| Adverse event | Randomised clinical trials: methylphenidate group (from Storebø 2015) | Randomised clinical trials: placebo or no intervention group (from Storebø 2015) | National Summary of Product Characteristics (UK, USA, DK) | Non‐comparative cohort studies and cohort studies from randomised trials (present review) | Non‐comparative cohort studies (present review) | Non‐comparative cohort studies from randomised trials (present review) |
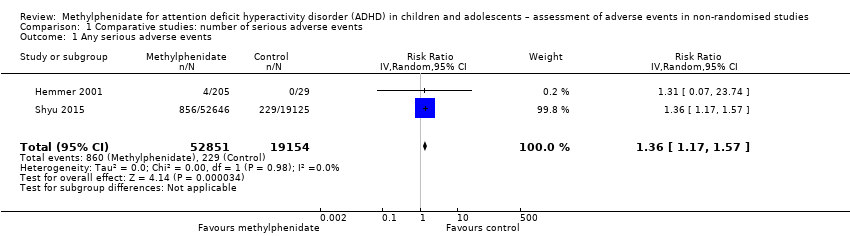
| Serious adverse events | 1.90% (95% CI 1.10% to 3.20%; 9 studies, 919 participants) | 2.80% (95% CI 1.70% to 4.80%; 9 studies, 613 participants) | No information | 1.20% (95% CI 0.70% to 2.00%; 51 studies, 162,434 participants) | 1.10% (95% CI 0.60% to 2.00%; 32 studies, 159,761 participants) | 1.60% (95% CI 1.00% to 2.30%; 18 studies, 2661 participants) |
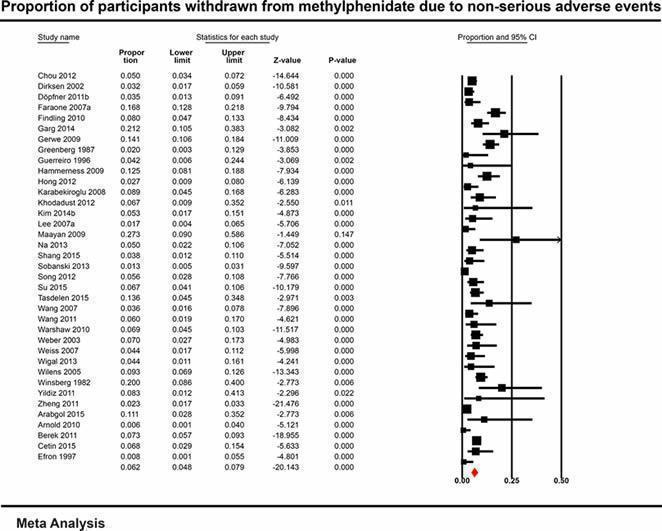
| Non‐serious adverse events | 51.4% (95% CI 41.9% to 60.9%; 21 studies, 1861 participants) | 38.3% (95% CI 30.3% to 47.0%; 21 studies, 1271 participants) | No information | 51.2% (95% CI 41.2% to 61.1%; 49 studies, 13,978 participants) | 47.1% (95% CI 35.6% to 58.9%; 36 studies, 13,035 participants) | 62.1% (95% CI 44.4% to 77.1%; 13 studies, 943 participants) |
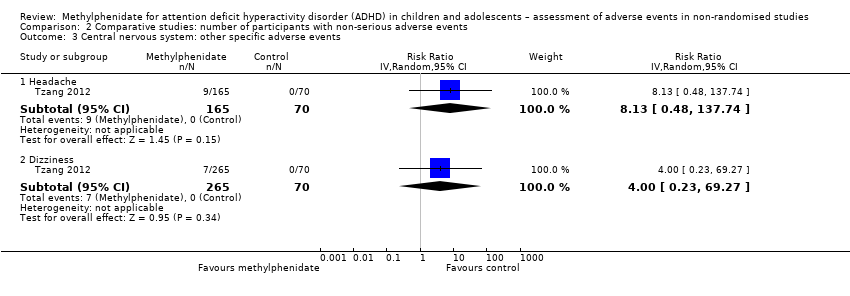
| Headache | 11.6% (95% CI 8.80% to 13.3%; 17 studies, 1642 participants) | 9.40% (95% CI 7.10% to 12.4%; 17 studies, 1082 participants) | 1% to 10% | 14.4% (95% CI 11.3% to 18.3%; 90 studies, 13,469 participants)a | 9.90% (95% CI 7.00% to 13.9%; 57 studies, 10,929 participants | 24.3% (95% CI 18.0% to 32.1%; 33 studies, 2540 participants) |
| Anxiety | 6.50% (95% CI 1.20% to 29.2%; 3 studies, 356 participants) | 12.4% (95% CI 8.30% to 18.0%; 3 studies, 240 participants) | 1% to 10% (UK and DK); no information (USA) | 18.4% (95% CI 11.3% to 28.7%; 22 studies, 1287 participants)a | 10.2% (95% CI 5.30% to 18.9%; 8 studies, 938 participants | 27.9% (95% CI 17.8% to 40.8%; 14 studies, 349 participants |
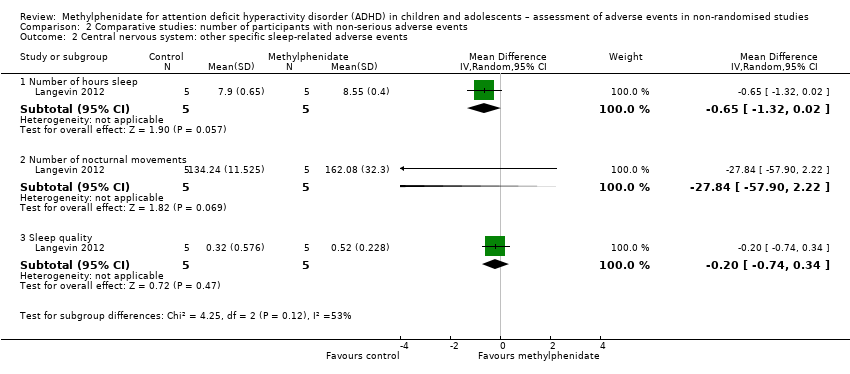
| Sleep difficulty | 8.00% (95% CI 5.80% to 11.1%; 13 studies, 1417 participants) | 8.30% (95% CI 6.40% to 10.7%; 13 studies, 999 participants) | 1% to 10% | 17.9% (95% CI 14.7% to 21.6%; 82 studies, 11,507 participants)a | 14.3% (95% CI 11.2% to 18.2%; 51 studies, 9073 participants | 25.4% (95% CI 18.2% to 34.4%, 31 studies, 2434 participants) |
| Irritability | 6.40% (95% CI 3.70% to 10.8%; 11 studies, 1038 participants) | 3.50% (95% CI 1.40% to 8.60%; 11 studies, 778 participants) | 1% to 10% | 17.2% (95% CI 11.5% to 25%; 35 studies, 4792 participants)a | 15.5% (95% CI 10.2% to 22.7%; 21 studies, 3298 participants | 20.6% (95% CI 7.90% to 44.1%; 14 studies, 1494 participants) |
| Tics | 2.30% (95% CI 1.00% to 5.20%; 7 studies, 684 participants) | 5.50% (95% CI 3.70% to 8.10%; 7 studies, 476 participants) | No information | 6.40% (95% CI 4.50% to 8.90%; 39 studies, 1980 participants)a | 5.60% (95% CI 3.80% to 8.10%; 29 studies, 1601 participants) | 10.6% (95% CI 5.30% to 19.9%; 10 studies, 379 participants) |
| Drowsiness | 7.30% (95% CI 2.40% to 20.2%; 4 studies, 510 participants) | 6.70% (95% CI 2.60% to 16.2%; 4 studies, 310 participants) | 1% to 10% | 9.50% (95% CI 5.20% to 16.6%; 17 studies, 1146 participants)a | 7.50% (95% CI 3.10% to 17.2%; 7 studies, 644 participants) | 11.3% (95% CI 5.00% to 23.3%; 10 studies, 502 participants) |
| Sadness | 5.70% (95% CI 1.30% to 21.9%; 4 studies, 382 participants) | 4.20% (95% CI 0.90% to 16.9%; 4 studies, 318 participants) | No information | 16.8% (95% CI 9.40% to 28.3%; 21 studies, 1802 participants)a | 13.1% (95% CI 6.60% to 24.1%; 9 studies, 626 participants) | 20.6% (95% CI 8.10% to 43.1%; 12 studies, 1176 participants) |
| Fatigue | 4.80% (95% CI 2.30% to 9.90%; 6 studies, 471 participants) | 6.50% (95% CI 4.30% to 9.60%; 6 studies, 387 participants) | 1% to 10% | 5.70% (95% CI 3.00% to 10.4%; 17 studies, 2182 participants) | 5.60% (95% CI 2.80% to 10.9%; 5 studies, 673 participants) | 7.80% (95% CI 5.80% to 10.5%; 12 studies, 1509 participants) |
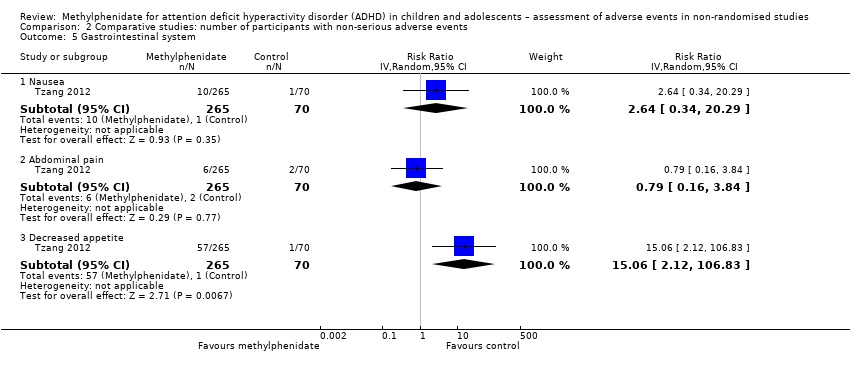
| Abdominal pain | 11.5% (95% CI 7.70% to 16.8%; 13 studies, 1406 participants) | 7.60% (95% CI 5.00% to 11.5%; 13 studies, 935 participants) | 0% to 10% | 10.7% (95% CI 8.60% to 13.3%; 79 studies, 11,750 participants)a | 7.60% (95% CI 5.70% to 10.0%; 46 studies, 9229 participants) | 16.3% (95% CI 11.6% to 22.4%; 33 studies, 2521 participants) |
| Decreased appetite | 17.3 (95% CI 12.3% to 24.2%; 16 studies, 1751 participants) | 4.30% (95% CI 2.40% to 7.40%; 16 studies, 1211 participants) | 1% to 10% | 31.1% (95% CI 26.5% to 36.2%; 84 studies, 11,594 participants)a | 28.8% (95% CI 23.0% to 33.5%; 57 studies, 9662 participants) | 39.7% (95% CI 27.0% to 54.0%; 27 studies, 1967 participants) |
| Vomiting | 5.70% (95% CI 4.00% to 8.00%; 11 studies, 1140 participants) | 5.10% (95% CI 3.5% to 7.4%; 11 studies, 776 participants) | 1% to 10% | 7.30% (95% CI 3.70% to 13.4%; 20 studies, 2731 participants)a | 7.10% (95% CI 2.80% to 17.0%; 11 studies, 1528 participants) | 7.20% (95% CI 3.30% to 15.1%; 9 studies, 1203 participants) |
| Nausea | 7.50% (95% CI 6.10% to 9.30%; 11 studies, 1174 participants) | 5.20% (95% CI 3.80% to 7.10%; 11 studies, 821 participants) | > 10% | 7.60% (95% CI 5.30% to 10.6%; 41 studies, 5612 participants)a | 8.00% (95% CI 7.00% to 9.10%; 22 studies, 3921 participants) | 10.4% (95% CI 5.80% to 17.9%; 19 studies, 1691 participants) |
| Decreased weight | 6.30% (95% CI 3.80% to 10.3%; 6 studies, 472 participants) | 2.40% (95% CI 1.00% to 5.70%; 6 studies, 387 participants) | 1% to 10% | 8.70% (95% CI 4.80% to 15.3%; 26 studies, 5182 participants)a | 6.60% (95% CI 3.10% to 13.3%; 17 studies, 4855 participants) | 16.6% (95% CI 8.70% to 30.6%; 9 studies, 327 participants) |