| Domain 1 ‐ Patient selection |
| Description | Describe methods of patient selection and included patients |
| Type of bias assessed | Selection bias, spectrum bias |
| Review Question | Women of reproductive age with clinically suspected endometriosis (symptoms, clinical examination ± presence of pelvic mass), scheduled for surgical exploration of pelvic/abdominal cavity for confirmation of the diagnosis ± treatment |
| Informaton collected | Study objectives, study population, selection (inclusion and exclusion criteria), study design, clinical presentation, age, number of participants enrolled and number of participants available for analysis, setting, place and period of the study |
| Signalling question 1 | Was a consecutive or random sample of patients enrolled? |
| Yes | If a consecutive sample or a random sample of the eligible patients was included in the study |
| No | If non‐consecutive sample or non‐random sample of the eligible patients was included in the study |
| Unclear | If this information was unclear |
| Signalling question 2 | Did the study avoid inappropriate exclusions? |
| Yes | If inclusion/exclusion criteria were presented and all patients with suspected endometriosis were included, with an exception for those who a) had a history of medical conditions or were on medical therapy that would have potentially interfered with interpretation of index test (e.g. malignancy, pregnancy, autoimmune disorders, infectious diseases, treatment with hormonal or immunomodulator substances); b) refused to participate in the study; or c) were unfit for surgery |
| No | If the study excluded the patients based on education level, psychosocial factors, genetic testing or phenotype or excluded patients with any co‐morbidities commonly present in general population, including a population that could have undergone a testing for endometriosis in clinical setting (hypertension, asthma, obesity, benign gastro‐intestinal or renal disease, etc) |
| Unclear | If the study did not provide clear definition of the selection (inclusion or exclusion) criteria and 'no' judgement was not applicable |
| Signalling question 3 | Was a 'two‐gate' design avoided? |
| Yes | If the study had a single set of inclusion criteria, defined by the clinical presentation (i.e. only participants in whom the target condition is suspected) ‐ a ‘single‐gate’ study design |
| No | If the study had more than one set of inclusion criteria in respect to clinical presentation (i.e. participants suspected of target condition and participants with alternative diagnosis in whom the target condition would not be suspected in clinical practice) ‐ a 'two‐gate' study design |
| Unclear | If it was unclear whether a 'two‐gate deign' was avoided or not |
| Risk of bias | Could the selection of patients have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for 3 of the above questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the included patients do not match the review question? |
| Low | If the study includes only clinically relevant population that would have undergone index test in real practice and includes representative form of target condition |
| High | If the study population differed from the population defined in the review question in terms of demographic features and co‐morbidity (e.g. studies with multiple sets of inclusion criteria with respect to clinical presentation including either healthy controls or alternative diagnosis controls that would not have undergone index test in real practice). Further, if target condition diagnosed in the study population was not representative of the entire spectrum of disease, such as limited spectrum of severity (e.g. only mild forms) or limited type of endometriosis (e.g. only DIE) |
| Unclear | If this information was unclear (e.g. severity of endometriosis was not reported) |
| Domain 2 ‐ Index test |
| Description | Describe the index test, how it was conducted and interpreted |
| Type of bias assessed | Test review bias, clinical review bias, interobserver variation bias |
| Review Question | Any type of urinary biomarkers |
| Informaton collected | Index test name, description of positive case definition by index test as reported, threshold for positive result, examiners (number, level of expertise, blinding), interobserver variability, conflict of interests |
| Signalling question 1 | Were the index test results interpreted without knowledge of the results of the reference standard? |
| Yes | If the operators performing or interpreting index test were unaware of the results of reference standard |
| No | If the operators performing or interpreting index test were not blinded to the results of reference standard |
| Unclear | If this information was unclear |
| Signalling question 2 | If a threshold was used, was it pre‐specified? |
| Yes | If study clearly provided a threshold for positive result and was defined before execution or interpretation of index test |
| No | If a threshold for positive result was not provided or not defined prior to test execution |
| Unclear | If it was unclear whether a threshold was pre‐specified or not |
| Signalling question 3 | Was a menstrual cycle phase considered in interpreting the index test? |
| Yes | If all the included participants were in the same phase of menstrual cycle or if the study reported subgroup analyses per cycle phase or if study reported the pooled estimates after impact of the cycle phase on biomarker expression was not detected |
| No | If study included participants in different phases of menstrual cycle, but effect of cycle phase on index test was not assessed |
| Unclear | If the cycle phase was not reported |
| Risk of bias | Could the conduct or interpretation of the index test have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the index test, its conduct, or interpretation differ from the review question? |
| Low | We considered all types of urinary biomarkers as eligible; therefore all the included studies were classified as 'low concern', unless 'unclear' judgement was applicable |
| High | We did not consider the studies where index tests other than urinary biomarkers were included (or excluded information on other index tests reported in addition to urine tests) or where index test looked at other target conditions not specified in the review (e.g. studies aimed at classifying pelvic masses as benign and malignant); therefore none of the included studies was classified as 'high concern' |
| Unclear | If study did not present sufficient information on at least one of the following: laboratory method, sample handling, reagents used, experience of the test operators |
| Domain 3 ‐ Reference standard |
| Description | Describe the reference standard, how it was conducted and interpreted |
| Type of bias assessed | Verification bias, bias in estimation of diagnostic accuracy due to inadequate reference standard |
| Review Question | Target condition ‐ pelvic endometriosis, ovarian endometriosis, DIE. Reference standard ‐ visualisation of endometriosis at surgery (laparoscopy or laparotomy) with or without histological confirmation |
| Informaton collected | Target condition, prevalence of target condition in the sample, reference standard, description of positive case definition by reference test as reported, examiners (number, level of expertise, blinding) |
| Signalling question 1 | Were the reference standards likely to correctly classify the target condition? |
| Yes | If the study reported at least one of the following: surgical procedure was described in sufficient details; or criteria for positive reference standard were stated; or diagnosis was confirmed by histopathology; or the procedure was performed by the team with high level of expertise in diagnosis/surgical treatment of target condition, including tertiary referral centres for endometriosis |
| No | If reference standard did not classify target condition correctly; considering the inclusion criteria and a nature of the reference standard, none of the studies were classified as 'no' for this item |
| Unclear | If information on execution of the reference standard, its interpretation or operators was unclear |
| Signalling question 2 | Were the reference standard results interpreted without knowledge of the results of the index tests? |
| Yes | If operators performing the reference test were unaware of the results of index test |
| No | If operators performing the reference test were aware of the results of index test |
| Unclear | If this information was unclear |
| Risk of bias | Could the reference standard, its conduct, or its interpretation have introduced bias? |
| Low | If 'yes' classification for all the above 2 questions |
| High | If 'no' classification for any of the above 2 questions |
| Unclear | If 'unclear' classification for any of the above 2 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the target condition as defined by the reference standard does not match the question? |
| Low | Considering the inclusion criteria, all the studies were classified as 'low concern', therefore all the included studies were classified as 'low concern' |
| High | We excluded the studies where participants did not undergo surgery for diagnosis of endometriosis, therefore none of the included studies were classified as 'high concern' |
| Unclear | Only studies were laparoscopy/laparotomy served as a reference test were included; therefore none of the included studies was classified as 'unclear concern' |
| Domain 4 ‐ Flow and timing |
| Description | Describe any patients who did not receive the index tests or reference standard or who were excluded from the 2 x 2 table, describe the interval and any interventions between index tests (sample collection) and the reference standard |
| Type of bias assessed | Disease progression bias, bias of diagnostic performance due to missing data |
| Review Question | Less than 12 months interval between index test (sample collection) and reference standard ‐ endometriosis may progress over time, so we had chosen an arbitrary time interval of 12 months as an acceptable time interval between the sample collection and surgical confirmation of diagnosis |
| Informaton collected | Time interval between index test (sample collection) and reference standard, withdrawals (overall number reported and if were explained) |
| Signalling question 1 | Was there an appropriate interval between index test (sample collection) and reference standard? |
| Yes | If time interval was reported and was less than 12 months |
| No | We excluded all the studies where time interval was longer than 12 months, therefore none of the included studies were classified as 'no' for this item |
| Unclear | If time interval was not stated clearly, but authors' description allowed to assume that the interval was reasonably short |
| Signalling question 2 | Did all women receive the same reference standard? |
| Yes | If all participants underwent laparoscopy or laparotomy as a reference standard; considering the inclusion criteria, all the studies were classified as 'yes' for this item, as anticipated |
| No | If all participants did not undergo surgery or had alternative reference standard or if only a subset of participants had surgery as reference standard, but the information on this population was not available in isolation; considering the inclusion criteria, none of the included studies were classified as 'no' for this item |
| Unclear | If this information was unclear; considering the inclusion criteria, none of the included studies were classified as 'unclear' for this item |
| Signalling question 3 | Were all women included in the analysis? |
| Yes | If all the women were included in the analysis or if women were excluded because they did not meet inclusion criteria prior to execution of index test or if the withdrawals were less than 5% of the enrolled population (arbitrary selected cut‐off) |
| No | If any patients were excluded from the analysis because of un interpretable results, inability to undergo either index test or reference standard or for unclear reasons |
| Unclear | If this information was unclear |
| Risk of bias | Could the patient flow have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |









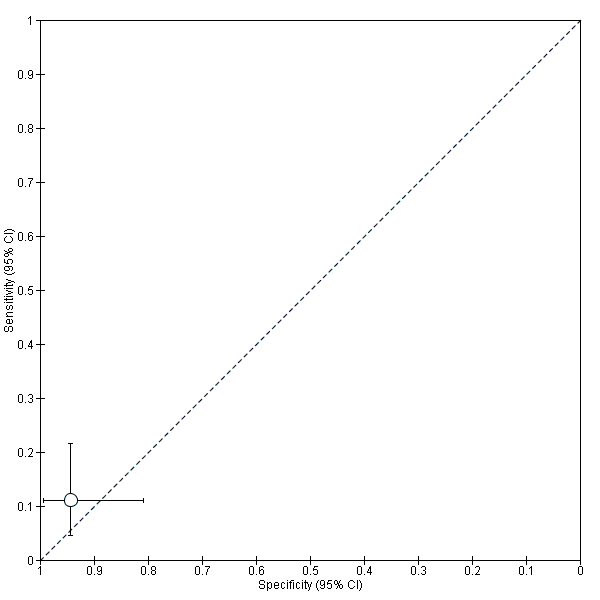






![Proteome by MALDI‐TOF‐MS (peptide m/z 1579.2 Da [collagen alpha 6(IV) chain precursor]; cut‐off not reported).](/es/cdsr/doi/10.1002/14651858.CD012019/media/CDSR/CD012019/image_n/nCD012019-TST-007.png)
![Proteome by MALDI‐TOF‐MS (peptide m/z 891.6 Da [collagen alpha1 chain precursor];; cut‐off not reported).](/es/cdsr/doi/10.1002/14651858.CD012019/media/CDSR/CD012019/image_n/nCD012019-TST-008.png)

![CK 19 [CYFRA 21‐1] (> 5.3 ng/ml).](/es/cdsr/doi/10.1002/14651858.CD012019/media/CDSR/CD012019/image_n/nCD012019-TST-010.png)
