 More women underwent hysterectomy (77%) in the colposuspension group compared with 33% in the mesh group. P < 0.001 | |
| Outcomes | Assessed at 3 months, 1 year, and 2 years Reports the following review outcomes at 2 years:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | At 2 years Gp A 32/35, Gp B 31/33 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funding by the Claude Bernard University. Authors no COI. Measures of variance very low for some outcomes; attempt to check data with primary authors unsuccessful |
| Methods | Double‐blind, triple‐arm RCT Randomisation, allocation concealment, N/S power 33 in each group 80% power to detect 35% difference with 5% type 2 error 2‐year review | |
| Participants | Inclusion criteria: women ≥ 18 years of age with a POPQ point Ba of ≥ 0 Exclusion criteria: N/S Concomitant surgery: hysterectomy, colpopexy, posterior repair, continence at surgeons discretion | |
| Interventions | 99 randomised Gp A: 32 standard AC using midline plication with delayed absorbable suture Gp B: 31 vaginal paravaginal repair using free‐hand formed porcine dermis graft (PelvicolTM) Gp C: 36 vaginal paravaginal repair using free‐formed polypropylene mesh. All graft material was secured to the arcus tendineus fascia pelvis using a CapioTM device with permanent monofilament suture | |
| Outcomes | Assessed at 2 years Reports the following review outcomes at 2 years:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) | Low risk | 2 years: Gp A 24/32; Gp B 26/31; Gp C 28/36 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | High risk | Authors report COI with companies producing product evaluated and funded by Boston Scientific, whose product Capio was being evaluated |
| Methods | RCT (computer‐generated number table, opaque envelopes) on posterior IVS and sacrospinous fixation for vault prolapse | |
| Participants | 66 randomised, no stratification | |
| Interventions | Gp A (33): infracoccygeal sacropexy (posterior IVS) using multifilament polypropylene tape | |
| Outcomes | Reports the following review outcomes at median 17‐ to 19‐month follow‐up:
| |
| Notes | Abstract and further data from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PC‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 100% reviewed |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
| Methods | Multi‐centre RCT (computer generated) on primary surgery anterior vaginal wall prolapse Power calculation: 90 in each arm required Follow‐up: 2 years ITT analysis: yes, including those women with missing data at 2 years but with 1 year follow‐up completed | |
| Participants | 206 randomised No differences between the 2 groups with respect to demographic and clinical characteristics At 2 years number available for analysis: 176 (A 91, B 85) ITT analysis: 201 analysed (A 103, B 98) | |
| Interventions | A (100): interrupted fascial plication polyglactin (Vicryl) 00 with porcine dermis graft (Pelvicol overlay) fixed with PDS suburethrally and uterosacral cardinal ligament distally | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | No participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | At 2 years: 91/100 native tissue versus biological 85/106 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
| Methods | Single‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 38 in each arm Type of randomisation: computer generated Blinding strategy: primary surgeon ‐ until the surgery day; women, research nurse, and medical assistant remained blinded Allocation concealment: sealed, opaque envelopes Definition of cure: Ant wall POPQ stage < 2, "Optimal support" = Aa and Ba at stage 0, "Satisfactory" = Aa and Ba at stage 1 and improved from preop staging Follow‐up: 12 months (full publication) and 24 months (abstract only) Prolapse assessment: POPQ | |
| Participants | Inclusion: 21 years and older with POPQ stage 2 or greater anterior prolapse requiring surgical correction Exclusion: pregnancy (present or contemplated), prior repair with graft, systemic infection, compromised immune system, uncontrolled diabetes mellitus, previous pelvic irradiation/cancer, polypropylene allergy, scheduled for concomitant Burch or pubovaginal sling Randomised: 76 Withdrawals: 1 Lost to follow‐up: 1 Analysed: 76 | |
| Interventions | Gp A (38): AC with delayed absorbable (PDS) sutures Gp B (38): AC + polypropylene 4‐armed mesh kit repair (Perigee, American Medical Systems) Concomitant surgery: vaginal hysterectomy, bilateral salpingo‐oophorectomy, uterosacral suspension, mid‐urethral tape, site‐specific rectocele repair, perineoplasty, Apogee mesh kit repair Concomitant prolapse and suburethral tape surgeries were performed in both groups | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Women blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | 1 year: Gp A 37/38, Gp B 37/38 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No statement about funding |
| Methods | Multi‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 101 in each arm Type of randomisation: computer generated Allocation concealment: opaque envelopes Blinding strategy: not specified, but lack of a non‐surgical blinded outcome reviewer Definition of cure: less than stage 2 prolapse at Aa or Ba Follow up: 24 months Prolapse assessment: POPQ | |
| Participants | Inclusion: postmenopausal women with symptomatic anterior vaginal wall prolapse to the hymen or beyond Exclusion: apical defect indicating vaginal fixation or SUI necessitating surgery or the main symptomatic prolapse component was in the posterior vaginal wall. Also women with gynaecological tumour or malignancy calling for laparotomy or laparoscopy, and those with untreated vaginal infection Randomised: 202 Withdrawals: 1 Lost to follow‐up: 1 Analysed: 200 No significant differences in baseline demographics, prior hysterectomy, or prolapse surgeries between the 2 groups | |
| Interventions | Gp A (96): AC using a 0 or 2/0 multifilament suture Gp B (104): AC + self tailored (from a 6 x 11 cm mesh patch) 4‐armed low‐weight polypropylene mesh Type of mesh: non‐absorbable monofilament polypropylene (Parietene light, Sofradim, France) Sutures for AC: absorbable 0 or 2/0 multifilament suture Concomitant surgery: vaginal hysterectomy, posterior repair, culdoplasty as required, no concomitant continence surgeries were performed | |
| Outcomes | Assessed at 2 months, 1, 2, and 3 years Reports the following review outcomes at 3 years:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 years: 95/104 (92%) vs 85/96 (89%) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Some inconsistencies in data across publications at different follow‐up times |
| Methods | Single‐centre RCT (computer‐generated randomisation by sealed envelopes with blinded research nurse) | |
| Participants | 106 women | |
| Interventions | Gp A (37): posterior colporrhaphy as per Maher 2‐0 Ethibond | |
| Outcomes | Assessed at 1 year and 2 years (few 2‐year data reported) Reports the following review outcomes at 1 year:
| |
| Notes | Ongoing study: initial full‐text review after 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded non‐surgeon reviewer |
| Incomplete outcome data (attrition bias) | Low risk | At 17 months 99/106 completed; gps unclear |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Unrestricted research grant from Organogenesis, whose product was being evaluated |
| Methods | Single‐centre RCT Jordan 57 in each group had 80% power to detect 25% difference between the groups with a 5% type 1 error with a 10% drop‐out rate No ITT analysis | |
| Participants | Inclusion criteria: symptomatic stage 3 or greater utero‐vaginal prolapse in all compartments: primary and recurrent Exclusion criteria: less than grade 3 prolapse in any compartment, any prior surgery with implants for pelvic floor defects, prior radiation, those wishing uterine preservation | |
| Interventions | AC group (n = 65): 2.0 PDS plication Mesh group (n = 64): self shaped polypropylene (Gynemesh) 15 x 3 cm with 2 arms retropubic space without suturing Concomitant continence surgery if needed and vaginal hysterectomy in those with uterine prolapse All underwent sacrospinous colpopexy and posterior colporrhaphy | |
| Outcomes | Assessed at 6 weeks, then every 6 months. Median follow‐up 28/29 months, range 6 to 10 Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated: POPQ assessment by independent investigator |
| Incomplete outcome data (attrition bias) | High risk | AC group: 63/65; mesh group 53/64 at median 28‐month review; follow‐up times variable |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | Funded by Cook Medical |
| Methods | Parallel‐group RCT | |
| Participants | Included: women with a cystocele requiring surgical management Excluded: allergy to graft material, immunocompromised, non‐English speaking, unavailable for follow‐up Concomitant surgery or previous non‐anterior prolapse surgery were not exclusion criteria. | |
| Interventions | Small intestine mesh‐augmented procedure vs same anterior repair without mesh | |
| Outcomes | Assessed at 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation through university obstetrics & gynaecology department data manager |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Follow‐up assessment by examining physician blinded to allocation with no involvement in clinical care |
| Incomplete outcome data (attrition bias) | Low risk | 55/57 women randomised (96%) were included in analysis for objective outcomes and 57/57 (100%) for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Reports expected review outcomes |
| Other bias | Low risk | Supplier of product (Cook) partially funded study, however the blinded nature of participants and reviewers overcomes potential biases |
| Methods | Multi‐centre (6) international RCT Nordic countries: Norway, Sweden, Denmark, and Finland: Block computer‐generated randomisation list Allocation concealment: opaque, sealed envelopes ITT analysis Sample size: 130 women allowed 80% power to detect 20% difference with an alpha error of 5% and a drop‐out rate of 15% Assessors: surgeons Women unblinded Surgeons trained to ensure uniform surgery performed | |
| Participants | Inclusion criteria: ≳ 55 years, anterior wall prolapse stage 2 POPQ Aa or Ba ≳ ‐1 Exclusion criteria: previous major pelvic surgery with the exception of a hysterectomy for reasons other than genital prolapse, previous vaginal surgery, or hysterectomy for POP; concomitant prolapse of the uterus or an enterocele of stage 1 or higher; previous incontinence sling surgery performed through the obturator membrane; current treatment with corticosteroids; or a history of genital or abdominal cancer All surgery covered intra‐operative antibiotics and pre‐ and post‐local oestrogens Concomitant surgery allowed posterior repair | |
| Interventions | AC group: interrupted absorbable suture fascial plication, vaginal trimming and closure with running unlocked absorbable suture Mesh group: biosynthetic system monofilament polypropylene mesh with central portion coated in absorbable hydrophylic porcine collagen film Bard Avaulta Plus anterior 169 available randomisation with 161 randomised AC: 79 randomised, 1 year 76 Mesh: 82 randomised, 1 year 78 | |
| Outcomes | Assessed at 3 months, 1 year, and 3 years Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked computer‐generated randomisation list for each of 4 countries |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded (unable to blind) |
| Blinding of outcome assessment (detection bias) | High risk | Surgeons evaluated |
| Incomplete outcome data (attrition bias) | Low risk | 1‐year evaluation/randomised AC 76/79, mesh 78/82 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI |
| Methods | Single‐centre RCT (computer‐generated number table) | |
| Participants | 143 women | |
| Interventions | Gp A (70): no mesh: Vicryl plication of anterior endopelvic fascia Standardised concomitant surgery | |
| Outcomes | Assessed at 2, 6, 12 weeks and 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | 143/170 (84%) completed 1‐year review |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No COI statement |
| Methods | Single‐centre RCT comparing polypropylene mesh surgery with site‐specific surgery in the treatment of cystocele CONSORT statement: yes Power calculation: 45 in each arm Type of randomisation: computer generated Blinding strategy: no (assessment was performed by non‐blinded reviewers) Allocation concealment: not specified Definition of cure/failure: "Acceptable cure" defined as cystocele less than ‐1 cm (stage 1 POPQ) Follow‐up: mean 12 months (range 8 to 16) Prolapse assessment: POPQ | |
| Participants | Inclusion: primary cystocele Exclusion: SUI, concomitant rectocele or enterocele or recurrent cystocele Randomised: 90 (45 to each arm) Analysed: 85 Lost to follow‐up: 5 | |
| Interventions | A (42): site‐specific polyglactin 910 anterior repair B (43): self styled 4‐armed polypropylene (Parietene, Sofradim, France) mesh, no anterior repair Concomitant surgery not standardised, management of concomitant apical prolapse was not specified in either group | |
| Outcomes | Assessed at 6 weeks, 6 months, and annually Reports the following review outcomes at mean follow‐up of 1 year (range 8 to 16 months):
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded reviewers; objective assessment was participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | Flow diagram: 1 year Gp A 42/45, Gp B 43/45 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No funding and no COI |
| Methods | 2‐centre, double‐blinded randomised control trial Allocation concealment: sealed envelopes Randomisation block and stratified site Women and assessors blinded (women unblinded 12 months) Based on a study by Kohli et al (Kohli 2003) assuming that graft use is associated with a 93% anatomic success rate, 63 women per group would be needed to detect a 20% difference at .05 and .20. We aimed to recruit 160 women (80 women per group) to account for drop‐out | |
| Participants | Inclusion criteria: women with stage 2 or greater symptomatic rectocele (defined as vaginal bulge, defecatory symptoms, or both) electing surgical repair were eligible | |
| Interventions | Gp A: 70 controls midline plication or site‐specific repair Gp B: 67 midline plication or site‐specific repair with 4 x 7 cm subintestinal submucosal graft over the repair and secured to levator ani fascia using interrupted No. 2‐0 polyglycolic acid and inferiorly to the perineal body using No. 2‐0 polyglycolic acid sutures. Excess vaginal tissue was trimmed in all women, and the posterior vaginal incision was closed using 2‐0 polyglycolic acid sutures. The deep and superficial transverse perineal muscles and bulbocavernosus muscles were re‐approximated using No. 0 | |
| Outcomes | Assessed at 6 months and 1 year Reports the following outcomes at median 12.2 to 12.5 months (range 10 to 43 months):
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded reviewers |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 year Gp A 70/80, Gp B 67/79 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No financial COI; grant funding National Institute of Child and Human Health |
| Methods | Single‐centre RCT Computer randomisation on patient hospitalisation numbers Allocation concealment: not stated Women unblinded Postop unblinded due to surgeries Sample size 30 in each group allowed 80% power to detect a 45% difference with an alpha error of 5% ITT analysis: not stated | |
| Participants | Inclusion criteria: symptomatic posthysterectomy patients with at least 2‐compartment prolapse (with affected apical/vault compartment, stage 2 or higher (POPQ)), requesting pelvic floor reconstructive surgery, and diagnosed with a complete unilateral or bilateral avulsion injury Exclusion criteria: nil further stated Assessment pre‐ and postoperative POPQ examination, 4D ultrasonography with acquisition of volume data sets at rest, during pelvic floor muscle contraction, and on maximum Valsalva manoeuvre, PISQ‐12, POPDI, UDI, CRADI 142 reviewed and 72 excluded (70 no avulsion, 2 refused) Sacrospinous fixation: 34, 1 year 31 Mesh: 36, 1 year 36 | |
| Interventions | Native tissue sacrospinous fixation: all cases: anterior repair with 2.0 polyglactin (Vicryl Plus) (Ethicon), posterior high levatorplasty Vicryl Plus 1: 2x Nurolon 1.0 (Ethicon) permanent R sacrospinous ligament Mesh: Prolift total (Ethicon): 3 arms each side with mesh secured to apex with Vicryl Plus 2.0 and to introitus posteriorly Primary outcome: failure defined: Ba, C, or Bp at hymen or below Uterosacral suspension definition ≳ 10 mm descent of the bladder below the lower margin of the symphysis pubis on maximum Valsalva | |
| Outcomes | Assessed at 3 months and at 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer randomisation based on hospital number? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | No, cannot be blinded |
| Blinding of outcome assessment (detection bias) | High risk | No, cannot blind |
| Incomplete outcome data (attrition bias) | Low risk | 1 year 31/34 sacrospinous fixation, mesh 36/36 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Funded by Czech Ministry of health and Charles University in Prague; 1 author financial COI |
| Methods | Single‐unit raffle randomisation prior to surgery No allocation concealment described Surgeons and women unblinded Unclear who performed assessments (blinded?) 2 surgeons performed 2 surgeries with mesh kit prior to surgery Sample size: 100 women to 80% power to detect 26% difference between the groups with alpha error of 5% with 20% loss to follow‐up at 2 years | |
| Participants | 122 reviewed, 100 randomised AC 55, 1 year 54, 2 years 50 Mesh 45, 1 year 43, 2 years 42 Inclusion criteria: 45 years old or older, with AVWP ≥ 2 (POPQ stage) without previous surgical correction or with previous surgical treatment of AVWP without Exclusion criteria: women who were previously treated (due to AVWP or SUI) using polypropylene mesh, who were receiving oncological treatment, with altered Papanicolaou smear exam or with uterine bleeding, with genital or acute urinary infection, women who didn't commit to ambulatory follow‐up or who refused the written informed consent All preop Urodynamics | |
| Interventions | Spinal anaesthesia with antibiotics Nazca TC kit (Promedon, Córdoba, Argentina) monofilament macroporous 4 arms (1 prepubic and 1 transobturator each side) concomitant surgery as required: hysterectomy, apical or posterior repair AC group 2.0 polyglactin (Vicryl) fascial plication mid‐urethral sling if SUI on preop Urodynamics (14/55) | |
| Outcomes | Assessed at 1 year and 2 years Reports the following review outcomes at 2 years:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Raffle randomisation 55 in AC and 45 in mesh |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded |
| Incomplete outcome data (attrition bias) | High risk | AC group 55 and 42 completed 2 years (42/55) Mesh group 45 and 42 completed 2 years (42/45) |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No COI reported |
| Methods | Multi‐centre and multi‐national RCT Randomisation and allocation concealment: N/S 90% power to detect 20% difference UDI prolapse domain at 1 year with 5% type 1 error with 38 in each group | |
| Participants | Gp A (48): AC Gp B (48): Perigee transobturator polypropylene mesh Gp A: 35 AC only, 5 SSF, 5 hysterectomy, 6 mid‐urethral sling Gp B: 34 Perigee only, 4 SSF, 8 hysterectomy, 1 mid‐urethral sling | |
| Interventions | Inclusion criteria: stage 2 or more cystocele Excluded if anterior was not the leading prolapse Concomitant surgery allowed Stage 2 or more uterine prolapse hysterectomy or SSF SUI mid‐urethral sling | |
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | No clear numbers supplied in abstract |
| Selective reporting (reporting bias) | Low risk | Reports 1 of our primary review outcomes |
| Other bias | Unclear risk | No statement about funding |
| Methods | Parallel‐group RCT | |
| Participants | Inclusion: grade 2 or 3 cystocele Exclusion: urinary incontinence, previous gynaecological operation, concomitant rectocele or enterocele, recurrent cystocele | |
| Interventions | Polypropylene mesh surgery (20 women) vs AC (20 women) | |
| Outcomes | Assessed at 6 weeks, 6 months, 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated by computer programme" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | All 40/40 randomised women were included in analysis |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Reports "no conflict of interest". No other potential bias identified |
| Methods | Multi‐centre RCT Randomisation was computerised, and stratification was performed for the presence of uterine descent ≥ 2. No blinding of group assignment was performed Allocation concealment: N/S Power 80 to detect 25% difference in groups with 5% type 1 error from sample size of 50 in each group | |
| Participants | Inclusion criteria: ≥ stage 2 cystocele Exclusion criteria: history of urogynaecological surgery for pelvic organ prolapse or incontinence, cancer or COPD, concomitant urinary stress incontinence with an indication for surgical correction, recurrent lower urinary tract infections (> 3 culture proven infections/year), maximum bladder capacity < 300 ml, an indication for hysterectomy, and women with childbearing potential and inadequate birth control measures Randomised: A 64, B 61 Withdrawals prior to surgery: A 2, B 2 12 months: A 51, B 53 | |
| Interventions | Gp A: AC Gp B: trocar‐guided transobturator synthetic mesh (Avaulta) | |
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Research nurse from online list |
| Blinding of participants and personnel (performance bias) | High risk | No |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers blinded by strapping thighs prior to review |
| Incomplete outcome data (attrition bias) | Low risk | 1 year AC 55/56, mesh 55/58 |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | No funding and no COI |
| Methods | RCT (computer‐generated random number tables. Sealed envelopes concealed assignment) comparing 3 surgical techniques | |
| Participants | 83 women | |
| Interventions | Gp A (33): anterior repair: midline plication without tension 0 PDS Number and level of surgeons unknown | |
| Outcomes | Assessed at 6 months, 1 year, and 2 years Reports the following review outcomes at median follow‐up 23 months (range 4.5 to 44.4 months)
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 83/114 randomised women included in analysis (73%) |
| Selective reporting (reporting bias) | Unclear risk | Main review outcomes reported, but no comparative data for most outcomes |
| Other bias | Unclear risk | No statement about funding. Significant disparity between total numbers in Table 1 and actual numbers with prolapse reported |
| Methods | Multi‐centre RCT 13 centres; 22 surgeons Randomisation list computer generated for each centre. Allocation concealment not discussed and woman, surgeon, and assessor (surgeons) not blinded Surgeons underwent specific Prolift mesh training Full power calculation completed | |
| Participants | Randomised: Gp A 99, Gp B 95 1‐year examination: A 84, B 83 Inclusion criteria: recurrent stage 2 or higher anterior or posterior wall prolapse, or both Exclusion criteria: pregnancy, future pregnancy, prior vaginal mesh repair, a compromised immune system or any other condition that would compromise healing, previous pelvic irradiation or cancer, blood coagulation disorders, renal failure, upper urinary tract obstruction, renal failure and upper urinary tract obstruction, or presence of large ovarian cysts or myomas | |
| Interventions | Gp A: conventional surgery was performed at the discretion of the surgeon, although absorbable sutures were specified and hysterectomies permitted Gp B: standardised and structured in the tension‐free vaginal mesh: performed as described by Fatton (Fatton 2007), and no hysterectomies were performed or T incisions allowed | |
| Outcomes | Assessed at 6 months and 1 year Reports the following review outcomes at 1 year:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described. Unfortunately, preoperatively group A is significantly different than group B, as demonstrated by having greater degree prolapse at Ap, Bp, and GH in Table 4; having significantly higher number with ≥ stage 2 apical compartment prolapse in those in Table I undergoing prior apical surgery, (36% (16/45) in group A versus 18% (10/56) in group B (P = 0.04, odds ratio 2.54)); and finally prior sacral colpopexy was 3 times as frequent in group B. Only the final anomaly is acknowledged |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded reviewers; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Unclear risk | Gp A 84/99, Gp B 83/98 |
| Selective reporting (reporting bias) | High risk | Primary outcome definition inconsistent |
| Other bias | High risk | Funded by university research fund; all authors reported financial support from Ethicon, which manufactures product being evaluated by non‐blinded reviewers |
AC = anterior colporrhaphy
AVWP = anterior vaginal wall prolapse
BW = Baden‐Walker
CI = confidence interval
COI = conflict of interest
CONSORT = Consolidated Standards of Reporting Trials
CRADI = Colorectal‐Anal Distress Inventory
Hb = haemoglobin
ICS = International Continence Society
ITT = intention to treat
IVS = intravaginal slingplasty
N/S = not specified
PDS = absorbable polydioxanone surgical suture
PFDI = Pelvic Floor Distress Inventory
PFIQ = Pelvic Floor Impact Questionnaire
PGI‐I = Patient Global Impression of Improvement
PISQ = Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire
POP = pelvic organ prolapse
POPDI = Pelvic Organ Prolapse Distress Inventory
POPIQ = Pelvic Organ Prolapse Impact Questionnaire
POPQ = Pelvic Organ Prolapse Quantification (according to ICS)
PQOL= Prolapse Quality of Life Questionnaire
QOL = quality of life
RCT = randomised controlled trial
SD = standard deviation
SSF = sacrospinous fixation
SUI = stress urinary incontinence (symptom diagnosis)
UDI = Urogenital Distress Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a RCT | |
| Not a RCT | |
| Assessment of impact of traction on uterine prolapse without any surgical intervention | |
| Juneja and colleagues compared hysterectomy (n = 9) versus no hysterectomy (n = 7) for uterine prolapse in conjunction with posterior infracoccygeal colpopexy in a pilot randomised study. Due to a predefined decision that papers with fewer than 20 women in each treatment group would not be included in the review, the manuscript was excluded | |
| Tincello et al report a pilot randomised patient preference study comparing colposuspension or tension‐free vaginal tape for urinary incontinence at time of anterior repair for prolapse. 31 women were recruited, however only 4 (2 in each arm) were randomised. Due to a predefined decision that papers with fewer than 20 women in each treatment group would not be included in the review, the manuscript was excluded |
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Puborectalis sling RCT ‐ a study on reducing pelvic organ prolapse recurrences following prolapse surgery |
| Methods | Multi‐centre RCT |
| Participants | Pelvic organ prolapse |
| Interventions | Vaginal repair and hysterectomy with and without mesh |
| Outcomes | Prolapse on uterosacral suspension |
| Starting date | 2012 |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000236897 |
| Notes | Ongoing? |
| Trial name or title | PROSPECT (PROlapse Surgery: Pragmatic Evaluation and randomised Controlled Trials) |
| Methods | RCT |
| Participants | Women having prolapse surgery |
| Interventions | Anterior and posterior repair (colporrhaphy) with or without non‐absorbable or biological mesh inlay, or mesh kit |
| Outcomes | Prolapse symptoms (POP‐SS), prolapse stage (POPQ), economic outcomes |
| Starting date | 01 09 2009 |
| Contact information | |
| Notes | Health Technology Assessment‐funded study in UK ongoing |
| Trial name or title | Anterior defect correction with mesh plus treatment of stress incontinence with transobturator or transvaginal approach |
| Methods | RCT |
| Participants | Prolapse and SUI |
| Interventions | Anterior mesh repair tension‐free vaginal tape compared to anterior mesh repair with transobturator tape |
| Outcomes | |
| Starting date | 2008 |
| Contact information | |
| Notes | Slow recruitment; study terminated |
| Trial name or title | Trial of small intestine submucosa (SIS) mesh for anterior repair |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair versus SIS biograft (Cook) |
| Outcomes | |
| Starting date | 2009 |
| Contact information | https://clinicaltrials.gov/show/NCT00955448 |
| Notes | Study completed; unable to identify publication as yet |
| Trial name or title | ATHENA |
| Methods | RCT |
| Participants | Women with occult urinary incontinence |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Sacrocolpopexy versus vaginal mesh procedure for pelvic prolapse (Elevate) |
| Methods | RCT |
| Participants | Vaginal prolapse |
| Interventions | Laparoscopic sacral colpopexy versus Elevate transvaginal mesh |
| Outcomes | |
| Starting date | 2010 |
| Contact information | |
| Notes | No longer recruiting |
| Trial name or title | The ELEGANT Trial: Elevate Transvaginal Mesh vs Anterior Colporrhaphy |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair versus Elevate (AMS) anterior mesh |
| Outcomes | |
| Starting date | 2011 |
| Contact information | http://ClinicalTrials.gov/show/NCT01497171 |
| Notes | Study ended due to funding termination |
| Trial name or title | Laparoscopic to vaginal surgery for uterine prolapse |
| Methods | RCT |
| Participants | Uterine prolapse |
| Interventions | Laparoscopic supracervical hysterectomy and sacral colpopexy versus vaginal hysterectomy and uterosacral colpopexy |
| Outcomes | |
| Starting date | 2012 |
| Contact information | |
| Notes | Terminated as unable to offer laparoscopy |
| Trial name or title | Prosthetic Pelvic Organ Prolapse Repair (PROSPERE) |
| Methods | RCT |
| Participants | Cystocele |
| Interventions | Lap sacral colpopexy versus vaginal mesh procedure (unspecified) |
| Outcomes | |
| Starting date | 2012 |
| Contact information | https://clinicaltrials.gov/show/NCT01637441 |
| Notes | Study active but not recruiting? |
| Trial name or title | Laparoscopic sacral colpopexy versus modified total pelvic floor reconstructive surgery for apical prolapse stage III‐IV |
| Methods | RCT |
| Participants | Uterine and vault prolapse |
| Interventions | Lap sacrocolpopexy versus vaginal mesh repair with Gynemesh |
| Outcomes | |
| Starting date | 2012 |
| Contact information | https://clinicaltrials.gov/show/NCT01762384 |
| Notes | Ongoing recruiting |
| Trial name or title | Study of Uterine Prolapse Procedures ‐ Randomised Trial (SUPeR) |
| Methods | RCT |
| Participants | Uterine prolapse |
| Interventions | Mesh hysteropexy (Uphold LITE) versus vaginal hysterectomy uterosacral suspension |
| Outcomes | |
| Starting date | 2013 |
| Contact information | https://clinicaltrials.gov/show/NCT01802281 |
| Notes | Ongoing |
| Trial name or title | CUPIDO 1 and CUPIDO 2 |
| Methods | RCT |
| Participants | Women with SUI (CUPIDO 1) and women with occult SUI (CUPIDO 2) |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Ongoing |
IIQ = Incontinence Impact Questionnaire
POP = pelvic organ prolapse
POPQ = Pelvic Organ Prolapse Quantification
POP‐SS = Prolapse Symptom Score
RCT = randomised controlled trial
SUI = stress urinary incontinence (symptom diagnosis)
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (1‐3 years) Show forest plot | 12 | 1614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.81] |
| Analysis 1.1  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 years). | ||||
| 1.1 Anterior compartment:mesh vs native tissue | 9 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 1.2 Multicompartment: mesh vs native tissue | 4 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 2 Repeat surgery (1‐3 years) Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 2 Repeat surgery (1‐3 years). | ||||
| 2.1 Prolapse | 12 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.88] |
| 2.2 Continence surgery | 9 | 1284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.83] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 7 | 867 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.51, 3.81] |
| 3 Recurrent prolapse (any) at 1‐3 years Show forest plot | 21 | 2494 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.53] |
| Analysis 1.3  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 3 Recurrent prolapse (any) at 1‐3 years. | ||||
| 3.1 Anterior compartment repair: mesh versus native tissue | 15 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.40] |
| 3.2 Multi‐compartment repair: mesh versus native tissue | 6 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 4 Injuries bladder or bowel Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 4 Injuries bladder or bowel. | ||||
| 4.1 Bladder injury | 11 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [1.62, 9.50] |
| 4.2 Bowel injury | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 78.81] |
| 5 Objective failure of anterior compartment (cystocoele) Show forest plot | 13 | 1406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.36, 0.55] |
| Analysis 1.5  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele). | ||||
| 5.1 Anterior compartment repair: mesh versus native tissue | 9 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.47] |
| 5.2 Multi‐compartment repair: mesh versus native tissue | 4 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.51, 1.06] |
| 6 Objective failure of posterior compartment (rectocoele) Show forest plot | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| Analysis 1.6  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele). | ||||
| 6.1 Mesh vs native tissue | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 7 POPQ assessment (any mesh) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 7 POPQ assessment (any mesh). | ||||
| 7.1 Point Ba POPQ | 10 | 1125 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.27, ‐0.59] |
| 7.2 Point C POPQ | 8 | 925 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.13, 0.23] |
| 7.3 Point Bp | 7 | 832 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.34, 0.44] |
| 7.4 total vaginal length | 5 | 611 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.25, 0.40] |
| 8 Bladder function: de novo stress urinary incontinence (1‐3 years) Show forest plot | 12 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.06, 1.82] |
| Analysis 1.8  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 8 Bladder function: de novo stress urinary incontinence (1‐3 years). | ||||
| 8.1 Anterior compartment: mesh vs native tissue | 8 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.11] |
| 8.2 Multi compartment : mesh vs native tissue | 4 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.92] |
| 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder Show forest plot | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.63] |
| Analysis 1.9  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder. | ||||
| 10 De novo dyspareunia (1‐3 years) Show forest plot | 11 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.47] |
| Analysis 1.10  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 10 De novo dyspareunia (1‐3 years). | ||||
| 10.1 Anterior compartment: mesh vs native tissue | 8 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.93] |
| 10.2 Multicompartment: mesh vs native tissue | 3 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.42] |
| 11 Sexual function (1‐3 years) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 11 Sexual function (1‐3 years). | ||||
| 11.1 PISQ score | 7 | 857 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.40, 0.13] |
| 12 Quality of life: continuous data (1‐2 years): Show forest plot | 7 | 665 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| Analysis 1.12  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 12 Quality of life: continuous data (1‐2 years):. | ||||
| 12.1 PQOL end score | 3 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.31, 0.49] |
| 12.2 Pelvic floor impact questionnaire end score | 4 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.34, 0.37] |
| 13 Quality of life: dichotomous data "much or very much better" Show forest plot | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| Analysis 1.13  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 13 Quality of life: dichotomous data "much or very much better". | ||||
| 13.1 PGI‐I | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| 14 Operating time (minutes) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 14 Operating time (minutes). | ||||
| 14.1 Anterior compartment: mesh vs native tissue | 10 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Multicompartment: mesh vs native tissue | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Blood transfusion Show forest plot | 6 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.88, 2.72] |
| Analysis 1.15  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 15 Blood transfusion. | ||||
| 16 Length of stay in hospital (days) Show forest plot | 7 | 953 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| Analysis 1.16  Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 16 Length of stay in hospital (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (2 year review) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.77, 1.44] |
| Analysis 2.1  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 1 Awareness of prolapse (2 year review). | ||||
| 2 Repeat surgery for prolapse (2 years) Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.40] |
| Analysis 2.2 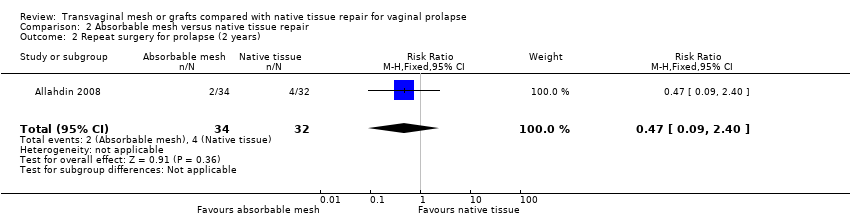 Comparison 2 Absorbable mesh versus native tissue repair, Outcome 2 Repeat surgery for prolapse (2 years). | ||||
| 3 Recurrent prolapse (3 months ‐2 years) Show forest plot | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.96] |
| Analysis 2.3  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 3 Recurrent prolapse (3 months ‐2 years). | ||||
| 3.1 Any site stage 2 or more | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.10, 2.70] |
| 3.2 Anterior compartment | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 4 Death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.4  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 4 Death. | ||||
| 4.1 absorbable mesh versus native tissue repair | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Objective failure of anterior compartment (cystocoele) Show forest plot | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| Analysis 2.5  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele). | ||||
| 5.1 Anterior compartment repair: absorbable mesh versus native tissue | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 5.2 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.93] |
| 6 Objective failure of posterior compartment (rectocoele) Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| Analysis 2.6  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele). | ||||
| 6.1 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| 7 Stress urinary incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 7 Stress urinary incontinence. | ||||
| 7.1 Postoperative SUI | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.95, 2.00] |
| 8 Quality of life (2 years) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Analysis 2.8  Comparison 2 Absorbable mesh versus native tissue repair, Outcome 8 Quality of life (2 years). | ||||
| 8.1 VAS QoL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (1‐3 year) Show forest plot | 7 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.43] |
| Analysis 3.1  Comparison 3 Biological repair versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 year). | ||||
| 1.1 Anterior compartment repair: biological graft vs native tissue | 4 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.23] |
| 1.2 Multicompartment repair: biological graft vs native tissue | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [1.04, 19.92] |
| 1.3 Posterior compartment repair: biological graft vs native tissue | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.41, 1.94] |
| 2 Repeat prolapse surgery (1‐2 years) Show forest plot | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.61, 2.44] |
| Analysis 3.2  Comparison 3 Biological repair versus native tissue repair, Outcome 2 Repeat prolapse surgery (1‐2 years). | ||||
| 3 Recurrent prolapse (1 year) Show forest plot | 7 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| Analysis 3.3  Comparison 3 Biological repair versus native tissue repair, Outcome 3 Recurrent prolapse (1 year). | ||||
| 3.1 Anterior compartment repair: biological graft vs native tissue | 5 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.05] |
| 3.2 Posterior compartment repair: biological graft vs native tissue | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.18, 3.70] |
| 4 Injuries to bladder or bowel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Biological repair versus native tissue repair, Outcome 4 Injuries to bladder or bowel. | ||||
| 4.1 bladder injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.40] |
| 4.2 bowel injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.57] |
| 5 Objective failure of anterior compartment (cystocele) Show forest plot | 6 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| Analysis 3.5  Comparison 3 Biological repair versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocele). | ||||
| 6 Objective failure of posterior compartment (rectocele) Show forest plot | 3 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.39, 3.51] |
| Analysis 3.6  Comparison 3 Biological repair versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocele). | ||||
| 7 POPQ assessment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Biological repair versus native tissue repair, Outcome 7 POPQ assessment. | ||||
| 7.1 Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.98, ‐0.02] |
| 7.2 Point C | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| 7.3 Bp POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.27, 0.47] |
| 7.4 total vaginal length | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.06, 1.14] |
| 8 De novo urinary stress incontinence Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.8  Comparison 3 Biological repair versus native tissue repair, Outcome 8 De novo urinary stress incontinence. | ||||
| 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder Show forest plot | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| Analysis 3.9  Comparison 3 Biological repair versus native tissue repair, Outcome 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder. | ||||
| 10 De novo dyspareunia (1 year) Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.20, 3.67] |
| Analysis 3.10  Comparison 3 Biological repair versus native tissue repair, Outcome 10 De novo dyspareunia (1 year). | ||||
| 11 Sexual function (1 year) Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| Analysis 3.11  Comparison 3 Biological repair versus native tissue repair, Outcome 11 Sexual function (1 year). | ||||
| 11.1 PISQ | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| 12 Quality of life (1 year) Show forest plot | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.38] |
| Analysis 3.12  Comparison 3 Biological repair versus native tissue repair, Outcome 12 Quality of life (1 year). | ||||
| 12.1 PQOL score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.63] |
| 12.2 PFDI‐20 | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.11, 0.39] |
| 13 Operating time (minutes) Show forest plot | 4 | 232 | Mean Difference (IV, Fixed, 95% CI) | 10.34 [6.31, 14.36] |
| Analysis 3.13  Comparison 3 Biological repair versus native tissue repair, Outcome 13 Operating time (minutes). | ||||
| 14 Blood transfusion Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.14, 32.90] |
| Analysis 3.14 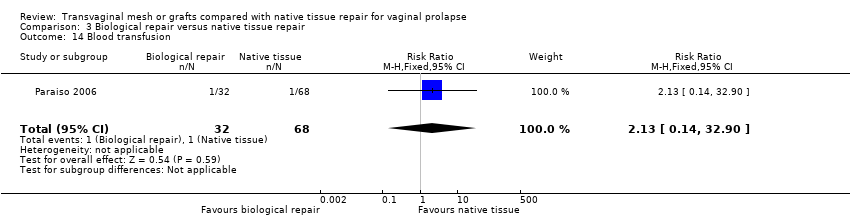 Comparison 3 Biological repair versus native tissue repair, Outcome 14 Blood transfusion. | ||||

PRISMA study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Any transvaginal permanent mesh versus native tissue repair, outcome: 1.1 Awareness of prolapse (1 to 3 years).

Forest plot of comparison: 3 Biological repair versus native tissue repair, outcome: 3.1 Awareness of prolapse (1 to 3 years).

Funnel plot of comparison: 1 Any transvaginal permanent mesh versus native tissue repair, outcome: 1.3 Recurrent prolapse (any) at 1 to 3 years.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 years).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 2 Repeat surgery (1‐3 years).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 3 Recurrent prolapse (any) at 1‐3 years.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 4 Injuries bladder or bowel.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 7 POPQ assessment (any mesh).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 8 Bladder function: de novo stress urinary incontinence (1‐3 years).
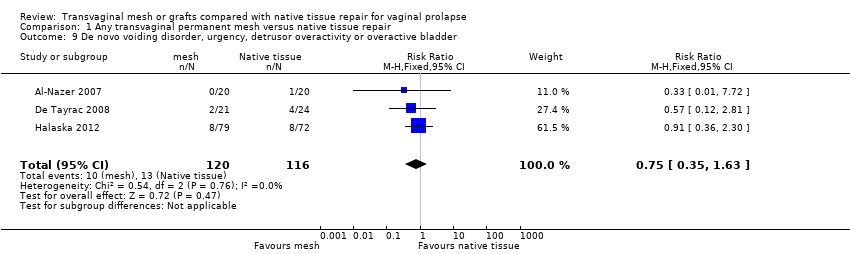
Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 10 De novo dyspareunia (1‐3 years).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 11 Sexual function (1‐3 years).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 12 Quality of life: continuous data (1‐2 years):.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 13 Quality of life: dichotomous data "much or very much better".

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 14 Operating time (minutes).

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 15 Blood transfusion.

Comparison 1 Any transvaginal permanent mesh versus native tissue repair, Outcome 16 Length of stay in hospital (days).

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 1 Awareness of prolapse (2 year review).

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 2 Repeat surgery for prolapse (2 years).
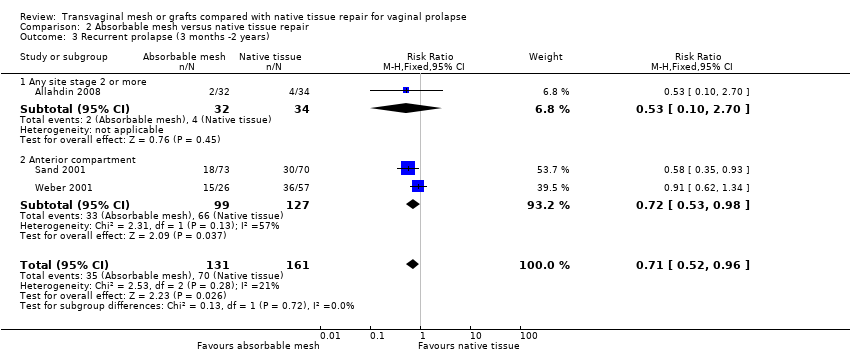
Comparison 2 Absorbable mesh versus native tissue repair, Outcome 3 Recurrent prolapse (3 months ‐2 years).

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 4 Death.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocoele).

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocoele).
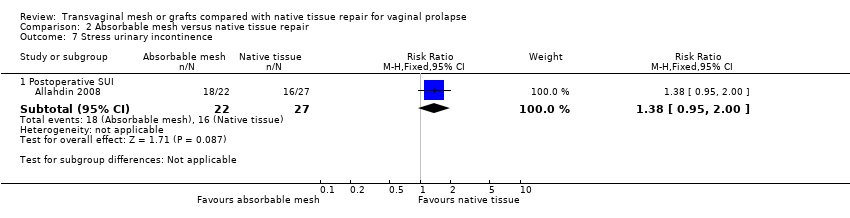
Comparison 2 Absorbable mesh versus native tissue repair, Outcome 7 Stress urinary incontinence.

Comparison 2 Absorbable mesh versus native tissue repair, Outcome 8 Quality of life (2 years).

Comparison 3 Biological repair versus native tissue repair, Outcome 1 Awareness of prolapse (1‐3 year).
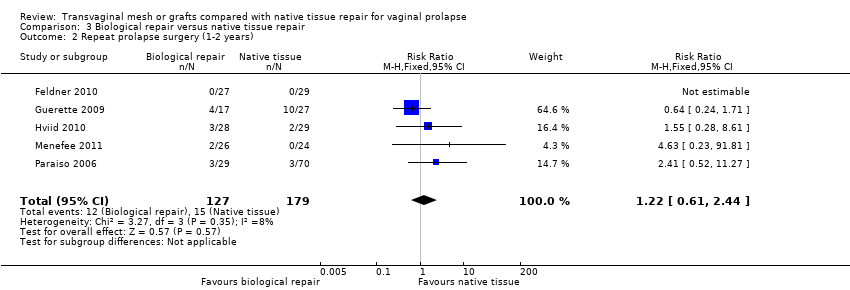
Comparison 3 Biological repair versus native tissue repair, Outcome 2 Repeat prolapse surgery (1‐2 years).

Comparison 3 Biological repair versus native tissue repair, Outcome 3 Recurrent prolapse (1 year).

Comparison 3 Biological repair versus native tissue repair, Outcome 4 Injuries to bladder or bowel.

Comparison 3 Biological repair versus native tissue repair, Outcome 5 Objective failure of anterior compartment (cystocele).

Comparison 3 Biological repair versus native tissue repair, Outcome 6 Objective failure of posterior compartment (rectocele).

Comparison 3 Biological repair versus native tissue repair, Outcome 7 POPQ assessment.

Comparison 3 Biological repair versus native tissue repair, Outcome 8 De novo urinary stress incontinence.

Comparison 3 Biological repair versus native tissue repair, Outcome 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder.

Comparison 3 Biological repair versus native tissue repair, Outcome 10 De novo dyspareunia (1 year).

Comparison 3 Biological repair versus native tissue repair, Outcome 11 Sexual function (1 year).

Comparison 3 Biological repair versus native tissue repair, Outcome 12 Quality of life (1 year).

Comparison 3 Biological repair versus native tissue repair, Outcome 13 Operating time (minutes).

Comparison 3 Biological repair versus native tissue repair, Outcome 14 Blood transfusion.
| Any transvaginal permanent mesh versus native tissue repair for vaginal prolapse | ||||||
| Population: women with vaginal prolapse | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Any transvaginal permanent mesh | |||||
| Awareness of prolapse review 1 to 3 years | 188 per 1000 | 124 per 1000 | RR 0.66 (0.54 to 0.81) | 1614 (12 RCTs) | ⊕⊕⊕⊝ | |
| Repeat surgery ‐ prolapse review 1 to 3 years | 32 per 1000 | 17 per 1000 | RR 0.53 | 1675 | ⊕⊕⊕⊝ | |
| Repeat surgery ‐ continence surgery | 26 per 1000 | 28 per 1000 (16 to 48) | RR 1.07 (0.62 to 1.83) | 1284 (9 RCTs) | ⊕⊕⊝⊝ | |
| Repeat surgery ‐ surgery for prolapse, SUI, or mesh exposure review 1 to 3 years | 48 per 1000 | 114 per 1000 | RR 2.40 | 867 | ⊕⊕⊕⊝ | |
| Recurrent prolapse review 1 to 3 years | 381 per 1000 | 152 per 1000 | RR 0.40 | 2494 | ⊕⊕⊝⊝ | I2 = 73% |
| Bladder injury | 5 per 1000 | 21 per 1000 | RR 3.92 | 1514 | ⊕⊕⊕⊝ | |
| De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years | 95 per 1000 | 88 per 1000 | RR 0.92 | 764 | ⊕⊕⊝⊝ | |
| De novo stress urinary incontinence review 1 to 3 years | 96 per 1000 | 133 per 1000 | RR 1.39 | 1512 | ⊕⊕⊝⊝ | |
| Quality of life review 1 to 2 years | The mean quality of life in the mesh groups was 0.05 standard deviations higher (0.20 lower to 0.30 higher). This is an imprecise finding that is consistent with a small benefit in either group, or else no difference between the groups | 665 (7 studies) | ⊕⊝⊝⊝ very low1, 2,4 | I2 = 60% | ||
| *The basis for the assumed risk is the median control group risk across studies The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious risk of bias: most of the studies were at unclear or high risk of bias associated with poor reporting of methods, including failure by many to describe satisfactory methods of allocation concealment or blinding. A minority of studies did not report use of blinding at all. 2Downgraded one level due to serious imprecision: findings compatible with benefit in either group or with no clinically meaningful difference between the groups. 3Downgraded one level due to serious imprecision: findings compatible with benefit in native tissue group or with no clinically meaningful difference between the groups. 4Downgraded one level due to serious inconsistency: substantial statistical heterogeneity. | ||||||
| Absorbable mesh versus native tissue repair for vaginal prolapse | ||||||
| Population: women with vaginal prolapse Control: native tissue repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Absorbable mesh | |||||
| Awareness of prolapse at 2 years | 724 per 1000 | 760 per 1000 | RR 1.05 | 54 | ⊕⊝⊝⊝ | |
| Repeat surgery for prolapse (stage 2 or more) at 2 years | 125 per 1000 | 59 per 1000 | RR 0.47 | 66 | ⊕⊝⊝⊝ | |
| Recurrent prolapse at 3 months to 2 years | 429 per 1000 | 304 per 1000 | RR 0.71 | 292 | ⊕⊕⊝⊝ | |
| Bladder injury | Not reported in the included studies | |||||
| De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years | Not reported in the included studies | |||||
| Stress urinary incontinence at 2 years | 593 per 1000 | 818 per 1000 | RR 1.38 | 49 | ⊕⊝⊝⊝ | |
| Quality of life at 2 years | The mean quality of life score was the same in both groups, when measured using a severity score of 1 to 10 (mean difference 0, 95% CI ‐2.82 to 2.82) | 54 | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious risk of attrition bias: at two years 18% not included in analysis. | ||||||
| Biological repair versus native tissue repair for vaginal prolapse | ||||||
| Population: women with vaginal prolapse Control: native tissue repair | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native tissue repair | Biological repair | |||||
| Awareness of prolapse at 1 to 3 years | 105 per 1000 | 102 per 1000 | RR 0.97 | 777 | ⊕⊕⊝⊝ | |
| Repeat prolapse surgery 1 to 2 years | 43 per 1000 | 52 per 1000 | RR 1.22 | 306 | ⊕⊕⊝⊝ | |
| Recurrent prolapse at 1 year | 295 per 1000 | 277 per 1000 | RR 0.94 | 587 | ⊕⊝⊝⊝ | |
| Bladder injury | Not estimable as only 1 event occurred (in the native tissue group) | 137 (1 study) | ||||
| Bowel injury | Not estimable as only 1 event occurred (in the biological repair group) | 137 (1 study) | ||||
| De novo dyspareunia (pain during sexual intercourse) review 1 to 3 years | 177 per 1000 | 150 per 1000 | RR 0.85 | 37 | ⊕⊝⊝⊝ | |
| De novo urinary stress incontinence at 1 year | Not estimable ‐ no events occurred | 56 | ||||
| Quality of life at 1 year | The mean quality of life in the biological repair group was 0.05 standard deviations lower (0.48 lower to 0.38 higher). This is an imprecise finding that is consistent with a small benefit in either group, or else no difference between the groups | 84 | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious risk of bias: four of the studies at high or unclear risk of bias associated with blinding status. 7Downgraded one level due to serious risk of bias: blinding status unclear. 8Downgraded two levels due to very serious imprecision: single small study, only six events. 9Downgraded one level due to serious risk of attrition bias, and a further two levels due to very serious imprecision: only 84 participants. | ||||||
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| Ali 2006 abstract | 0 | 43 | 3 | 46 |
| 0 | 23 | 1 | 21 | |
| 0 | 182 | 21 | 183 | |
| 0 | 60 | 5 | 62 | |
| 0 | 81 | 18 | 88 | |
| 0 | 39 | 2 | 40 | |
| 0 | 54 | 4 | 44 | |
| 0 | 72 | 16 | 79 | |
| 0 | 33 | 5 | 32 | |
| 0 | 35 | 2 | 33 | |
| 0 | 24 | 2 | 28 | |
| 0 | 38 | 2 | 37 | |
| 0 | 96 | 18 | 104 | |
| Qatawneh 2013 | 0 | 63 | 4 | 53 |
| 0 | 42 | 3 | 43 | |
| Thijs 2010 abstract | 0 | 48 | 9 | 48 |
| 0 | 20 | 3 | 20 | |
| 0 | 51 | 2 | 53 | |
| 0 | 84 | 14 | 83 | |
| Total | 134 | 1097 |
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| Ali 2006 abstract | 0 | 43 | 3 | 46 |
| 0 | 23 | 1 | 21 | |
| 0 | 182 | 21 | 183 | |
| 0 | 39 | 2 | 40 | |
| 0 | 54 | 4 | 44 | |
| 0 | 35 | 2 | 33 | |
| 0 | 24 | 2 | 28 | |
| 0 | 38 | 2 | 37 | |
| 0 | 96 | 18 | 104 | |
| Qatawneh 2013 | 0 | 63 | 4 | 53 |
| 0 | 42 | 3 | 43 | |
| Thijs 2010 abstract | 0 | 48 | 9 | 48 |
| 0 | 20 | 3 | 20 | |
| 0 | 51 | 2 | 53 | |
| Total | 76 | 753 |
| Study ID | Repair events | Repair total | Exposure events | Exposure total |
| 0 | 60 | 5 | 62 | |
| 0 | 81 | 18 | 88 | |
| 0 | 72 | 16 | 79 | |
| 0 | 33 | 5 | 32 | |
| 0 | 84 | 14 | 83 | |
| Total | 58 | 344 |
| Study ID | Surgery for mesh exposure | Total number of women in mesh group |
| 6 | 186 | |
| 3 | 62 | |
| 7 | 88 | |
| 4 | 66 | |
| 2 | 40 | |
| 2 | 44 | |
| 10 | 79 | |
| 3 | 32 | |
| 2 | 33 | |
| 2 | 37 | |
| 14 | 104 | |
| Qatawneh 2013 | 4 | 53 |
| 5 | 78 | |
| 3 | 43 | |
| 2 | 36 | |
| 7 | 42 | |
| Thijs 2010 abstract | 4 | 48 |
| 3 | 20 | |
| 2 | 53 | |
| 5 | 83 | |
| Total | 100 | 1227 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (1‐3 years) Show forest plot | 12 | 1614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.81] |
| 1.1 Anterior compartment:mesh vs native tissue | 9 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 1.2 Multicompartment: mesh vs native tissue | 4 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 2 Repeat surgery (1‐3 years) Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 12 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.88] |
| 2.2 Continence surgery | 9 | 1284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.83] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 7 | 867 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.51, 3.81] |
| 3 Recurrent prolapse (any) at 1‐3 years Show forest plot | 21 | 2494 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.53] |
| 3.1 Anterior compartment repair: mesh versus native tissue | 15 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.40] |
| 3.2 Multi‐compartment repair: mesh versus native tissue | 6 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 4 Injuries bladder or bowel Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Bladder injury | 11 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [1.62, 9.50] |
| 4.2 Bowel injury | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 78.81] |
| 5 Objective failure of anterior compartment (cystocoele) Show forest plot | 13 | 1406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.36, 0.55] |
| 5.1 Anterior compartment repair: mesh versus native tissue | 9 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.47] |
| 5.2 Multi‐compartment repair: mesh versus native tissue | 4 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.51, 1.06] |
| 6 Objective failure of posterior compartment (rectocoele) Show forest plot | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 6.1 Mesh vs native tissue | 3 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 7 POPQ assessment (any mesh) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Point Ba POPQ | 10 | 1125 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.27, ‐0.59] |
| 7.2 Point C POPQ | 8 | 925 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.13, 0.23] |
| 7.3 Point Bp | 7 | 832 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.34, 0.44] |
| 7.4 total vaginal length | 5 | 611 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.25, 0.40] |
| 8 Bladder function: de novo stress urinary incontinence (1‐3 years) Show forest plot | 12 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.06, 1.82] |
| 8.1 Anterior compartment: mesh vs native tissue | 8 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.11] |
| 8.2 Multi compartment : mesh vs native tissue | 4 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.92] |
| 9 De novo voiding disorder, urgency, detrusor overactivity or overactive bladder Show forest plot | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.63] |
| 10 De novo dyspareunia (1‐3 years) Show forest plot | 11 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.47] |
| 10.1 Anterior compartment: mesh vs native tissue | 8 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.93] |
| 10.2 Multicompartment: mesh vs native tissue | 3 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.42] |
| 11 Sexual function (1‐3 years) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 PISQ score | 7 | 857 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.40, 0.13] |
| 12 Quality of life: continuous data (1‐2 years): Show forest plot | 7 | 665 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 12.1 PQOL end score | 3 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.31, 0.49] |
| 12.2 Pelvic floor impact questionnaire end score | 4 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.34, 0.37] |
| 13 Quality of life: dichotomous data "much or very much better" Show forest plot | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| 13.1 PGI‐I | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.80, 1.25] |
| 14 Operating time (minutes) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Anterior compartment: mesh vs native tissue | 10 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Multicompartment: mesh vs native tissue | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Blood transfusion Show forest plot | 6 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.88, 2.72] |
| 16 Length of stay in hospital (days) Show forest plot | 7 | 953 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (2 year review) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.77, 1.44] |
| 2 Repeat surgery for prolapse (2 years) Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.40] |
| 3 Recurrent prolapse (3 months ‐2 years) Show forest plot | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.96] |
| 3.1 Any site stage 2 or more | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.10, 2.70] |
| 3.2 Anterior compartment | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 4 Death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 absorbable mesh versus native tissue repair | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Objective failure of anterior compartment (cystocoele) Show forest plot | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 5.1 Anterior compartment repair: absorbable mesh versus native tissue | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 5.2 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.93] |
| 6 Objective failure of posterior compartment (rectocoele) Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| 6.1 Multi‐compartment repair: absorbable mesh versus native tissue | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.19] |
| 7 Stress urinary incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative SUI | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.95, 2.00] |
| 8 Quality of life (2 years) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| 8.1 VAS QoL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (1‐3 year) Show forest plot | 7 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.43] |
| 1.1 Anterior compartment repair: biological graft vs native tissue | 4 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.23] |
| 1.2 Multicompartment repair: biological graft vs native tissue | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [1.04, 19.92] |
| 1.3 Posterior compartment repair: biological graft vs native tissue | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.41, 1.94] |
| 2 Repeat prolapse surgery (1‐2 years) Show forest plot | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.61, 2.44] |
| 3 Recurrent prolapse (1 year) Show forest plot | 7 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 3.1 Anterior compartment repair: biological graft vs native tissue | 5 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.05] |
| 3.2 Posterior compartment repair: biological graft vs native tissue | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.18, 3.70] |
| 4 Injuries to bladder or bowel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 bladder injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.40] |
| 4.2 bowel injury | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 75.57] |
| 5 Objective failure of anterior compartment (cystocele) Show forest plot | 6 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| 6 Objective failure of posterior compartment (rectocele) Show forest plot | 3 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.39, 3.51] |
| 7 POPQ assessment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.98, ‐0.02] |
| 7.2 Point C | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| 7.3 Bp POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.27, 0.47] |
| 7.4 total vaginal length | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.06, 1.14] |
| 8 De novo urinary stress incontinence Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 De novo voiding disorders, urgency, detrusor overactivity or overactive bladder Show forest plot | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| 10 De novo dyspareunia (1 year) Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.20, 3.67] |
| 11 Sexual function (1 year) Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| 11.1 PISQ | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.33, 4.33] |
| 12 Quality of life (1 year) Show forest plot | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.38] |
| 12.1 PQOL score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.63] |
| 12.2 PFDI‐20 | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.11, 0.39] |
| 13 Operating time (minutes) Show forest plot | 4 | 232 | Mean Difference (IV, Fixed, 95% CI) | 10.34 [6.31, 14.36] |
| 14 Blood transfusion Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.14, 32.90] |

