Короткие курсы пероральных стероидов в качестве дополнительной терапии при хроническом риносинусите
Abstract
Background
This review is one of a suite of six Cochrane reviews looking at the primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is a common condition involving inflammation of the lining of the nose and paranasal sinuses. It is characterised by nasal blockage and nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Oral corticosteroids are used to control the inflammatory response and improve symptoms.
Objectives
To assess the effects of a short course of oral corticosteroids as an adjunct ('add‐on') therapy in people with chronic rhinosinusitis who are already on standard treatments.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 7); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 11 August 2015.
Selection criteria
Randomised controlled trials (RCTs) comparing a short course (up to 21 days) of oral corticosteroids to placebo or no treatment, where all patients were also receiving pharmacological treatment for chronic rhinosinusitis.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity, and the adverse event of mood or behavioural disturbances. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score, and the adverse events of insomnia, gastrointestinal disturbances and osteoporosis. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
Two trials with a total of 78 participants met the inclusion criteria. Both the populations and the 'standard' treatments differed in the two studies.
Oral steroids as an adjunct to intranasal corticosteroids
One trial in adults with nasal polyps included 30 participants. All participants used intranasal corticosteroids and were randomised to either short‐course oral steroids (oral methylprednisolone, 1 mg/kg and reduced progressively over a 21‐day treatment course) or no additional treatment. None of the primary outcome measures of interest in this review were reported by the study. There may have been an important reduction in the size of the polyps (measured by the nasal polyps score, a secondary outcome measure) in patients receiving oral steroids and intranasal corticosteroids, compared to intranasal corticosteroids alone (mean difference (MD) ‐0.46, 95% confidence interval (CI) ‐0.87 to ‐0.05; 30 participants; scale 1 to 4) at the end of treatment (21 days). This corresponds to a large effect size, but we are very uncertain about this estimate as we judged the study to be at high risk of bias. Moreover, longer‐term data were not available and the other outcomes of interest were not reported.
Oral steroids as an adjunct to antibiotics
One trial in children (mean age of eight years) without nasal polyps included 48 participants. The trial compared oral corticosteroids (oral methylprednisolone, 1 mg/kg and reduced progressively over a 15‐day treatment course) with placebo in participants who also received a 30‐day course of antibiotics. This study addressed one of the primary outcome measures (disease severity) and one secondary outcome (CT score). For disease severity the four key symptoms used to define chronic rhinosinusitis in children (nasal blockage, nasal discharge, facial pressure, cough) were combined into one score. There was a greater improvement in symptom severity 30 days after the start of treatment in patients who received oral steroids and antibiotics compared with placebo and antibiotics (MD ‐7.10, 95% CI ‐9.59 to ‐4.61; 45 participants; scale 0 to 40). The observed mean difference corresponds to a large effect size. At the same time point there was a difference in CT scan score (MD ‐2.90, 95% CI ‐4.91 to ‐0.89; 45 participants; scale 0 to 24). We assessed the quality of the evidence to be low.
There were no data available for the longer term (three months).
Authors' conclusions
There might be an improvement in symptom severity, polyps size and condition of the sinuses when assessed using CT scans in patients taking oral corticosteroids when these are used as an adjunct therapy to antibiotics or intranasal corticosteroids, but the quality of the evidence supporting this is low orvery low (we are uncertain about the effect estimate; the true effect may be substantially different from the estimate of the effect). It is unclear whether the benefits of oral corticosteroids as an adjunct therapy are sustained beyond the short follow‐up period reported (up to 30 days), as no longer‐term data were available.
There were no data in this review about the adverse effects associated with short courses of oral corticosteroids as an adjunct therapy.
More research in this area, particularly research evaluating longer‐term outcomes and adverse effects, is required.
PICO
Резюме на простом языке
Краткосрочное применение пероральных кортикостероидов в дополнение к другим методам лечения хронического риносинусита
Вопрос обзора
Мы рассмотрели доказательства о пользе и вреде добавления короткого курса (как правило, до 14 дней) кортикостероидов, принимаемых через рот, людям с хроническим риносинуситом, которые также получают другой вид лечения (например, кортикостероиды, доставляемые через нос).
Актуальность
Хронический риносинусит ‐ распространенное состояние, характеризующееся воспалением носа и околоносовых пазух (группа заполненных воздухом пространств позади носа, глаз и щек). У пациентов с хроническим риносинуситом встречаются, по меньшей мере, два или более из следующих симптомов, по меньшей мере, в течение 12 недель: заложенность носа, выделения из носа или насморк, боль или чувство давления в области лица и/или пониженное обоняние (гипосмия). У некоторых людей также бывают полипы в носу, представляющие собой похожие на виноград набухания нормальной слизистой оболочки внутри носовых ходов и носовых пазух.
Короткие курсы пероральных кортикостероидов широко используют для лечения хронического риносинусита. Они действуют, регулируя воспалительный ответ, и при наличии полипов они быстро уменьшают размер полипов и уменьшают выраженность симптомов. Нежелательные эффекты кортикостероидов могут включать в себя бессонницу, изменения настроения и желудочно‐кишечные расстройства (например, боли в желудке, изжогу, диарею, запор, тошноту и рвоту). Когда кортикостероиды применяют в течение длительного времени или применяют многократно короткими курсами также возможно развитие остеопороза (хрупкие кости).
Характеристика исследований
Этот обзор включает в себя доказательства по состоянию на 11 августа 2015 года. Мы включили два рандомизированных контролируемых клинических испытания с общим числом участников 78.
В одном исследовании приняли участие 30 взрослых с носовыми полипами. Участники получали либо интраназальные и пероральные кортикостероиды, либо только интраназальные кортикостероиды. Единственным результатом, представляющим интерес для этого обзора, о котором сообщалось, был ответ на вопрос, уменьшается ли размеров полипов носа по завершении этих сравниваемых вариантов лечения (три недели).
В еще одном исследовании участвовали 48 детей (средний возраст восемь лет) с хроническим риносинуситом, но без носовых полипов. Участники получали либо антибиотики и пероральные кортикостероиды либо только антибиотики и плацебо (таблетки сахара). Пероральные кортикостероиды и плацебо давали в течение 15 дней, антибиотики ‐ в течение 30 дней. В клиническом испытании сообщили о результатах,по завершении лечения антибиотиками (через один месяц).
Основные результаты
В конце трехнедельного курса лечения, люди, которые принимали как интраназальные кортикостероиды, так и пероральные стероиды, возможно, имели меньшие носовые полипы, чем люди, которые получали только интраназальные кортикостероиды. В клиническом испытании не следили за людьми, для определения увеличения размеров полипа после его окончания. Клиническое испытание не предоставило информацию о неблагоприятных событиях или других исходах, важных для пациентов, таких как тяжесть симптомов или качество жизни.
Дети, получавшие и антибиотики, и пероральные кортикостероиды, казалось, имели более низкий общий балл оценки симптомов и лучшие баллы, получаемые при компьютерно‐томографическом сканировании (КТ), после лечения по сравнению с детьми, которые получали антибиотики и контрольное лечение. Отчетность о побочных эффектах в этом исследовании была не очень ясной, и поэтому трудно сказать, испытывал ли кто‐либо из участников желудочно‐кишечные расстройства, изменения настроения или трудности со сном.
Качество доказательств
Мы оценили качество доказательств для пероральных стероидов в комбинации с интраназальными стероидами у взрослых с полипами в носу ‐ как очень низкое (мы очень не уверены в оценке), так как доказательство исходит из одного клинического испытания, которое имеет малое число участников. Клиническое испытание имело высокий риск смещения из‐за способа его проведения. Клиническое испытание не сообщало о побочных эффектах и не сообщало о результатах после окончания лечения.
Мы оценили качество доказательств для пероральных стероидов в комбинации с антибиотиками у детей ‐ как низкое (необходимы дальнейшие исследования, которые, весьма вероятно, окажут значительное влияние на нашу уверенность в оценке эффекта и, вероятно, изменят оценку), так как доказательства исходят от одного небольшого испытания. Клиническое испытание не имело высокого риска смещения, но оно включало только детей, без носовых полипов, которые не могут иметь те же результаты, что и взрослые с носовыми полипами. Клиническое испытание не сообщило о результатах после окончания лечения и информация о побочных эффектах лечения была плохо представлена.
Authors' conclusions
Summary of findings
| Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both arms) for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis | ||||||
| Outcomes No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life | — | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score | — | No RCT reported this outcome | ||||
| Adverse effect: mood or behavioural disturbances | — | No RCT reported this outcome | ||||
| Health‐related quality of life | — | No RCT reported this outcome | ||||
| Adverse effect: insomnia | — | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances ‐ not measured | — | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Short‐course oral corticosteroids compared to placebo (antibiotics in both groups) for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis | |||||
| Outcomes No of participants (studies) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | |||
| Disease‐specific health‐related quality of life | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score, assessed with: 4 individual symptoms measured on 0 to 10 visual analogue scale summed to provide a range of 0 to 40 No. of participants: 45 | The mean disease severity score without oral steroids was 15.2 | The mean disease severity score with oral steroids was 3.6 | The mean disease severity score in the intervention group was 7.10 lower (9.59 lower to 4.61 lower) | ⊕⊕⊝⊝ | A lower score indicates less severe symptoms. The results relate to a standardised mean difference of 1.61 standard deviations lower (‐2.29 to 0.93 lower), corresponding to a large difference. |
| Adverse effect: mood or behavioural disturbances | No RCT reported this outcome | ||||
| Health‐related quality of life | No RCT reported this outcome | ||||
| Adverse effect: insomnia | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Study completed only in children (mean age 8 years old). Study follow‐up time was less than 3 months (1 month). Scales were not validated and were completed by "parents and children". 2Symptoms included in this score were: purulent nasal discharge, nasal obstruction, cough and facial pain/headache. | |||||
Background
Description of the condition
Chronic rhinosinusitis is defined as inflammation of the nose and paranasal sinuses characterised by two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip). The other possible symptoms include facial pain/pressure, reduction or loss of sense of smell (in adults) or cough (in children). Symptoms must have continued for at least 12 weeks. In addition, people must have either mucosal changes within the ostiomeatal complex and/or sinuses as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms, including nasal obstruction, nasal discharge, facial pain, anosmia and sleep disturbance, have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment and intracranial infection.
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore potential differences between them.
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline.
Description of the intervention
Short courses of oral steroids are widely used in medicine for a variety of inflammatory conditions. In patients with chronic rhinosinusitis they are often used with a view to gaining a rapid improvement in symptoms and to allow improved access for topically applied agents. They are often given over a seven‐ to 21‐day period and may be at a fixed dose or a reducing dose over the course. This strategy is thought to reduce the risk of adverse effects (Mygind 1996). A wide spectrum of adverse events are reported with systemic steroid usage (see Table 1); however, data on the incidence in association with chronic rhinosinusitis are lacking. While it is possible to extrapolate findings from trials in other diseases, there is a risk that the incidence is disease‐specific; for example, a high incidence of avascular necrosis is seen with high‐dose steroid use in systemic lupus erythematosus, which is in part attributed to the underlying disease process and severity as well as the higher dosages prescribed in severe disease (Da Silva 2006).
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare; appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent, occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
References: Da Silva 2006; Naber 1996; Stanbury 1998
NSAIDs: non‐steroidal anti‐inflammatory drugs
Adverse effects associated with short‐term oral steroid use are said to include gastrointestinal disturbances, insomnia and altered mental states. However, there are few or no published data on the frequency of these effects when short‐term courses are prescribed. Adverse effects associated with long‐term use of oral steroids are also listed in Table 1.
How the intervention might work
Short courses of oral steroids are most often used in patients with chronic rhinosinusitis and nasal polyps. The intention is to reduce the inflammation in order to produce a rapid reduction in the size of the polyps, to improve symptoms and allow better penetration of topical treatments into the nasal cavity. They may be used in a similar way for patients with chronic rhinosinusitis without polyps, who have severe nasal obstruction or complete anosmia (loss of sense of smell). The initial effect of treatment is expected to be immediate. Any observed improvement may continue, especially if one effect of the intervention is to improve the bio‐availability of an adjunct treatment.
There is, however, a lack of evidence regarding the optimal treatment regimen of oral steroids with respect to indication, dose and duration. The optimum usage of steroids is clinically important as it may reduce the need for surgery by providing good symptomatic control.
Why it is important to do this review
Short courses of oral steroids are widely used as a form of add‐on therapy in patients with chronic rhinosinusitis. This review and a closely related new review of 'Short‐course oral steroids alone for chronic rhinosinusitis', Head 2016a, update and expand a previous Cochrane review that looked at this treatment in patients with nasal polyps (Martinez‐Devesa 2011). This review seeks to establish the additional benefits (and harms) of steroids, when added on to existing therapies for chronic rhinosinusitis. In contrast, the companion review will seek to establish the relative effectiveness (and harms) of oral steroids when compared to no treatment or other commonly used agents for chronic rhinosinusitis (such as intranasal corticosteroids).
This review is one of a suite of Cochrane reviews looking at common management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b), and we use the same outcome measures across the reviews. We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on attempting to assess the impact of the intervention on the surgical procedure or to modify the post‐surgical results.
Objectives
To assess the effects of a short course of oral corticosteroids as an adjunct ('add‐on') therapy in people with chronic rhinosinusitis who are already on standard treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
-
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only to be included if the data from the first phase were available); and
-
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
-
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
-
perioperative studies, where the sole purpose of the study was to investigate the effect of the intervention on surgical outcome.
Types of participants
Patients with chronic rhinosinusitis, whether with polyps or without polyps.
We excluded studies that included a majority of patients with:
-
cystic fibrosis;
-
allergic fungal sinusitis/eosinophilic fungal/mucinous rhinosinusitis;
-
aspirin‐exacerbated respiratory disease;
-
antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus);
-
malignant polyps;
-
primary ciliary dyskinesia;
-
gross immunodeficiency (congenital or acquired);
-
a history of surgery for nasal polyps within six weeks of entry to the study.
Types of interventions
We included all short (see below) courses of oral steroids, regardless of dose. This includes:
-
prednisone;
-
prednisolone;
-
methylprednisolone;
-
hydrocortisone;
-
cortisone acetate.
Short courses of oral steroids are defined as lasting up to, but not exceeding, 21 days.
The main comparators were:
-
oral steroids plus intranasal corticosteroids versus placebo or no treatment plus intranasal corticosteroids.
Other possible comparison pairs included:
-
oral steroid plus co‐intervention X versus placebo/no treatment plus co‐intervention X ('co‐intervention X' refers to any of the other possible co‐interventions).
This review is part of a larger series of six reviews for the treatment of chronic rhinosinusitis:
-
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis (Chong 2016b).
-
Different types of intranasal steroids for chronic rhinosinusitis (Chong 2016a). This review compares different classes, doses and delivery methods of intranasal corticosteroids for chronic rhinosinusitis.
-
Short‐course oral steroids alone for chronic rhinosinusitis (Head 2016a). This review compares short‐course oral steroids alone with placebo or no intervention, or against other pharmacological interventions such as antibiotics or nasal saline irrigation.
-
Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis (this review). This review compares oral steroids where they have been used as add‐on therapy to other treatments for chronic rhinosinusitis (such as intranasal corticosteroids, antibiotics or saline solution).
-
Saline irrigation for chronic rhinosinusitis (Chong 2016c). This review compares nasal saline irrigation for chronic rhinosinusitis with both placebo/no intervention and with intranasal corticosteroids or antibiotics.
-
Systemic and topical antibiotics for chronic rhinosinusitis (Head 2016b). This review compares both topical and systemic antibiotics with placebo/no treatment, two different antibiotics with each other and antibiotics with intranasal corticosteroids.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Both short‐term (at the end of treatment) and long‐term effects are important, therefore we evaluated outcomes at the end of treatment or within three weeks thereof in addition to three to six months, six to 12 months and more than 12 months. For adverse events, we analysed data from the longest time periods.
Primary outcomes
-
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
-
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales). In the absence of validated symptom score data, patient‐reported individual symptom scores were reported for the following symptoms: nasal obstruction/blockage/congestion, nasal discharge (rhinorrhoea), facial pressure/pain, loss of sense of smell (adults), cough (children).
-
Significant adverse effect: mood or behavioural disturbances.
Secondary outcomes
-
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
-
Other adverse effects: gastrointestinal disturbances.
-
Other adverse effects: insomnia.
-
Other adverse effects: osteoporosis (where follow‐up was at least six months).
-
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
-
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
The adverse events that we aimed to collect from studies including one of the various comparators listed above were the same as those adverse events identified in the methods section of the companion reviews assessing the effects of those interventions as primary treatments. For example, for studies in which all participants received intranasal corticosteroids, the list of adverse events will also include those specifically for intranasal corticosteroids as found in Chong 2016a and Chong 2016b.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 11 August 2015.
Electronic searches
The Information Specialist searched:
-
the Cochrane Register of Studies ENT Trials Register (searched 11 August 2015);
-
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7);
-
Ovid MEDLINE (1946 to July week 5 2015);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 11 August 2015);
-
PubMed (as a top up to searches in Ovid MEDLINE) (searched 11 August 2015);
-
-
Ovid EMBASE (1974 to 2015 week 32);
-
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies) (searched 11 August 2015);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 11 August 2015);
-
Google Scholar (searched 11 August 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. The same two review authors evaluated the full text of each potentially relevant study to determine if it met the inclusion and exclusion criteria for this review.
We resolved differences by discussion and consensus, with the involvement of a third author for clinical and/methodological input where necessary.
Data extraction and management
Two review authors independently extracted data from each study using a standardised data collection form (see Appendix 2). If we had found a study that had more than one publication, we would have aimed to retrieve all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We would have contacted the original study authors for clarification or for missing data whenever needed. If differences had have been found between publications of a study, we would have contacted the original authors for clarification. We would have used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, these included:
-
presence or absence of nasal polyps;
-
baseline nasal polyp score (where appropriate);
-
whether the patient has had previous sinus surgery;
-
number of previous courses of oral steroids.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
-
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
-
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
-
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may report data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'medium‐term' follow‐up periods, our time point is defined as 'three to six months' post‐randomisation. If a study reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, personnel and outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Measures of treatment effect
If dichotomous outcomes were found, we would have summarised the effects (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with CIs. For the key outcomes that we presented in the 'Summary of findings' table, we would have also expressed the results as absolute numbers and compared to the assumed risk. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011).
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD) or as standardised mean difference (SMD) if different scales have been used to measure the same outcome. We provided a clinical interpretation of the SMD values.
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we would have analysed these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We would have tried to contact study authors via email whenever the outcome of interest was not reported, if the methods of the study had suggested that the outcome had been measured, or where data presented in the paper were in graphical format, in order to try to obtain the study values for the study results. We would have done the same if not all data required for meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we would have approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If it was impossible to estimate these, we would have contacted the study authors. Apart from imputations for missing standard deviations, no other imputations were planned. However, we had to carry out calculations relating to disease severity (reported as symptom scores) as most of the data were not measured using validated instruments nor reported in a way that was comparable across studies (see 'Imputing total symptom scores' below).
We extracted and analysed all data using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but did present data for the results of individual symptoms, we used the symptoms covering the important domains of the EPOS chronic rhinosinusitis diagnosis criteria, EPOS 2012, to calculate a total symptom score. The EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge; other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where mean final values or changes from baseline were presented in the paper for the individual symptoms we summed these to calculate a 'total symptom score'. We calculated standard deviations for the total symptom score as if the symptoms were independent, random variables that were normally distributed. We acknowledge that there is likely to be a degree of correlation between the individual symptoms, however we used this process because the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Assessment of heterogeneity
If we had found more than one study for each of the comparisons, we would have assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
If heterogeneity had been detected we would have assessed it by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. When the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias is likely to occur in a meta‐analysis. We sought further information from the study authors. If no further information could be obtained, we noted this as being a 'high' risk of bias. Quite often there was insufficient information to judge the risk of bias; we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We would have assessed funnel plots if sufficient trials (more than 10) were available for an outcome. If asymmetry of the funnel plot was observed, we would have conducted more formal investigation using the methods proposed by Egger 1997.
Data synthesis
If we had found more than one study in within a comparison pair, we would have conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). If we had found dichotomous data, we would have analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We would have analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if we had found that data were from the same scale, we might have pooled mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measure, we would not have pooled change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
Had we found more than one study for each comparison pair, we would have conducted the following subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers:
-
whether patients had chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, were a mixed group or the status of polyps is not known or not reported.
We would have undertaken the subgroup analysis as although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; Fokkens 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence pointing to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011).
We would have presented the main analyses of this review according to the subgroups of phenotypes of chronic rhinosinusitis. We would have presented all other subgroup analysis results in tables.
None of the studies had a mixed group of patients. If a mixed group of patients had been found within the paper, we would have analysed the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients had belonged to one category.
In addition to the subgroups above, we had planned to conduct the following subgroup analyses in the presence of statistical heterogeneity:
-
patient age (children versus adults);
-
dose;
-
duration of treatment.
Sensitivity analysis
Had we found more than one study for each comparison, we would have carried out sensitivity analyses to determine whether the findings are robust to the decisions made in the course of identifying, screening and analysing the trials. We had planned to conduct sensitivity analysis for the following factors, whenever possible:
-
impact of model chosen: fixed‐effect versus random‐effects model;
-
risk of bias of included studies: excluding studies with high risk of bias (we define these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed);
-
how outcomes were measured: we would have investigated the impact of including data where the validity of the measurement is unclear.
If any of these investigations had found a difference in the size of the effect or heterogeneity, we would have mentioned this in the Effects of interventions section.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence for each outcome using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision;
-
publication bias.
The 'Summary of findings' table presents only the six top priority outcomes (disease‐specific health‐related quality of life, disease severity score, generic quality of life and three adverse effects: mood disturbances, gastrointestinal disturbance and insomnia). We did not include the outcomes of endoscopic score, CT scan score or the adverse effect osteoporosis in the 'Summary of findings' table. Similarly we did not present the results for the individual symptoms in the 'Summary of findings' table.
Results
Description of studies
Results of the search
The searches retrieved a total of 2470 references. We screened the titles and abstracts and subsequently removed 2424 studies. We assessed 46 full texts for eligibility and excluded 43 studies. We included two studies and identified one ongoing study. No studies are awaiting assessment.
A flow chart of study retrieval and selection is provided in Figure 1.
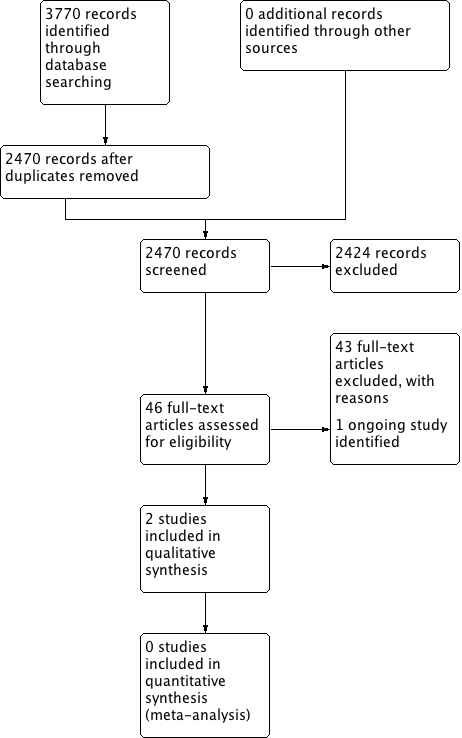
Process for sifting search results and selecting studies for inclusion.
Included studies
Two studies are included in the review (Bülbül 2013; Ozturk 2011). See Characteristics of included studies.
Design
Bülbül 2013 is an unblinded, parallel‐group, quasi‐randomised controlled trial (randomised by order of presentation). Ozturk 2011 is a double‐blind, parallel‐group, randomised, placebo‐controlled trial.
Setting
Both studies were conducted in Turkey. Bülbül 2013 was a single‐site study conducted in a university hospital outpatient ear, nose and throat department, whereas Ozturk 2011 was conducted in the paediatric ear, nose and throat outpatients clinics of two university hospitals.
Participants and sample size
In Bülbül 2013, the two study arms that met the inclusion criteria for this review consisted of 30 adults (mean age 34.73 ± 16.72) with a diagnosis of bilateral nasal polyps on endoscopic examination. Ozturk 2011 included 48 children with a mean age in the oral steroids group of 8.5 ± 2.9 years and a mean age in the placebo group of 8.0 ± 2.3 years. All patients had chronic rhinosinusitis without nasal polyps. The diagnosis of chronic rhinosinusitis was based of sinonasal symptoms and signs present for a period of more than three months in the presence of abnormalities on coronal sinus CT scans.
Interventions
Bülbül 2013 compared intranasal steroids alone (budesonide), oral steroids alone (methylprednisolone) and treatment with both intranasal steroids and oral steroids. No placebo was used. The treatment time was 21 days for all arms. For this review, the only comparison of interest was the group receiving oral steroids and intranasal steroids compared with the group receiving intranasal steroid alone (i.e. oral steroids compared with no treatment, with both groups receiving concurrent treatment with an intranasal steroid).
In Ozturk 2011, methylprednisolone was prescribed for 15 days at a dose adjusted to the weight of the child (1 mg/kg/day, maximum of 40 mg for 10 days) and reduced over the treatment time. Patients in the control arm received identical‐looking lactose tablets as placebo. In addition, children in both arms of the study received broad‐spectrum antibiotics (oral amoxicillin/clavulanate at 45/6.3 mg/kg/day, maximum of 2000/285 mg/day) for 30 days as concurrent treatment.
Outcomes
Bülbül 2013 aimed to investigate the effects of glucocorticoids on chronic rhinosinusitis with nasal polyps with detection of inflammatory response by measurement of nitric oxide levels in nasal polyp tissue. The only outcome of interest to this review was the secondary outcome of endoscopic nasal polyp score measured with Rasp criteria. No information about this scale was presented in the paper but Côté 2011 indicates that it is a four‐point scale, graded from 1 to 4 (1 = least severe, 4 = most severe). The outcomes were reported at 21 days, at the end of the treatment course. The study did not mention whether they measured any adverse events.
Ozturk 2011 presents the primary outcome of total symptom score (comprising a cumulative score for the individual symptoms of purulent nasal discharge, nasal obstruction, postnasal drainage, halitosis, cough and facial pain/headache measured using a visual analogue scale (VAS) of 0 to 10 (0 = none, 10 = most severe, non‐validated) and a CT scan score measured with the Lund‐Mackay scoring system (0 to 24; 0 = least severe, 24 = most severe). The study also specified that "tolerability was evaluated by means of medical history, physical examination, and measurement of adverse events. Hypertension, edema, weight gain, increase in appetite, gastrointestinal disturbances, nervousness, agitation, psychosis, headache, mood swings, delirium, euphoria, moon face, skin atrophy, bruising, hyperpigmentation, muscle weakness, joint pain, and allergic reactions were defined as clinically significant adverse events".
Excluded studies
We excluded 43 papers after reviewing the full paper. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. We identified 19 of these from the excluded papers list in the previous version of the Cochrane review (Martinez‐Devesa 2011). The reasons for exclusion from the previous review were found to be still valid under the updated inclusion criteria developed for this review (Alobid 2005; Blomqvist 2001; Blomqvist 2009; Bonfils 1998; Bonfils 2003; Bonfils 2006; Chi Chan 1996; Damm 1999; Hessler 2007; Jankowski 2003a; Jankowski 2003b; Kroflic 2006; Lildholdt 1988; Lildholdt 1989; Nores 2003; Ragab 2006; Rasp 2000; Sieskiewicz 2006; Stevens 2001).
Thirteen papers were reporting RCTs comparing oral steroid treatment with placebo or no treatment, but study participants did not receive any other concurrent treatment (Alobid 2006; Alobid 2012; Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2011; Kirtsreesakul 2012; Martinez‐Anton 2008; Vaidyanathan 2011; Van Zele 2008; Van Zele 2010). These studies are included in the Cochrane review of oral steroids alone for chronic rhinosinusitis (Head 2016a). We found three protocols for ongoing RCTs: none of these studies appeared to use oral steroids as an adjunct to other treatment (Chi 2011; NCT00841802; NCT02367118).
Of the remaining eight papers, one included a population of people with allergic fungal rhinosinusitis (Rupa 2010), one compared oral steroids with intranasal steroids, but participants did not receive any background treatments (Reychler 2015), and six were either non‐randomised studies or commentaries on existing RCTs (Grammer 2013; Rasp 1997; Remer 2005; Sousa 2009; Tuncer 2003; van Camp 1994).
Ongoing studies
One ongoing study is being conducted to investigate the efficacy of oral steroids (prednisone 40 mg in reducing doses for 20 days) followed by intranasal steroids (mometasone), compared with intranasal steroids alone (mometasone) in adults with chronic rhinosinusitis without nasal polyps (NCT01676415). Both groups also had a concurrent course of antibiotics lasting for three weeks. The study is due to report results during 2016. See Characteristics of ongoing studies.
Risk of bias in included studies
For details of the risk of bias in the included studies see the 'Risk of bias' tables (Characteristics of included studies). Details of the risk of bias for each study can be found in Figure 2. A 'Risk of bias' graph shows our judgements about each risk of bias item presented as percentages across all included studies (Figure 3).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
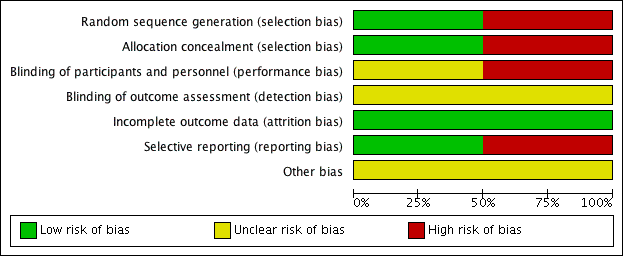
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Both the included studies were randomised and controlled. We assessed Bülbül 2013 as high risk of bias for sequence generation as it was quasi‐randomised, with the allocation to the arm of the trial being completed based on the order of presentation. Ozturk 2011 used a random allocation chart based on a table of random numbers to generate the sequence.
Allocation concealment
We assessed Bülbül 2013 as high risk of bias for allocation concealment as the allocation to the arm of the study was completed by order of presentation, which allows the people allocating participants to treatment group to know exactly which arm participants are being allocated to. We assessed Ozturk 2011 as having a low risk of bias for allocation concealment as sealed envelopes were used to prevent the healthcare personnel from influencing allocation of participants in the study.
Blinding
As Bülbül 2013 lacked a placebo arm, we assessed that there was a high risk of bias for blinding of participants and personnel. In addition, it did not report whether outcome assessment was blinded; we therefore assessed this to be an unclear risk. We assessed Ozturk 2011 to be of unclear risk of bias for blinding of participants and personnel. Although the methods for blinding were well explained in the paper and efforts were made to keep the size and appearance of the placebo the same as the active treatment, there was no discussion on the taste of the tablets. Methylprednisolone has a distinctive, bitter taste that is different to the slightly sweet taste of lactose. This may have allowed the blinding to be compromised and it is noted that one patient did drop out of the study due to the unpalatability of the active tablet. The method for standardising reporting by outcome assessors for CT scans was mentioned in the paper but the risk was unclear for the other outcomes, since these are mostly patient/parent‐reported and the effectiveness of blinding using the placebo was unclear.
Incomplete outcome data
We assessed both studies to have a low risk of bias for incomplete outcome data. Bülbül 2013 reported that there were no patients who dropped out of the study whereas Ozturk 2011 reported a low rate of participant drop‐out (3 out of 48 (6%)).
Selective reporting
We found no protocols for either study (Bülbül 2013; Ozturk 2011).
We assessed Bülbül 2013 to have a low risk of reporting bias. The outcomes listed in the methods section were all presented in the results section although the study did not present any information about adverse events.
We assessed Ozturk 2011 to be at a high risk of bias for selective reporting. There appears to be a discrepancy between the adverse events that were planned to be reported in the methods section and the results section, which stated that "No clinically significant adverse events were reported". However, the paper follows this statement by reporting a number of patients with increase in appetite and weight gain, which were classified as "clinically significant adverse events" in the methods section of the paper. No information was provided about other types of adverse events and we cannot be certain that there were no events. In addition, the summed scores for the individual symptom scores as presented in the paper do not add up to the total symptom score as presented and no information is presented with regard to any adjustments or weighting that may have been made.
Other potential sources of bias
Use of validated outcome measures
The validation of outcomes was one area of potential bias that we identified as relevant at the start of the review. Bülbül 2013 did not provide information regarding the validation of the outcome measures. Similarly, Ozturk 2011 did not mention whether the measures they used for assessing outcomes were validated and this is particularly a concern when symptom severity was "... assessed in the patients and their parents" using visual analogue scales.
Funding and conflict of interests in trials
No funding information was presented for either trial (Bülbül 2013; Ozturk 2011). With regards to conflicts of interest, Bülbül 2013 stated that there were "None declared" and Ozturk 2011 declared that "The authors have declared that they have no conflicts of interest".
Baseline characteristics
Bülbül 2013 was a poorly reported study and did not present details of the baseline characteristics for each group. There was a non‐significant difference between the groups for the severity of nasal polyps, with the placebo group containing the more severely affected patients. The small size of the trial makes it difficult to draw conclusions.
The baseline characteristics for the two groups in Ozturk 2011 were similar with no significant differences between the groups in any of the characteristics presented including age, duration of symptoms or presence of atopy.
Effects of interventions
See: Summary of findings for the main comparison Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both groups) for chronic rhinosinusitis; Summary of findings 2 Short‐course oral corticosteroids compared to placebo (antibiotics in both arms) for chronic rhinosinusitis
Two trials comprising two different comparison pairs were included in this review. Bülbül 2013 compared oral steroids with a background of intranasal corticosteroids in 30 adults with chronic rhinosinusitis and nasal polyps. Ozturk 2011 investigated oral steroids with a background of antibiotics in 48 children with chronic rhinosinusitis without nasal polyps. The results of these two comparison pairs are discussed separately.
Where the range of scales and values for minimal important differences were unclear, we used the standardised mean difference (SMD) as a guide to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD, or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1988).
Oral steroids as an adjunct to intranasal corticosteroids
See also summary of findings Table for the main comparison.
Primary outcomes
1. Disease‐specific health‐related quality of life
The study did not report this as an outcome.
2. Disease severity ‐ symptoms score
The study did not report this as an outcome. Individual symptom scores were not reported.
3. Significant adverse effect: mood or behavioural disturbances
The study did not report this as an outcome.
Secondary outcomes
1. General health‐related quality of life
The study did not report this as an outcome.
2. Other adverse event: gastrointestinal disturbances
The study did not report this as an outcome.
3. Other adverse event: insomnia
The study did not report this as an outcome.
4. Other adverse event: osteoporosis
The study did not report this as an outcome.
5. Endoscopic scores (including nasal polyps score)
Nasal polyps scores were measured after treatment (21 days) in Bülbül 2013 using the Rasp criteria, although no explanation of, or reference to, the criteria or validation thereof was made within the paper. Further investigation into this scale appears to indicate that the Rasp criteria rate the severity of nasal polyps on a four‐point scale (1 to 4, 1 = least severe) (Côté 2011). The results were available for 30 patients and showed that there might be an improvement in mean nasal polyp size for the population that received oral and intranasal steroids, compared with the group receiving intranasal steroids alone (mean difference (MD) ‐0.46, 95% confidence interval (CI) ‐0.87 to ‐0.05) (Analysis 1.1). The observed mean difference corresponds to a large effect size (SMD of 0.79). This result is not presented in the 'Summary of findings' table as we did not consider it to be a priority outcome.
6. Computerised tomography (CT) scan score
The study did not report this outcome.
Oral steroids as an adjunct to antibiotics
See also summary of findings Table 2.
Primary outcomes
1. Disease‐specific health‐related quality of life
The study did not report this as an outcome.
2. Disease severity ‐ symptoms score
Ozturk 2011 presented results for a total symptom score after treatment (30 days) as assessed by patient and parents. The symptoms measured were purulent nasal discharge, nasal obstruction, postnasal drainage, halitosis, cough and facial pain/headache using a visual analogue scale (range of 0 to 10, 0 = "none", 10 = "most severe"). We combined the four individual scores that related to elements of the EPOS 2012 diagnostic criteria (purulent nasal discharge, nasal obstruction, cough and facial pain/headache) to make a total symptom score with a range from 0 to 40 at the end of treatment. Results for 45 children were available and showed a lower score in the oral steroids group at 30 days (MD ‐7.10, 95% CI ‐9.59 to ‐4.61) (Analysis 2.1). The observed mean difference corresponds to a large effect size (SMD of 1.61).
Ozturk 2011 also presents results for individual symptom scores for each of the four domains from the EPOS 2012 definition criteria for chronic rhinosinusitis: purulent nasal discharge, nasal obstruction, cough and facial pain or headache.
Nasal obstruction/congestion/blockage
Ozturk 2011 (45 participants) at the end of the trial (30 days) showed an improvement in nasal blockage in favour of the group receiving oral steroid and antibiotics compared with the group that received antibiotics alone (MD ‐3.50, 95% CI ‐4.71 to ‐2.29) (Analysis 2.2).
Nasal discharge
Ozturk 2011 measured 'purulent nasal discharge' in 45 participants and showed no improvement in discharge between the groups at the end of the trial (MD ‐0.20, 95% CI ‐1.54 to 1.14) (Analysis 2.3).
Facial pain or headache
Ozturk 2011 (45 participants) showed an improvement in facial pain or headache in favour of the group with oral steroids in addition to antibiotics at the end of the trial (MD ‐1.30, 95% CI ‐2.55 to ‐0.05) (Analysis 2.4).
Cough
Ozturk 2011 (45 participants) showed an improvement in cough in favour of the oral steroid group at the end of the trial (MD ‐2.10, 95% CI ‐3.35 to ‐0.85) (Analysis 2.5).
None of the results for individual symptoms are presented in the GRADE 'Summary of findings' table as we did not consider them to be priority outcomes.
3. Significant adverse effect: mood or behavioural disturbances
Ozturk 2011 states that "No clinically significant adverse events were reported", but does not provide further information for any of the pre‐specified adverse events in the protocol.
Secondary outcomes
1. General health‐related quality of life
The study did not report this as an outcome.
2. Other adverse event: gastrointestinal disturbances
Ozturk 2011 listed "gastrointestinal disturbances" as a "clinical significant adverse event". Although the report states that "No clinically significant adverse events were reported", the authors noted that "increase in appetite" was reported in 16/24 patients in the oral steroids and antibiotics group and 11/24 patients in the antibiotics alone group. In addition, there was a larger "weight gain" (which was also listed as "clinically significant adverse event") reported in the children receiving oral steroids and antibiotics compared with those receiving antibiotics alone at the end of treatment (30 days) (0.42 ± 0.26 kg and 0.27 ± 0.30 kg, respectively).
3. Other adverse event: insomnia
Ozturk 2011 states that "No clinically significant adverse events were reported", but it is uncertain whether there were any events reported (see "gastrointestinal disturbances" above).
4. Other adverse event: osteoporosis
The study had not listed this as an outcome to be monitored or reported.
5. Endoscopic scores (including nasal polyps score)
The study did not report this as an outcome.
6. Computerised tomography (CT) scan score
Ozturk 2011 reported the CT scan score at 30 days, as measured using the Lund‐Mackay scoring system (range: 0 to 24, higher = more severe). The results (45 participants) showed an improvement in CT score in favour of the oral steroid group at the end of the trial (MD ‐2.90, 95% CI ‐4.91 to ‐0.89) (Analysis 2.6). This is not presented in the GRADE 'Summary of findings' table as we did not consider it to be a priority outcome.
Discussion
Summary of main results
The main findings of the review are as follows.
Short‐course oral steroids as an adjunct to intranasal corticosteroid treatment
One small study (30 adults with chronic rhinosinusitis with nasal polyps) with a high risk of bias showed an improvement in nasal polyp size (score measured by endoscopy) at 21 days for the group receiving oral steroids and intranasal steroids, compared with the group that received intranasal steroids alone. There were no data available for any other efficacy outcome or adverse effects.
Short‐course oral steroids as an adjunct to antibiotic treatment
One small study (48 children, with chronic rhinosinusitis without nasal polyps) showed an improvement in total symptom severity score (low quality evidence) and computerised tomography (CT) scan score with short‐course oral steroids at one month for participants who received oral steroids and antibiotics, compared with those who received placebo tablets and antibiotics.
Overall completeness and applicability of evidence
The evidence for oral steroids as an adjunct to other treatments is incomplete. We identified only two small studies (78 participants) (Bülbül 2013; Ozturk 2011). Neither of them followed patients beyond the end of the treatment and thus they only reflect the short‐term outcomes of the treatment. There are no data on whether the short‐term benefits over just using intranasal corticosteroids or antibiotics are sustainable over the longer term.
The populations included within the trials were also limited; the inclusion criteria for Bülbül 2013 were based solely on the diagnosis of nasal polyps. The severity of the other signs and symptoms of chronic rhinosinusitis was unclear. We felt that the population included in Ozturk 2011 was a very small, unusual group of the chronic rhinosinusitis population, being children with chronic rhinosinusitis (well defined) but without the presence of nasal polyps. The underlying pathology in children with chronic rhinosinusitis is different from that in adults with nasal polyps.
The short time frame of evaluation (less than three months) in the included studies also severely limits the completeness and applicability of the evidence in this review.
Neither of the studies included in this review adequately reported adverse effects, despite the adverse effects being one of the major concerns with the use of this type of medication. This is a trend repeated in other conditions and reliable data for adverse events associated with short‐term steroid use have not been well recorded in the literature (Burton 2008).
Quality of the evidence
The quality of the evidence for polyp size measured by endoscopic score was very low for short‐course oral corticosteroids with intranasal steroids compared with intranasal steroids alone (summary of findings Table for the main comparison). We assessed the quality as very low on account of serious risk of bias introduced by the methodology of the trial (quasi‐randomised, unblinded), the very small study sample and the short‐term nature of the outcomes (21 days rather than three months).
For the comparison of short‐course oral steroids with antibiotics compared with antibiotics alone (summary of findings Table 2), we assessed the quality of the evidence as low. There were concerns about whether disease severity was measured using a validated outcome, the sample size was very small and we considered the directness of the results of the trial to be reduced because of the short‐term nature of the outcomes (one month rather than three months).
Potential biases in the review process
Although many clinicians suggest that the typical maximum duration for a short course of oral corticosteroids should 14 days, we decided to use 21 days in the inclusion criteria for this review, which is at the higher end of the acceptable duration. We still considered the evidence for short courses up to 21 days to be relevant and relaxing the inclusion criteria allowed more data to be included. If we had limited the evidence to 14 days in this review, we would have excluded both included studies. The studies had used oral steroids for 15 and 21 days respectively (Bülbül 2013; Ozturk 2011).
Validated symptom scores to measure patient‐important outcomes such as disease‐specific health‐related quality of life and disease severity are often not used in chronic rhinosinusitis trials and we identified this at the protocol stage as a potential bias (Chong 2015), which could affect the validity and interpretation of the results. The problem is more serious for symptom scores used to measure disease severity. Different trials measure different types of chronic rhinosinusitis symptoms, often with more emphasis on certain types of symptoms (e.g. asking a few questions related to nasal discharge), by omitting certain types of symptoms, or both.
We had to make a decision on whether to exclude all of these data (which is the majority of the data for disease severity across the suite of reviews) (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b), or to try to distinguish scores that seem valid (based on face validity) compared to those that do not. We did this by making an assumption that if a scale is to be considered as having face validity, it should measure symptoms relevant to most chronic rhinosinusitis patients and not have symptoms that are not relevant to most chronic rhinosinusitis patients. Where a study did not use a score that is known to be validated to measure disease severity in patients with chronic rhinosinusitis and the study report did not provide information that suggested the score had been validated, we included the total symptom score reported in our analysis if at least two of the of the symptoms identified in the EPOS 2012 diagnostic criteria for chronic rhinosinusitis were measured, i.e. nasal blockage, nasal discharge, facial pain or pressure, and loss of sense of smell (for adults) or cough (for children). However, when a study included other symptoms, we tried to exclude those and only included the scores for the main chronic rhinosinusitis symptoms. For Ozturk 2011, we used the sum of scores of four chronic rhinosinusitis symptoms rather than the total symptom score reported for two main reasons: the total score reported in the study included items that may not be relevant to chronic rhinosinusitis patients and there were discrepancies (i.e. errors) in the total score at endpoint compared with a summation of the individual scores. To account for the lack of validated scales used and lack of validated methods to sum the scores, we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Agreements and disagreements with other studies or reviews
Neither of the included studies in this review were included in the previous Cochrane review on this subject (Martinez‐Devesa 2011). The EPOS 2012 document included one of the studies, Ozturk 2011, to provide evidence for the use of antibiotics and combination treatment in children with chronic rhinosinusitis, but did not include Bülbül 2013 (which was published after the guideline).
Both of the studies in this review only presented short‐term data and the longest duration of follow‐up was 30 days. As part of this suite of chronic rhinosinusitis Cochrane reviews, we have investigated the use of short‐course oral steroids alone for chronic rhinosinusitis (Head 2016a). This review concluded that, in general, health‐related quality of life and patient‐reported symptoms were improved with short‐course oral steroids compared with placebo or no treatment during the treatment period (14 to 21 days). However, these improvements in results were not sustained after the course of oral steroid treatment had finished. The results for the outcomes in the treatment and control groups showed no conclusive results for any of the efficacy outcomes at three to six months after treatment. This review did find limited evidence for three of the pre‐specified adverse events: mood disturbances, insomnia and gastrointestinal disturbances. Of these, there was moderate quality evidence of increases in insomnia and gastrointestinal disturbances in the group given oral steroids compared with the comparison group. The evidence for mood disturbances was less conclusive (low quality) and there was no evidence of osteoporosis in any of the studies.
As the included studies did not report the incidence of adverse events and the risk of side effects may vary according to the condition that they are used to treat, it is important to consider data from similar conditions where possible. A recent review of systemic steroids in acute rhinosinusitis identified five trials including 1193 participants, receiving either oral steroids (prednisolone at dosages ranging from 24 mg to 80 mg for three to seven days) or placebo, where adverse events were reported (Venekamp 2014; Venekamp 2015). There was no difference between the active and control arms in terms of the risk of adverse events, with respect to mild or severe events, or the risk of discontinuation of treatment.
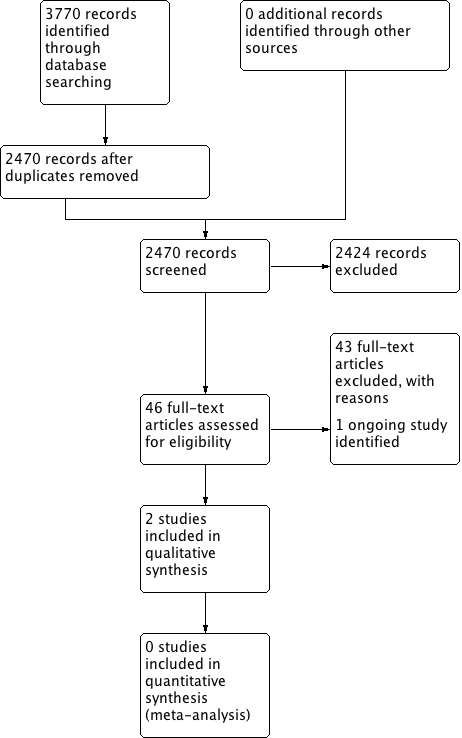
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
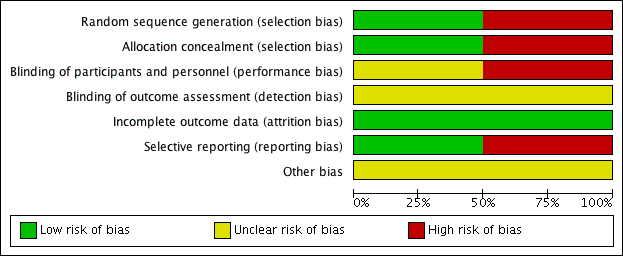
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
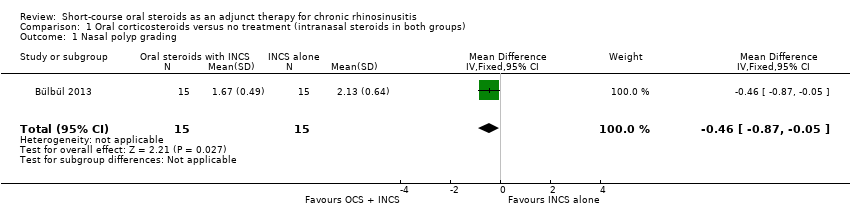
Comparison 1 Oral corticosteroids versus no treatment (intranasal steroids in both groups), Outcome 1 Nasal polyp grading.
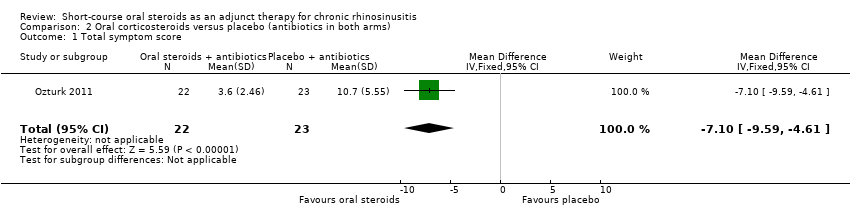
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 1 Total symptom score.
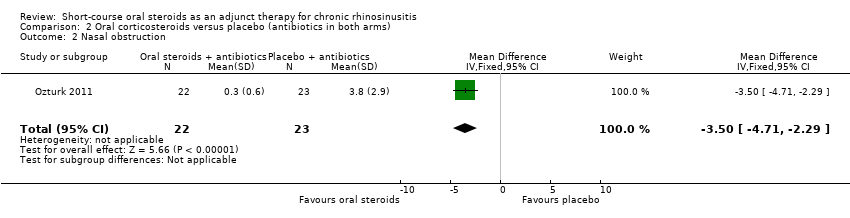
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 2 Nasal obstruction.
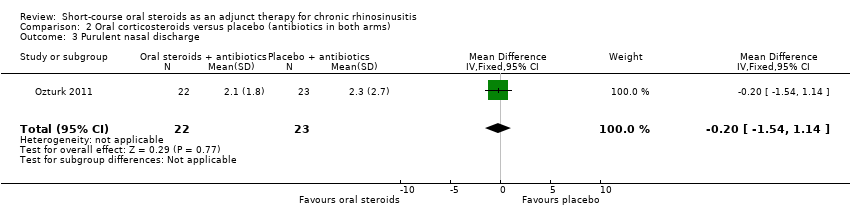
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 3 Purulent nasal discharge.
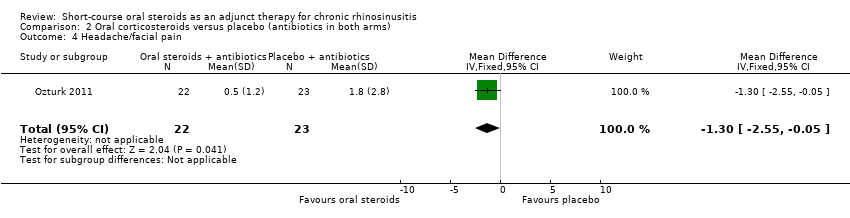
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 4 Headache/facial pain.
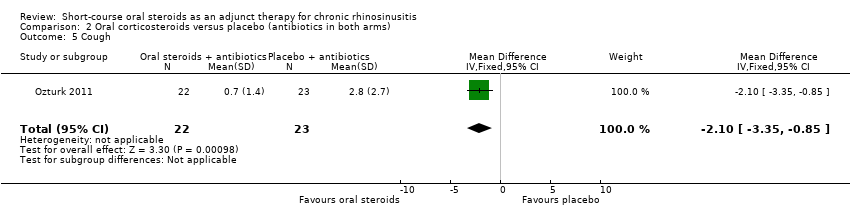
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 5 Cough.
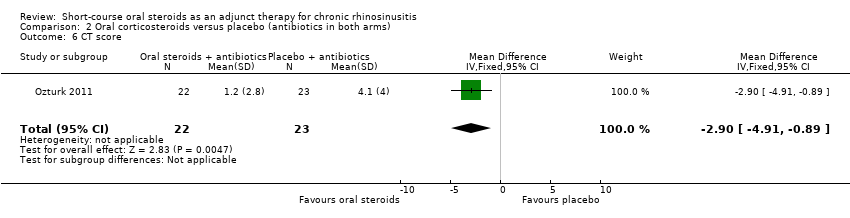
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 6 CT score.
| Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both arms) for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis | ||||||
| Outcomes No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life | — | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score | — | No RCT reported this outcome | ||||
| Adverse effect: mood or behavioural disturbances | — | No RCT reported this outcome | ||||
| Health‐related quality of life | — | No RCT reported this outcome | ||||
| Adverse effect: insomnia | — | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances ‐ not measured | — | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Short‐course oral corticosteroids compared to placebo (antibiotics in both groups) for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis | |||||
| Outcomes No of participants (studies) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | |||
| Disease‐specific health‐related quality of life | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score, assessed with: 4 individual symptoms measured on 0 to 10 visual analogue scale summed to provide a range of 0 to 40 No. of participants: 45 | The mean disease severity score without oral steroids was 15.2 | The mean disease severity score with oral steroids was 3.6 | The mean disease severity score in the intervention group was 7.10 lower (9.59 lower to 4.61 lower) | ⊕⊕⊝⊝ | A lower score indicates less severe symptoms. The results relate to a standardised mean difference of 1.61 standard deviations lower (‐2.29 to 0.93 lower), corresponding to a large difference. |
| Adverse effect: mood or behavioural disturbances | No RCT reported this outcome | ||||
| Health‐related quality of life | No RCT reported this outcome | ||||
| Adverse effect: insomnia | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Study completed only in children (mean age 8 years old). Study follow‐up time was less than 3 months (1 month). Scales were not validated and were completed by "parents and children". 2Symptoms included in this score were: purulent nasal discharge, nasal obstruction, cough and facial pain/headache. | |||||
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare; appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent, occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
| References: Da Silva 2006; Naber 1996; Stanbury 1998 NSAIDs: non‐steroidal anti‐inflammatory drugs | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nasal polyp grading Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total symptom score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.59, ‐4.61] |
| 2 Nasal obstruction Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐4.71, ‐2.29] |
| 3 Purulent nasal discharge Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.54, 1.14] |
| 4 Headache/facial pain Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐2.55, ‐0.05] |
| 5 Cough Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.35, ‐0.85] |
| 6 CT score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.91, ‐0.89] |

