Entrenamiento preoperatorio con ejercicios para pacientes con cáncer de pulmón de células no pequeñas
Resumen
Antecedentes
La resección quirúrgica para el cáncer de pulmón de células no pequeñas (CPCNP) en estadio inicial ofrece las mejores probabilidades de curación, pero se asocia con riesgo de complicaciones pulmonares posoperatorias (es decir, neumonía [un nuevo infiltrado acompañado de fiebre (> 38º C) y secreciones purulentas, o fiebre y recuento de leucocitos > 11 000], fístula broncopleural, atelectasia grave que requiere fisioterapia torácica o broncoscopia, y ventilación mecánica prolongada (> 48 horas)). Actualmente, no está claro si el entrenamiento preoperatorio con ejercicios, y la posible mejoría resultante en la capacidad de ejercicio, pueden mejorar también los resultados posoperatorios como el riesgo de desarrollar complicaciones pulmonares posoperatorias, la duración del drenaje intercostal posoperatorio o la duración de la estancia hospitalaria.
Objetivos
Los objetivos primarios de este estudio fueron determinar el efecto del entrenamiento preoperatorio con ejercicios sobre resultados posoperatorios como el riesgo de desarrollar una complicación pulmonar posoperatoria y la duración posoperatoria del uso de catéteres intercostales en adultos programados para ser sometidos a resección pulmonar por CPCNP. Los objetivos secundarios de este estudio fueron determinar el efecto del entrenamiento preoperatorio con ejercicios sobre la duración de la estancia hospitalaria, la fatiga, la disnea, la capacidad de ejercicio, la función pulmonar y la mortalidad posoperatoria.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE (PubMed), Embase Ovid, PEDro, y en SciELO el 28 de noviembre 2016.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) en los que los participantes que se programaron para ser sometidos a resección pulmonar por CPCNP se asignaron a recibir entrenamiento preoperatorio con ejercicios o ningún entrenamiento con ejercicios.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron los estudios e identificaron los adecuados para inclusión. Se realizaron metanálisis para los resultados: riesgo de desarrollar una complicación pulmonar postoperatoria; duración postoperatoria del catéter intercostal; duración de la estancia hospitalaria; capacidad de ejercicio posterior a la intervención (6 minutos de caminata) y capacidad vital forzada posterior a la intervención (CVF). Aunque tres estudios informaron el volumen espiratorio forzado posintervención en un segundo (FEV1), no se realizó el metanálisis de este resultado debido a la heterogeneidad estadística significativa (I² = 93%) entre los estudios. No estuvieron disponibles datos sobre la fatiga ni la disnea. Un estudio no informó la mortalidad posoperatoria hospitalaria de los grupos de ejercicio ni ningún ejercicio.
Resultados principales
Se identificaron cinco ECA que incluyeron a 167 participantes (la media de la edad varió de 54 a 72,5 años; el tamaño de la muestra varió entre 19 y 60 participantes). En general, se encontró que el riesgo de sesgo de los estudios incluidos fue alto y la calidad de la evidencia de todos los resultados fue baja. Los datos combinados de cuatro estudios demostraron que el entrenamiento preoperatorio con ejercicios redujo el riesgo de desarrollar una complicación pulmonar posoperatoria en el 67% (riesgos relativos [RR] 0,33; IC del 95%: 0,17 a 0,61). El número de días que los pacientes del grupo de ejercicio necesitaron un catéter intercostal fue inferior al del grupo sin ejercicio (diferencia de medias (DM) ‐3,33 días, IC del 95%: ‐5,35 a ‐1,30 días; dos estudios); la duración de la estancia hospitalaria postoperatoria también fue inferior en el grupo de ejercicio (DM ‐4,24 días, IC del 95%: ‐5,43 a ‐3,06 días; cuatro estudios). Los datos combinados de dos estudios demostraron que, en comparación con el grupo de ningún ejercicio, la distancia de caminata de seis minutos posintervención (DM 18,23 m; IC del 95%: 8,50 a 27,96 m) y la CVF posintervención (DM prevista 2,97%; IC del 95% previsto: 1,78% a 4,16%) fueron mayores en el grupo de ejercicio.
Conclusiones de los autores
El entrenamiento preoperatorio con ejercicios puede reducir el riesgo de desarrollar una complicación pulmonar posoperatoria, la duración del uso de los catéteres intercostales, la duración posoperatoria de la estancia hospitalaria, y mejorar la capacidad de ejercicio y la CVF en los pacientes sometidos a resección pulmonar por CPCNP. Los resultados de esta revisión sistemática se deben interpretar con cuidado debido a las disparidades entre los estudios, el riesgo de sesgo y los tamaños pequeños de la muestra. Esta revisión enfatiza la necesidad de ECA más grandes.
PICO
Resumen en términos sencillos
Entrenamiento con ejercicios antes de la cirugía pulmonar en pacientes con cáncer de pulmón de células no pequeñas
Pregunta de la revisión
Se examinó la evidencia acerca del efecto del entrenamiento con ejercicios realizado antes de la cirugía pulmonar sobre el riesgo de desarrollar una complicación pulmonar posoperatoria, el número de días que se necesitó un drenaje torácico después de la cirugía, la duración de la estancia hospitalaria, el nivel del estado físico y la función pulmonar en los pacientes con cáncer de pulmón de células no pequeñas (CPCNP).
Antecedentes
La cirugía pulmonar por CPCNP les ofrece a los pacientes una probabilidad de curación; sin embargo, la cirugía pulmonar se asocia con un aumento en el riesgo de complicaciones pulmonares posoperatorias. El entrenamiento preoperatorio con ejercicios, a través de la mejoría en los niveles del estado físico, puede reducir el riesgo de complicaciones pulmonares posoperatorias y mejorar otros resultados posoperatorios como el número de días que los pacientes necesitaron un drenaje torácico y la duración de la estancia hospitalaria. Sin embargo, no están claros los efectos del entrenamiento preoperatorio con ejercicios sobre resultados posoperatorios en los pacientes con CPCNP.
Características de los estudios
La evidencia está actualizada hasta noviembre 2016. Esta revisión incluyó datos de 167 participantes (la edad media osciló entre 54 y 72,5 años) en cinco estudios (el tamaño de la muestra de los estudios incluidos osciló entre 19 y 60 participantes).
Resultados clave
Los resultados de la presente revisión mostraron que, en comparación con un grupo control que no hizo ejercicios antes de la cirugía pulmonar, los pacientes con CPCNP que hicieron ejercicios antes de la cirugía pulmonar tuvieron 67% menos riesgo de desarrollar una complicación pulmonar posoperatoria. Según este resultado, se esperaría que de 100 pacientes con CPCNP que realizan ejercicios antes de la cirugía pulmonar, siete presentarán una complicación pulmonar posoperatoria, en comparación con 22 pacientes con CPCNP que presentarán una complicación pulmonar posoperatoria si no realizan ejercicios antes de la cirugía pulmonar. Además, en comparación con los del grupo control, los pacientes con CPCNP que hicieron ejercicios antes de la cirugía pulmonar tuvieron un drenaje torácico durante menos días (tres días menos), tuvieron una duración más corta de la estancia hospitalaria (cuatro días menos) y mejoraron la distancia de caminata de seis minutos (18 metros más), así como la función pulmonar antes de la cirugía (3% mejor).
Calidad de la evidencia
La calidad general de la evidencia fue baja para todos los resultados, principalmente debido al escaso número de estudios encontrados, el número pequeño de participantes en los estudios incluidos y las limitaciones en los métodos de los estudios.
Authors' conclusions
Summary of findings
| Preoperative exercise training compared to no exercise training for patients scheduled to undergo lung resection for non‐small cell lung cancer | ||||||
| Patient or population: patients scheduled to undergo lung resection for non‐small cell lung cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no exercise training | Risk with preoperative exercise training | |||||
| Number of patients who developed postoperative pulmonary complications | Study population | RR 0.33 | 158 | ⊕⊕⊝⊝ | ||
| 22 per 100 | 7 per 100 | |||||
| Number of days patients needed an intercostal catheter | The mean number of days patients needed an intercostal catheter in the control groups ranged from 7.4 to 8.8 days | The number of days patients needed an intercostal catheter in the intervention groups was, on average, 3.33 fewer days | ‐ | 38 | ⊕⊕⊝⊝ | |
| Postoperative length of hospital stay | The mean postoperative length of hospital stay in the control groups ranged from 9.7 to 12.2 days | The postoperative length of hospital stay in the intervention groups was, on average, 4.34 fewer days (95% CI 5.65 to 3.03 fewer days) | ‐ | 158 | ⊕⊕⊝⊝ | |
| Post‐intervention exercise capacity assessed with: 6‐minute walk distance (6MWD) | The mean post‐intervention exercise capacity in the control groups ranged from 340 to 434 metres in 6 minutes. | The post‐intervention exercise capacity in the intervention groups was, on average, 18.23 metres more | ‐ | 81 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant risk of bias across the studies 2 Small sample sizes across the studies, some with wide confidence intervals | ||||||
Background
Description of the condition
Lung cancer is the leading cause of cancer death worldwide (Ferlay 2013). Despite improvements in the medical treatment of lung cancer over recent decades, the five‐year survival rate remains poor, at approximately 13% (AIHW 2011; Ferlay 2013). Lung cancer is the third most commonly diagnosed cancer worldwide (Ferlay 2013), and non‐small cell lung cancer (NSCLC) accounts for the majority of cases (85%) AIHW 2011).
Surgical resection of the tumour provides the best chance of cure for NSCLC (NCCN 2015). Lung resection is suitable for patients with early stage disease, and those with sufficient cardiopulmonary reserve to withstand the surgery (NCCN 2015). International clinical practice guidelines recommend that patients undergo routine preoperative evaluation, consisting of lung function tests plus the addition of exercise tests, if forced expiratory volume in one second (FEV1) or impaired diffusing capacity of the lung for carbon monoxide, is reduced (Brunelli 2009a). For patients assessed to be unfit for surgery, or those with advanced disease, alternative treatments include chemotherapy, radiotherapy, targeted agents, or a combination (NCCN 2015). Although lung resection offers a chance of cure, it also results in an immediate insult to the cardiorespiratory system. There is a known, immediate reduction in peak oxygen consumption (VO2peak) of approximately 12% post‐lobectomy, and 18% post‐pneumonectomy (Brunelli 2009). Postoperative pulmonary complications are common. These include: respiratory failure (such as prolonged mechanical ventilation, re‐intubation, or acute respiratory distress syndrome), pneumonia, atelectasis requiring bronchoscopy, myocardial infarction, and arrhythmias (Benzo 2007). The incidence of postoperative pulmonary complications is higher in patients treated with an open thoracotomy approach (4% to 15%) than minimally invasive video‐assisted thoracic surgery (VATS (2%) Agostini 2010; Lugg 2016; McKenna 2006; Reeve 2010). Independent risk factors for the development of postoperative pulmonary complications after lung resection include: age over 75 years, body mass index over 30 kg/m², a diagnosis of chronic obstructive pulmonary disease (COPD), and being a current smoker (Agostini 2010; Lugg 2016). Postoperative pulmonary complications following lung resection are associated with longer length of hospital stay, higher rate of intensive care unit (ICU) admissions, higher 30‐day readmissions, and reduced overall survival (Lugg 2016); hence, prevention is of significant importance.
People with lung cancer experience a high disease burden, physical hardship, and morbidity over the disease trajectory. The adverse physical and psychological impairments in lung cancer occur as a result of multiple processes, including the disease, the cancer treatment, and individual patient factors such as multiple co‐morbidities, and a history of poor lifestyle behaviours (Jones 2009; Schmitz 2010). Common symptoms in lung cancer include dyspnoea, cough, fatigue, and pain; these commonly occur as complex symptom clusters, and are particularly debilitating to the patient (Cooley 2000; Hung 2011; Pan 2012). The majority (85% to 90%) of cases of lung cancer are caused by voluntary or involuntary exposure to cigarette smoke (NCCN 2015), and not surprisingly, 40% to 70% of patients also have COPD (Dela Cruz 2011). Many patients have a history of sedentary behaviour. At time of diagnosis, prior to treatment, patients with lung cancer are generally worse than their healthy, age‐matched peers in physical activity levels, exercise capacity, muscle strength, and health‐related quality of life (HRQoL; Coups 2009; Granger 2014; Novoa 2009). Following diagnosis and treatment, the subsequent vicious cycle of inactivity and functional decline is common (Granger 2014; Novoa 2009). Activity limitations, participation restrictions, and reduced HRQoL commonly ensue (Cavalheri 2015; Hung 2011; Pan 2012; Schmitz 2010; Tanaka 2002).
Description of the intervention
Exercise training is the intervention in this review. Exercise training is "a subset of physical activity that is planned, structured, and repetitive, and has as a final or an intermediate objective, the improvement or maintenance of physical fitness" (Caspersen 1985). This includes aerobic training, resistance training, or respiratory muscle training. Exercise training is not currently standard clinical practice in the preoperative or postoperative management of patients with NSCLC (Cavalheri 2013).
How the intervention might work
Numerous Cochrane and non‐Cochrane systematic reviews have demonstrated that exercise training is associated with improvements in exercise capacity, muscle strength, physical function, HRQoL, depression, and symptoms for the general cancer population (Cramp 2012; Rock 2012; Schmitz 2010; Speck 2010). There is also growing evidence of the effectiveness of exercise training specifically for the postoperative NSCLC population (Cavalheri 2013a; Cavalheri 2014; Crandall 2014; Granger 2011). Consistent evidence links higher physical activity levels after cancer diagnosis (breast, colon, and prostate) with reduced cancer‐specific and all‐cause mortality (Ballard‐Barbash 2012; Lee 2014). In NSCLC, patients who are more physically active have better exercise capacity and HRQoL, and fewer symptoms (Coups 2009; Granger 2014). Preliminary evidence suggests that higher exercise capacity at the time of a diagnosis of NSCLC is related to prolonged survival (Denehy 2013; Jones 2012). Postulated mechanisms linking exercise with improved survival in lung cancer include: the modulation of circulating metabolic and sex‐steroid hormone concentrations, immune surveillance, and reduced systemic inflammation and oxidative damage (McTiernan 2008). It is currently unclear if preoperative exercise training, and the potential resultant improvement in exercise capacity, may also improve postoperative outcomes, such as reduced postoperative pulmonary complications, duration of intercostal catheter, length of hospital stay, and mortality.
Exercise training is standard clinical practice for people with many other chronic respiratory diseases, as part of their pulmonary rehabilitation (McCarthy 2015; Spruit 2013). Exercise training, the cornerstone of pulmonary rehabilitation programmes, includes aerobic and resistance training, delivered in a supervised environment. For patients with COPD, it has been demonstrated to improve exercise capacity, HRQoL, dyspnoea, and fatigue (McCarthy 2015). Given the commonalities between COPD and lung cancer, and the common co‐occurrence of these two conditions, it is possible that exercise training may result in similar outcomes for those undergoing lung resection for NSCLC.
Why it is important to do this review
The primary objective of this review was to determine the effect of preoperative exercise training in adults scheduled to undergo lung resection for NSCLC, on preoperative and postoperative clinical and patient‐related outcomes. If the results of this review were positive, it would provide evidence to support preoperative exercise training, and justify the need to change clinical practice. Results of this review would also be able to direct future research, by mapping the evidence gaps, and highlighting areas of critical limitations that exist in the studies completed to date.
Objectives
The primary aims of this study were to determine the effect of preoperative exercise training on postoperative outcomes, such as risk of developing a postoperative pulmonary complication, and postoperative duration of intercostal catheter, in adults scheduled to undergo lung resection for NSCLC. The secondary aims of this study were to determine the effect of preoperative exercise training on length of hospital stay, fatigue, dyspnoea, exercise capacity, lung function, and postoperative mortality, in adults scheduled to undergo lung resection for NSCLC.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs) of preoperative exercise training compared with no exercise training for patients with NSCLC. We considered studies published in any language.
Types of participants
We included studies with patients who were scheduled to undergo lung resection for NSCLC. We included lung resection of any extent, that is, wedge resection, segmentectomy, lobectomy, bi‐lobectomy, or pneumonectomy. We also included studies with patients who underwent both VATS and open thoracotomy.
Types of interventions
Preoperative exercise training was the intervention, and was compared to no exercise (usual care). We included studies if the intervention group received a minimum of seven exercise sessions completed over a minimum of one week in the preoperative setting. We set up this short arbitrary cut‐off point because long exercise programmes are unlikely to be conducted, due to concerns from both patients and multidisciplinary medical teams related to delaying lung resection for long periods of time following the diagnosis of cancer (Benzo 2011; Morano 2013). The exercise sessions could be supervised, unsupervised, or both, and include aerobic, resistance or respiratory muscle training, or a combination. We recorded specific details of the exercise programme, including type of exercise, setting of exercise, supervision, frequency, duration, monitoring, and safety.
Types of outcome measures
Primary outcomes
The primary outcome measures of our review were:
-
Risk of developing a postoperative pulmonary complication (i.e. pneumonia (new infiltrate coupled with either fever (> 38º C) and purulent secretions, or fever and white cell count > 11,000), bronchopleural fistula, severe atelectasis that requires chest physiotherapy, or bronchoscopy and prolonged mechanical ventilation (> 48 hours)); and
-
Number of days patients needed an intercostal catheter following surgery.
Secondary outcomes
-
Postoperative length of hospital stay;
-
Post‐intervention fatigue (e.g. the Functional Assessment of Chronic Illness Therapy ‐ Fatigue Subscale);
-
Post‐intervention dyspnoea (e.g. the Borg scale or Medical Research Council scale);
-
Post‐intervention and postoperative exercise capacity (e.g. six‐minute walk distance (6MWD), performance during the stair climbing test, maximum work rate (Wmax), or peak rate of oxygen uptake (VO2peak);
-
Post‐intervention lung function (e.g. volumes ‐ FEV1 and forced vital capacity (FVC), flows and diffusing capacity);
-
Postoperative mortality.
Search methods for identification of studies
Electronic searches
We searched the following databases to identify RCTs:
-
The Cochrane Central Register of Controlled Trials (CENTRAL; Issue 11, 2016) in the Cochrane Library (searched 28 November 2016);
-
MEDLINE (PubMed; 1966 to 28 November 2016);
-
Embase Ovid (1974 to 28 November 2016);
-
PEDro (Physiotherapy Evidence database; 1980 to 28 November 2016); and
-
SciELO (the Scientific Electronic Library Online; 1978 to 28 November 2016).
We listed the search terms and strategies used to search for studies using CENTRAL, MEDLINE and Embase in Appendix 1, Appendix 2 and Appendix 3. The MEDLINE search string was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximising version as referenced in Chapter 6.4.11.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted the strategy for EMBASE. We also adapted both the terms and the strategies for use in PEDro and SciELO. We placed no restrictions on language or date of publication.
Searching other resources
Other searching sources included: (i) screening reference lists of all RCTs included in the review; (ii) contacting experts in the field for additional references; and (iii) hand searching abstracts from the Thoracic Society of Australia and New Zealand, European Respiratory Society, and American Thoracic Society scientific meetings (2010 to March 2016).
Data collection and analysis
Selection of studies
The two review authors independently examined the studies identified in the literature search using Covidence (Covidence 2017). First, we excluded studies based on their title and abstract and recorded the reason for exclusion. Subsequently, the two investigators independently examined the full text of the remaining studies and coded them as (i) 'include'; (ii) 'unclear' or (iii) 'exclude', based on the review criteria. We resolved disagreements by consensus and kept a full record of the decisions.
Data extraction and management
The two review authors independently extracted data from the included studies using a standardised form. We resolved any discrepancies by consensus. We attempted to contact authors of the included studies to provide any missing data detected during the process. One of the review authors (VC) then entered data into Review Manager 5.3 (RevMan 2014). In order to interpret the findings, we created a GRADE 'Summary of findings' table (Atkins 2004; Guyatt 2008). The outcomes that were included in the 'Summary of findings' table were: (i) risk of developing a postoperative pulmonary complication; (ii) number of days patients needed an intercostal catheter; (iii) length of hospital stay; and (iv) post‐intervention exercise capacity. We used both the 'Summary of findings' screen for numerical data and the 'quality assessment' screen to grade the evidence. We assessed the quality of evidence for each outcome by downgrading or upgrading the evidence according to the GRADE criteria. We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 11; Higgins 2011).
Assessment of risk of bias in included studies
The two review authors independently appraised the risk of bias of the included studies using the Cochrane 'seven evidence‐based domains' tables. We resolved disagreements by consensus. We judged risk of bias as either high, low, or unclear for selection bias (i.e. random sequence generation and allocation concealment), performance bias (i.e. blinding of participants and personnel), detection bias (i.e. blinding of outcome assessor), attrition bias (i.e. incomplete outcome data), reporting bias (i.e. selective outcome reporting), and other potential sources of bias. The judgement was accompanied by a direct quote, specific details of the study, or both, in the 'Risk of bias' table. We contacted study authors, where applicable, to seek clarification on issues regarding bias. We also contacted authors of unpublished studies to provide us with information pertaining to bias, and we added notes in the 'Risk of bias' table. We generated both the 'Risk of bias' graph (i.e. bar chart) and the 'Risk of bias' summary (i.e. traffic lights). We also used the GRADE approach to rate the overall quality of evidence for each outcome (Atkins 2004; Guyatt 2008a).
Measures of treatment effect
For the primary outcome (i.e. risk of developing a postoperative pulmonary complication), we used the risk ratio (RR). We also used the risk difference (RD), in order to calculate the number needed to treat to benefit (NNTB). For continuous outcomes, we used either the mean difference (MD) or standardised mean difference (SMD). We also calculated 95% confidence intervals (CIs).
Unit of analysis issues
For studies that presented two or more follow‐up data for a given outcome (e.g. exercise capacity post‐intervention, at three months postoperatively, six months postoperatively, or some combination), we did not combine the results from the different time points in a single meta‐analysis.
Dealing with missing data
We attempted to contact authors of the included studies for missing data. When our attempts to contact a study author were unsuccessful, we limited presentation of the outcome(s) of that specific study to a narrative discussion.
Assessment of heterogeneity
We assessed statistical heterogeneity across the studies using the I² statistic. We considered values of I² that were greater than 50% as substantial heterogeneity (Higgins 2011). If substantial statistical heterogeneity was detected, we investigated whether clinical or methodological heterogeneity were the potential causes. If substantial statistical heterogeneity was detected in meta‐analysis, we undertook a sensitivity analysis.
Assessment of reporting biases
We searched online trial registries in order to investigate potential publication bias and to assess potential outcome reporting bias in the included studies.
Data synthesis
We used Review Manager 5.3 for statistical analyses and to generate forest plots (RevMan 2014). For studies published by the same research group which used the same sample of participants, we only included data from one of the published studies in meta‐analyses. We analysed pooled data using a random‐effects model. We meta‐analysed the results of homogeneous studies using the inverse variance DerSimonian and Laird method (DerSimonian 1986). For I² values ranging between 50% and 60%, data aggregation was kept if the magnitude and direction of the studies' effects were not conflicting. Where data aggregation was not possible, due to clinical, methodological, or statistical heterogeneity, we used narrative discussion.
Subgroup analysis and investigation of heterogeneity
Where possible, we had planned to conduct subgroup analysis to evaluate the effect of the intervention in the following groups: (i) different exercise training regimens (e.g. aerobic versus resistance training); (ii) extent of lung resection (e.g. lobectomy versus pneumonectomy); (iii) type of surgical approach (e.g. open thoracotomy versus VATS); (iv) stage of NSCLC (e.g. stage I NSCLC versus stage II NSCLC) and (v) comorbidities (e.g. patients diagnosed with COPD versus patients not diagnosed with COPD, or patients with coronary artery disease versus patients without coronary artery disease). We assessed heterogeneity and the extent of inconsistency between studies by visual inspection of the forest plots, and by using the Chi² test, and the I² statistic.
Sensitivity analysis
We performed sensitivity analyses where we found significant heterogeneity among the studies. We investigated the effects of methodological differences on the results.
Results
Description of studies
Results of the search
We searched all the databases until 28 November 2016. The search yielded a total of 571 records: 81 from CENTRAL; 95 from MEDLINE; 323 from Embase; 29 from PEDro, and 43 from ScIELO. After removing duplicates, we had a total of 432 records. We excluded 413 based on the title and abstract, and assessed 19 full‐text articles and conference abstracts for eligibility. We excluded 11 studies: seven did not meet the review criteria, the authors of two reports did not reply to several contact attempts to confirm eligibility, and the authors of two conference abstracts did not reply to several contact attempts to confirm eligibility (Figure 1). We were able to contact the authors of two studies eligible for this review to obtain missing data.

Flow diagram of references identified, excluded, and included in review
Included studies
Refer to Characteristics of included studies for further details.
Study
This review included five RCTs (eight references) involving 167 participants (Benzo 2011; Lai 2017; Morano 2013; Pehlivan 2011; Stefanelli 2013).
Population
Four of the five studies only included participants with non‐small cell lung cancer (NSCLC) undergoing lung resection (Lai 2017; Morano 2013; Pehlivan 2011; Stefanelli 2013). One study did not specify the type of lung cancer of the participants (Benzo 2011). Two studies specifically included participants with NSCLC and a diagnosis of chronic obstructive pulmonary disease (COPD; Benzo 2011; Stefanelli 2013). Three studies included participants undergoing lung resection via either open thoracotomy or video‐assisted thoracic surgery (VATS; Benzo 2011; Lai 2017; Morano 2013). Stefanelli 2013 only included participants undergoing lung resection via open thoracotomy. One study did not specify the type of surgical technique used for the lung resection (Pehlivan 2011). The sample sizes ranged from 19 to 60, with the mean age of the participants ranging from 54 to 72.5 years.
Setting
The studies were based in the USA, China, Brazil, Turkey, and Italy.
Intervention
The type, frequency, and intensity of the exercise programs varied considerably across the included studies. The frequency and duration of exercise training programs varied from three times per day for one week (Pehlivan 2011), to five times per week for four weeks (Morano 2013). Aerobic exercise training was prescribed in all five studies. Only one study included resistance training (Benzo 2011); two studies included inspiratory muscle training and education (Benzo 2011; Morano 2013); four studies included breathing exercises (Benzo 2011; Lai 2017; Pehlivan 2011; Stefanelli 2013); and one study included stretches as well (Morano 2013).
The control groups received usual care with no formal exercise training. In one study, participants in the control group received instructions about lung expansion breathing techniques (Morano 2013).
Outcomes
The number of participants who developed a postoperative pulmonary complication was reported in four studies (Benzo 2011; Lai 2017; Morano 2013; Pehlivan 2011). The number of days participants needed an intercostal catheter following surgery was reported in two studies (Benzo 2011; Morano 2013). Four studies reported on postoperative length of hospital stay (Benzo 2011; Lai 2017; Morano 2013; Pehlivan 2011). Post‐intervention fatigue was not measured in any of the five included studies and post‐intervention dyspnoea was only reported by Stefanelli 2013. Post‐intervention exercise capacity was reported in three studies (Lai 2017; Morano 2013; Stefanelli 2013), and post‐intervention lung function was reported in three studies (Morano 2013; Pehlivan 2011; Stefanelli 2013). Mortality was only reported by Pehlivan 2011, and this was only in‐hospital mortality.
Excluded studies
Of the 19 studies for which the full texts were reviewed, 11 were excluded. The reasons for the exclusion of the 11 studies are summarised in Characteristics of excluded studies.
Risk of bias in included studies
Two out of the seven domains included in the Cochrane 'seven evidence‐based domains' table were identical across the five studies (allocation concealment and blinding of participants and personnel). None of the studies reported blinding participants or personnel. Intention‐to‐treat analysis was reported by Lai 2017 and Morano 2013. Further details can be found in the 'Risk of bias' tables (Characteristics of included studies), with summaries in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
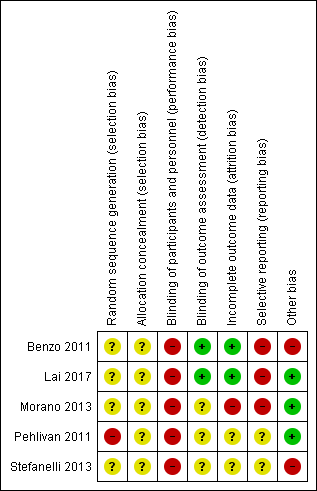
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged one study to be at high risk of selection bias (random sequence generation) because their allocation was based on hospital record number (Pehlivan 2011). We judged the other four studies at unclear risk, since they failed to report sufficient information about the random sequence generation process to permit judgement. We judged all studies to be at unclear risk of selection bias (allocation concealment), since they failed to report sufficient information about allocation concealment to permit judgement.
Blinding
We rated all studies at a high risk of performance bias, since neither the participants nor the personnel responsible for delivering the intervention were blinded to group allocation in any of the studies. Therefore, some of our results may be influenced by a placebo effect. Blinding of the outcome assessor was fully ensured in two studies, which were rated at low risk of detection bias (Benzo 2011; Lai 2017). Two studies did not describe blinding of outcome assessors, and were rated as unclear (Pehlivan 2011; Stefanelli 2013). Postoperative outcomes were obtained by a physical therapist blinded to the treatment assignment in Morano 2013. However, it was not clear whether post‐intervention outcome measures were taken by a blinded assessor, therefore, we judged the risk to be unclear.
Incomplete outcome data
We rated two studies at low risk of attrition bias because missing outcome data were balanced in numbers between the intervention and control groups, with similar reasons for missing data across groups (Benzo 2011), and because all participants successfully completed the training program and assessments (Lai 2017). One study was rated at high risk of bias, due mainly to a large loss to follow‐up (25%) in the control group (reasons were given; (Morano 2013)). We rated Pehlivan 2011 and Stefanelli 2013) as unclear risk of attrition bias due to insufficient reporting of attrition and exclusions.
Selective reporting
We rated three studies as high risk of reporting bias as (i) reported outcomes were not pre‐specified in the trial registration, (ii) not all of the pre‐specified outcomes were reported, and (iii) inclusion criteria were differently reported between trial register and published report (Benzo 2011; Lai 2017; Morano 2013). Two studies were judged to be at unclear risk of reporting bias because of insufficient information (Pehlivan 2011; Stefanelli 2013).
Other potential sources of bias
Two of the five included studies were rated at high risk of bias due to other sources of bias. Benzo 2011 reported findings of two studies they had undertaken; one study was stopped early due to poor recruitment; (ii) Stefanelli 2013 did not report numbers of patients allocated to each group.
Effects of interventions
See: summary of findings Table for the main comparison.
I. Primary outcome: risk of developing a postoperative pulmonary complication
Four studies reported the number of patients who developed a postoperative pulmonary complication (Benzo 2011; Lai 2017; Morano 2013; Pehlivan 2011; Table 1). Low‐quality evidence suggested that exercise training reduced the risk of developing a postoperative pulmonary complication by 67% (RR 0.33, 95% CI 0.17 to 0.61; Analysis 1.1; Figure 4). It is expected that one less person will develop a postoperative pulmonary complication for every four participants receiving preoperative exercise training rather than usual care (RD ‐0.25, 95% CI ‐0.37, ‐0.13; NNTB = 4).

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.1 Risk of developing a postoperative pulmonary complication.
| Study | Results |
| Number of patients who developed a postoperative pulmonary complication: Intervention group (IG): 3 of 9 (33%) Control Group (CG): 5 of 8 (63%) P = 0.23 (between‐group) Number of days patients needed a chest tube: IG: 4.3 ± 2.1 days CG: 8.8 ± 5.3 days P = 0.03 (between‐group) Postoperative length of hospital stay: IG: 6.3 ± 3.0 days CG: 11.0 ± 6.3 days P = 0.058 (between‐group) | |
| Number of patients who developed a postoperative pulmonary complication: IG: 4 of 30 (13%) CG: 11 of 30 (37%) P = 0.037 (between‐group) Postoperative length of hospital stay: IG: 6.9 ± 4.4 days CG: 10.7 ± 6.4 days P = 0.01 (between‐group) Exercise capacity: Six‐Minute Walk Distance (6MWD), in metres: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: baseline and post‐intervention: Mean ± standard deviation (SD): IG: 431.7 ± 102.8 m to 460.3 ± 93.6 m; CG: 434.5 ± 86.2 m to 443.9 ± 88.4 m P = 0.029 (between‐group) | |
| Number of patients who developed a postoperative pulmonary complication: IG: 2 of 12 (17%) CG: 7 of 9 (78%) P = 0.01 (between‐group) Number of days patients needed a chest tube: IG: 4.5 ± 2.9 days CG: 7.4 ± 2.6 days P = 0.03 (between‐group) Postoperative length of hospital stay: IG: 7.8 ± 4.8 days CG: 12.2 ± 3.6 days P = 0.04 (between‐group) Exercise capacity: Six‐Minute Walk Distance (6MWD), in metres: IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: Mean ± standard deviation (SD): IG: 425.5 ± 85.3 m to 475 ± 86.5 m (P < 0.01); CG: 339.6 ± 107 m to 335 ± 107 m (P > 0.05) P < 0.001 (between‐group) Lung function: (i) Forced expired volume in one second (FEV1; % predicted): IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 48.1 ± 13.9% to 54.8 ± 22.4% (P = 0.08); CG: 51.7 ± 9.8% to 58.8 ± 13.0% (P = 0.23) Between‐group difference was not calculated (ii) Forced vital capacity (FVC; % predicted): IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: Median (interquartile range): IG: 62.5% (49 to 71) to 76% (65 to 79.7); P = 0.02; CG: 62.5% (56 to 92) to 71% (63.2 to 89); P = 0.37 Between‐group difference was not calculated | |
| Number of patients who developed a postoperative pulmonary complication: IG: 1 of 30 (3%) CG: 5 of 30 (17%) P = 0.04 (between‐group) Postoperative length of hospital stay: IG: 5.4 ± 2.7 days CG: 9.7 ± 3.1 days P < 0.001 (between‐group) Lung function: (i) FEV1; % predicted: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: change from baseline to post‐intervention: IG: 15.84 ± 2.10%; CG: 9.92 ± 3.5% P = 0.3 (between‐group) (ii) FVC; % predicted: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 19.26 ± 2.33%; CG: 16.3 ± 2.4% P = 0.6 (between‐group) | |
| Exercise capacity: Peak rate of oxygen uptake (VO2peak), in ml/kg/min: IG: 20 participants completed; CG: 20 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 14.9 ± 2.3 ml/kg/min to 17.8 ± 2.1 ml/kg/min; CG: 14.8 ± 1.4 ml/kg/min to 14.5 ± 1.2 ml/kg/min P < 0.001 (between‐group) Lung function: FEV1; % predicted: IG: 20 participants completed; CG: 20 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 57.4 ± 19.1% to 59.8 ± 19.2%; CG: 57.6 ± 16.9% to 57.5 ± 17.0% P > 0.05 (between‐group) |
Intervention group (IG), Control Group (CG)
II. Primary outcome: number of days patients needed an intercostal catheter following surgery
Low‐quality evidence from two studies reported the number of days patients needed an intercostal catheter following surgery (Benzo 2011; Morano 2013; Table 1). Compared to the non‐exercise group, the number of days patients in the exercise group needed an intercostal catheter following surgery was lower (MD ‐3.33 days, 95% CI ‐5.35 to ‐1.30 days; Analysis 1.2; Figure 5).

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.2 Number of days patients needed an intercostal catheter.
III. Secondary outcome: postoperative length of hospital stay
Low‐quality evidence from four studies reported postoperative length of hospital stay (Benzo 2011; Lai 2017; Morano 2013; Pehlivan 2011; Table 1). Compared to the non‐exercise group, postoperative length of hospital stay was lower in the exercise group (MD ‐4.24 days, 95% CI ‐5.43 to ‐3.06 days; Analysis 1.3; Figure 6).
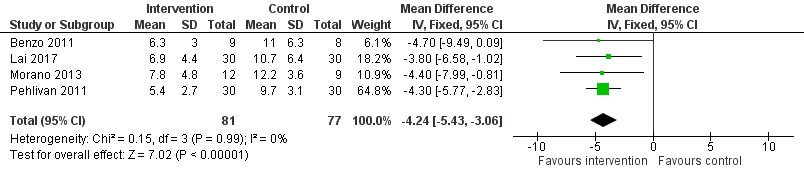
Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.3 Postoperative length of hospital stay.
IV. Secondary outcomes: post‐intervention fatigue and dyspnoea
Data were not available for these outcomes.
V. Secondary outcome: post‐intervention and postoperative exercise capacity
Three studies reported post‐intervention exercise capacity (Table 1). Two used the 6MWD (Lai 2017; Morano 2013), one used VO2peak (Stefanelli 2013). Data from the three studies were not pooled due to significant methodological and statistical heterogeneity (I² = 69%) across the studies. However, we conducted a meta‐analysis with data from the two studies that measured the 6MWD (Lai 2017; Morano 2013). There was low‐quality evidence that post‐intervention 6MWD was higher in the exercise group than in the non‐exercise group (MD 18.23 m, 95% CI 8.50 to 27.96 m; Analysis 1.4).
Stefanelli 2013 reported an improvement in VO2peak from baseline to post‐intervention in the exercise group (14.9 ± 2.3 ml/kg/min to 17.8 ± 2.1 ml/kg/min; P < 0.01), but no change in VO2peak in the non‐exercise group (14.8 ± 1.4 ml/kg/min to 14.5 ± 1.2 ml/kg/min; P > 0.05). Between‐group difference was reported as P > 0.05.
Only one study reported postoperative exercise capacity (Stefanelli 2013). This study found that exercise capacity decreased from immediately before surgery (post‐intervention time point) to 60 days postoperatively in both groups (VO2peak exercise group: 17.8 ± 2.1 to 15.1 ± 2.4; P < 0.01; non‐exercise group: 14.5 ± 1.2 to 11.4 ± 1.2; P < 0.01), however, there was no significant between‐group difference.
VI. Secondary outcome: post‐intervention lung function
Three studies reported post‐intervention FEV1 (Morano 2013; Pehlivan 2011; Stefanelli 2013; Table 1). We did not conduct a meta‐analysis due to significant statistical heterogeneity (I² = 93%) across the studies. None of the three studies reported between‐group difference in FEV1.
Two studies reported post‐intervention FVC (Morano 2013; Pehlivan 2011; Table 1). Compared to the non‐exercise group, post‐intervention FVC was greater in the exercise group (MD 2.97% predicted, 95% CI 1.78 to 4.16% predicted; Analysis 1.5).
VII. Secondary outcome: postoperative mortality
Only one study reported postoperative mortality (Pehlivan 2011). This study reported no in‐hospital postoperative mortality in either the intervention or the control group.
Discussion
Our meta‐analyses found that compared to no exercise training (i.e. usual care), preoperative exercise training conferred a 67% reduction in the risk of developing a postoperative pulmonary complication (RR 0.33, 95% CI 0.17 to 0.61), a three‐day reduction in intercostal catheter duration (MD ‐3.33 days, 95% CI ‐5.35 to ‐1.30 days), a four‐day reduction in postoperative length of hospital stay (MD ‐4.24 days, 95% CI ‐5.43 to ‐3.06 days), and improved preoperative 6MWD (MD 18.23 m, 95% CI 8.50, 27.96 m). None of the three studies that assessed FEV1 reported a change in FEV1 following preoperative exercise trainwhereas the meta‐analysis demonstrated that FVC improved 2.97% more in the exercise group compared to the non‐exercise group (MD 2.97% predicted, 95% CI 1.78 to 4.16% predicted). There were no data available for fatigue or dyspnoea, and only limited data available on postoperative mortality (one study only and no in‐hospital postoperative mortality in either group). Our findings should be viewed with caution as overall, we ratedi the quality of evidence as low. This was due to the significant risk of bias of the included studies and small sample sizes. Further higher quality trials are required to confirm the efficacy of preoperative exercise training.
Measurement of maximal exercise capacity (i.e. VO2peak) is recommended before lung resection in high risk patients (i.e. those with FEV1 and/or diffusing capacity for carbon monoxide < 80% of predicted values) to determine their eligibility for surgery (Brunelli 2009a). Patients with a VO2peak > 20ml/kg/min are considered operable and those with a VO2peak < 10ml/kg/min are considered inoperable. Patients with a VO2peak < 16ml/kg/min are at higher risk for peri or postoperative complications (Loewen 2007). Only one of our included studies (Stefanelli 2013) reported data on VO2peak. The mean VO2peak of participants in the intervention and control group in that study was 14.9 ± 2.3 ml/kg/min and 14.8 ± 1.4 ml/kg/min, respectively. That is, according to the cut‐off proposed by Loewen et al (Loewen 2007), they were at higher risk for peri or postoperative complications. Importantly, Stefanelli et al demonstrated that participants in the intervention group significantly improved their VO2peak to 17.8 ± 2.1 ml/kg/min, a value that is higher than the cut‐off for increased risk of peri or postoperative complications. Additionally, our meta‐analysis demonstrated an improvement in 6MWD in the intervention group that was over and above changes seen in the control group (MD 18.23 m; 95% CI 8.50, 27.96 m). Further studies are needed in order to investigate relationships between a significant improvements in exercise capacity following preoperative exercise training and better postoperative outcomes. However, we suggest that patients within the lower range of VO2peak (10‐15ml/kg/min) should be referred to preoperative exercise training as an attempt to decrease their risk of postoperative pulmonary complications.
The interventions provided in the studies included in our review varied in nature of exercise training. All studies included aerobic exercise training and supplemented this with either resistance training (Benzo 2011), respiratory muscle training (Benzo 2011; Morano 2013) and or breathing exercises (Benzo 2011; Pehlivan 2011; Stefanelli 2013). We cannot attribute one component of the exercise training to the benefits observed, and therefore until further studies are completed comparing respective types of exercise training, or study numbers increase significantly to allow us to undertake subgroup analyses, the optimal preoperative exercise prescription remains unknown. The studies included in the review did not report harm associated with preoperative exercise training. There is the potential that patients may experience short term temporary general muscle soreness after exercising, especially if they are unaccustomed to the specific types of exercises undertaken (Armstrong 1984). However, this is a usual response to exercise and not associated with permanent impairment.
Summary of main results
This review aimed to determine the effect of preoperative exercise training on outcomes such as risk of developing a postoperative pulmonary complication, number of days patients needed an intercostal catheter following surgery, postoperative length of hospital stay, post‐intervention fatigue and dyspnoea, post‐intervention and postoperative exercise capacity, lung function, and postoperative mortality in adults scheduled to undergo lung resection for non‐small cell lung cancer (NSCLC). We included data from five RCTs with 167 participants. This review showed that in patients who were scheduled to undergo lung resection for NSCLC, preoperative exercise training decreased the risk of postoperative pulmonary complications by 63%, reduced both intercostal catheter duration (by three days) and length of hospital stay (by four days), and improved preoperative exercise capacity and FVC. The evidence we found did not find that preoperative exercise training improved other outcomes including FEV1or postoperative mortality; there were no data for fatigue or dyspnoea .
The ability to reduce postoperative pulmonary complications is of significant value to patients and to the healthcare system. We found a number needed to treat for an additional beneficial outcome (NNTB) of four, meaning that for every four participants receiving preoperative exercise training, one less patient will develop a postoperative pulmonary complication.
Overall completeness and applicability of evidence
Data from a survey that described the management of people undergoing lung resection for lung cancer in 47 hospitals across Australia and New Zealand indicated that preoperative exercise training was provided in only four hospitals, to a 'few' patients (Cavalheri 2013). Our review suggests that patients scheduled for lung resection for NSCLC might benefit from a preoperative exercise training program. We did not find any evidence that surgery should be delayed to allow patients to undertake a preoperative exercise training program and therefore, delivery of the exercise program should take place in the available time before surgery. Preoperative exercise training has the potential to improve important patient‐focused postoperative outcomes.
Previous work demonstrated that, compared to patients undergoing lung resection who did not develop a postoperative pulmonary complication, those who developed a postoperative pulmonary complication required increase healthcare utilisation (Lugg 2016). This included more admissions to intensive care unit, longer length of hospital stay and higher readmission rates. Therefore, it could be suggested that preventing postoperative pulmonary complications may have a significant financial impact on patients and the healthcare system, although this was not a focus of our review.
Quality of the evidence
We rated the quality of evidence as low, mainly due to significant risk of bias and small sample sizes (the largest study only included 60 participants). We rated all of the studies as unclear risk of selection bias, as they did not provide sufficient information on allocation concealment, and high risk of performance bias, since none of the studies blinded study personnel or participants. Of note, blinding of personnel and participants cannot be achieved in studies of exercise training, as the personnel are required to deliver the exercise intervention, and participants are often aware of whether they are receiving usual care or exercise training. Lastly, intention‐to‐treat analysis was only reported in two studies.
Our review only included five RCTs, and since not all outcomes were measured in every study, each meta‐analysis included data from only two to four of the studies. Therefore, the low number of small studies impacted the overall quality of evidence. The low number of studies also prevented us from undertaking the planned subgroup analyses. Further RCTs are required to add data to improve the quality of evidence.
Potential biases in the review process
Our review was strengthened by a number of systematic processes followed to ensure rigor and completeness. This included the registration and publication of our protocol prior to starting the search; the use of broad search terms not restricted to language; the inclusion of two independent assessors to determine study inclusion, as well as assessing their agreement for study inclusion; and multiple attempts to contact authors of studies to clarify their suitability for inclusion, methodological details for assessment of risk of bias, and missing or unpublished outcome data. The limitation of this review was the exclusion of two studies where authors could not be contacted to clarify details required for inclusion, which added potential selection bias.
Agreements and disagreements with other studies or reviews
This is the first Cochrane review of preoperative exercise training in lung cancer. We found five published systematic reviews investigating exercise training in lung cancer (Crandall 2014; Granger 2011; Pouwels 2015; Rodriguez‐Larrad 2014; Sebio Garcia 2016). Two of these reviews also included studies examining exercise in the postoperative period for people following lung resection (Crandall 2014; Granger 2011), and one review also included perioperative physiotherapy interventions (i.e. not limited to exercise training; Rodriguez‐Larrad 2014). In contrast to our review, these previously published reviews included a wide range of study designs (i.e. RCTs, non‐RCTs, single group studies, and retrospective cohort studies), and therefore, their results should be interpreted with caution. The two most recently published systematic reviews specifically investigated the effectiveness of preoperative exercise training in people scheduled to undergo lung resection for NSCLC (Pouwels 2015; Sebio Garcia 2016). Pouwels 2015 did not include one RCT included in our review, and did not undertake meta‐analyses. Sebio Garcia 2016 included all the RCTs included in our review, and undertook meta‐analyses for lung function, length of hospital stay, and postoperative pulmonary complications. Consistent with our findings, Sebio Garcia 2016 reported a significant reduction of postoperative pulmonary complications (MD 0.55, 95% CI 0.34 to 0.89), and hospital length of stay (MD ‐4.83 days, 95% CI ‐5.90 to ‐3.76 days) with preoperative exercise training. The magnitude of difference of their findings was different to our findings, and this was likely because they included prospective non‐RCT studies and retrospective cohort studies, in addition to RCTs. Our review is the first to show the positive effect of preoperative exercise training on risk of developing a postoperative pulmonary complication, number of days patients needed an intercostal catheter postoperatively, length of hospital stay, and preoperative exercise capacity and FVC for people with NSCLC.
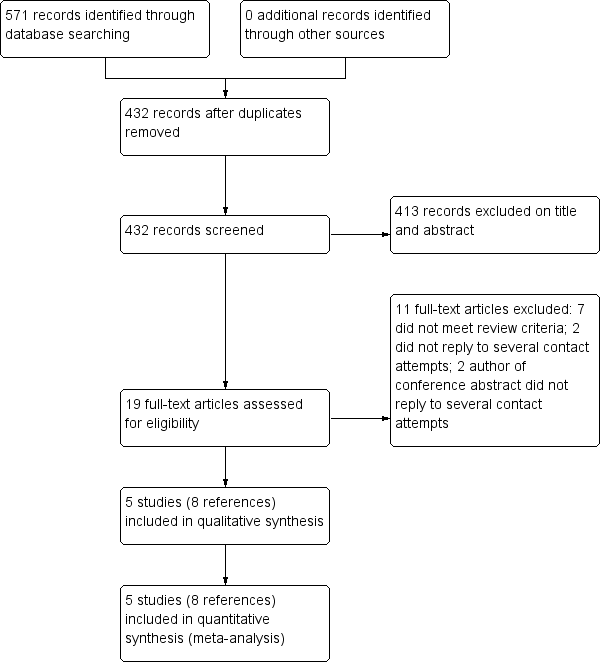
Flow diagram of references identified, excluded, and included in review
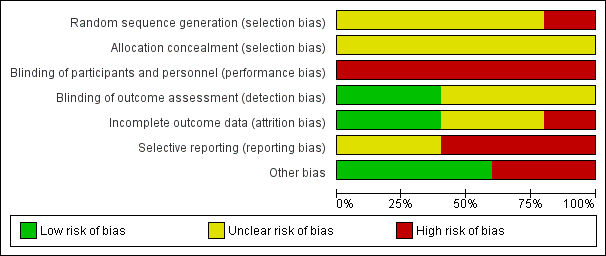
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.1 Risk of developing a postoperative pulmonary complication.
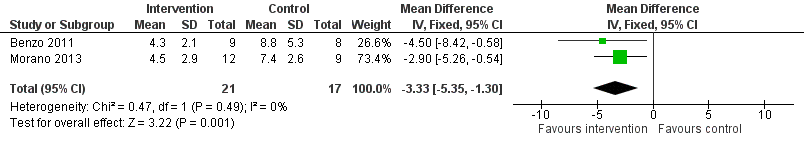
Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.2 Number of days patients needed an intercostal catheter.
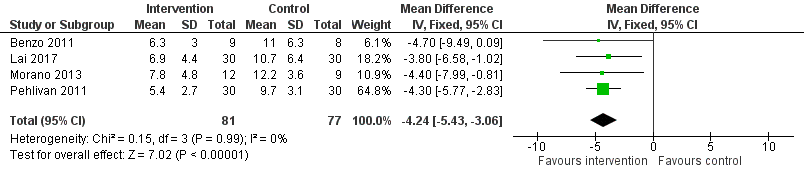
Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.3 Postoperative length of hospital stay.

Comparison 1 Intervention group versus control group, Outcome 1 Risk of developing a postoperative pulmonary complication.

Comparison 1 Intervention group versus control group, Outcome 2 Number of days patients needed an intercostal catheter.

Comparison 1 Intervention group versus control group, Outcome 3 Postoperative length of hospital stay.
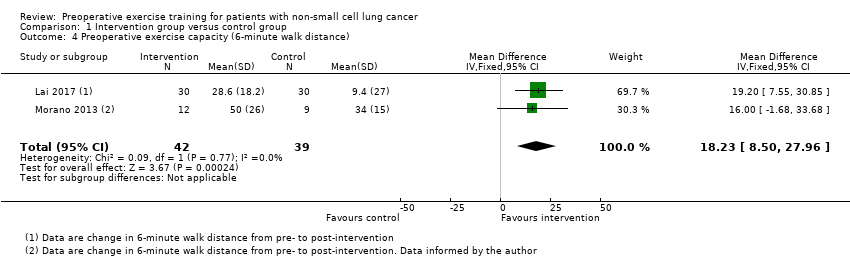
Comparison 1 Intervention group versus control group, Outcome 4 Preoperative exercise capacity (6‐minute walk distance).

Comparison 1 Intervention group versus control group, Outcome 5 Forced vital capacity (% pred).
| Preoperative exercise training compared to no exercise training for patients scheduled to undergo lung resection for non‐small cell lung cancer | ||||||
| Patient or population: patients scheduled to undergo lung resection for non‐small cell lung cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no exercise training | Risk with preoperative exercise training | |||||
| Number of patients who developed postoperative pulmonary complications | Study population | RR 0.33 | 158 | ⊕⊕⊝⊝ | ||
| 22 per 100 | 7 per 100 | |||||
| Number of days patients needed an intercostal catheter | The mean number of days patients needed an intercostal catheter in the control groups ranged from 7.4 to 8.8 days | The number of days patients needed an intercostal catheter in the intervention groups was, on average, 3.33 fewer days | ‐ | 38 | ⊕⊕⊝⊝ | |
| Postoperative length of hospital stay | The mean postoperative length of hospital stay in the control groups ranged from 9.7 to 12.2 days | The postoperative length of hospital stay in the intervention groups was, on average, 4.34 fewer days (95% CI 5.65 to 3.03 fewer days) | ‐ | 158 | ⊕⊕⊝⊝ | |
| Post‐intervention exercise capacity assessed with: 6‐minute walk distance (6MWD) | The mean post‐intervention exercise capacity in the control groups ranged from 340 to 434 metres in 6 minutes. | The post‐intervention exercise capacity in the intervention groups was, on average, 18.23 metres more | ‐ | 81 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant risk of bias across the studies 2 Small sample sizes across the studies, some with wide confidence intervals | ||||||
| Study | Results |
| Number of patients who developed a postoperative pulmonary complication: Intervention group (IG): 3 of 9 (33%) Control Group (CG): 5 of 8 (63%) P = 0.23 (between‐group) Number of days patients needed a chest tube: IG: 4.3 ± 2.1 days CG: 8.8 ± 5.3 days P = 0.03 (between‐group) Postoperative length of hospital stay: IG: 6.3 ± 3.0 days CG: 11.0 ± 6.3 days P = 0.058 (between‐group) | |
| Number of patients who developed a postoperative pulmonary complication: IG: 4 of 30 (13%) CG: 11 of 30 (37%) P = 0.037 (between‐group) Postoperative length of hospital stay: IG: 6.9 ± 4.4 days CG: 10.7 ± 6.4 days P = 0.01 (between‐group) Exercise capacity: Six‐Minute Walk Distance (6MWD), in metres: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: baseline and post‐intervention: Mean ± standard deviation (SD): IG: 431.7 ± 102.8 m to 460.3 ± 93.6 m; CG: 434.5 ± 86.2 m to 443.9 ± 88.4 m P = 0.029 (between‐group) | |
| Number of patients who developed a postoperative pulmonary complication: IG: 2 of 12 (17%) CG: 7 of 9 (78%) P = 0.01 (between‐group) Number of days patients needed a chest tube: IG: 4.5 ± 2.9 days CG: 7.4 ± 2.6 days P = 0.03 (between‐group) Postoperative length of hospital stay: IG: 7.8 ± 4.8 days CG: 12.2 ± 3.6 days P = 0.04 (between‐group) Exercise capacity: Six‐Minute Walk Distance (6MWD), in metres: IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: Mean ± standard deviation (SD): IG: 425.5 ± 85.3 m to 475 ± 86.5 m (P < 0.01); CG: 339.6 ± 107 m to 335 ± 107 m (P > 0.05) P < 0.001 (between‐group) Lung function: (i) Forced expired volume in one second (FEV1; % predicted): IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 48.1 ± 13.9% to 54.8 ± 22.4% (P = 0.08); CG: 51.7 ± 9.8% to 58.8 ± 13.0% (P = 0.23) Between‐group difference was not calculated (ii) Forced vital capacity (FVC; % predicted): IG: 12 participants completed; CG: 12 participants completed; Preoperative measurements: baseline and post‐intervention: Median (interquartile range): IG: 62.5% (49 to 71) to 76% (65 to 79.7); P = 0.02; CG: 62.5% (56 to 92) to 71% (63.2 to 89); P = 0.37 Between‐group difference was not calculated | |
| Number of patients who developed a postoperative pulmonary complication: IG: 1 of 30 (3%) CG: 5 of 30 (17%) P = 0.04 (between‐group) Postoperative length of hospital stay: IG: 5.4 ± 2.7 days CG: 9.7 ± 3.1 days P < 0.001 (between‐group) Lung function: (i) FEV1; % predicted: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: change from baseline to post‐intervention: IG: 15.84 ± 2.10%; CG: 9.92 ± 3.5% P = 0.3 (between‐group) (ii) FVC; % predicted: IG: 30 participants completed; CG: 30 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 19.26 ± 2.33%; CG: 16.3 ± 2.4% P = 0.6 (between‐group) | |
| Exercise capacity: Peak rate of oxygen uptake (VO2peak), in ml/kg/min: IG: 20 participants completed; CG: 20 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 14.9 ± 2.3 ml/kg/min to 17.8 ± 2.1 ml/kg/min; CG: 14.8 ± 1.4 ml/kg/min to 14.5 ± 1.2 ml/kg/min P < 0.001 (between‐group) Lung function: FEV1; % predicted: IG: 20 participants completed; CG: 20 participants completed; Preoperative measurements: baseline and post‐intervention: IG: 57.4 ± 19.1% to 59.8 ± 19.2%; CG: 57.6 ± 16.9% to 57.5 ± 17.0% P > 0.05 (between‐group) | |
| Intervention group (IG), Control Group (CG) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Risk of developing a postoperative pulmonary complication Show forest plot | 4 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.61] |
| 2 Number of days patients needed an intercostal catheter Show forest plot | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐5.35, ‐1.30] |
| 3 Postoperative length of hospital stay Show forest plot | 4 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐4.24 [‐5.43, ‐3.06] |
| 4 Preoperative exercise capacity (6‐minute walk distance) Show forest plot | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | 18.23 [8.50, 27.96] |
| 5 Forced vital capacity (% pred) Show forest plot | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | 2.97 [1.78, 4.16] |

