Corticosteroides orales de corta duración como tratamiento adyuvante para la rinosinusitis crónica
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE |
| #1 MeSH descriptor: [Sinusitis] explode all trees #2 MeSH descriptor: [Rhinitis] this term only #3 MeSH descriptor: [Rhinitis, Atrophic] this term only #4 MeSH descriptor: [Rhinitis, Vasomotor] this term only #5 MeSH descriptor: [Paranasal Sinus Diseases] this term only #6 MeSH descriptor: [Paranasal Sinuses] explode all trees #7 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis #8 kartagener* near syndrome* #9 inflamm* near sinus* #10 (maxilla* or frontal*) near sinus* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Chronic Disease] explode all trees #13 MeSH descriptor: [Recurrence] explode all trees #14 chronic or persis* or recurrent* #15 #12 or #13 or #14 #16 #11 and #15 #17 CRSsNP #18 (sinusitis or rhinitis) near (chronic or persis* or recurrent*) #19 #16 or #17 or #18 #20 MeSH descriptor: [Nasal Polyps] explode all trees #21 MeSH descriptor: [Nose] explode all trees #22 MeSH descriptor: [Nose Diseases] explode all trees #23 #21 or #22 #24 MeSH descriptor: [Polyps] explode all trees #25 #23 and #24 #26 (nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp*) #27 rhinopolyp* or CRSwNP #28 #19 or #20 or #25 or #26 or #27 #29 MeSH descriptor: [Steroids] explode all trees #30 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #31 MeSH descriptor: [Glucocorticoids] explode all trees #32 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #33 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #34 #32 not #33 #35 steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* #36 beclomethasone or beclometasone or beclamet or beclocort or becotide #37 betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon #38 dexamethasoneor dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten #39 flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone #40 methylprednisolone or medrol or metripred or urbason #41 mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten #42 paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen #43 corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or tri next nasal or aristocort or Ciclesonide #44 #29 or #30 or #31 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 #45 #28 and #44 | 1 exp Sinusitis/ 2 paranasal sinus diseases/ or rhinitis/ or rhinitis, atrophic/ or rhinitis, vasomotor/ 3 exp Paranasal Sinuses/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj5 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp Recurrence/ 11 (chronic or persis* or recurrent*).ab,ti. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.ab,ti. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).ab,ti. 16 13 or 14 or 15 17 exp Nasal Polyps/ 18 exp Nose/ or exp Nose Diseases/ 19 exp Polyps/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).ab,ti. 22 (rhinopolyp* or CRSwNP).ab,ti. 23 16 or 17 or 20 or 21 or 22 24 exp Steroids/ 25 exp Adrenal Cortex Hormones/ 26 exp Glucocorticoids/ 27 exp Anti‐Inflammatory Agents/ 28 exp Anti‐Inflammatory Agents, Non‐Steroidal/ 29 27 not 28 30 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* orbeclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).ab,ti. 31 (corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or (tri adj3 nasal) or aristocort or Ciclesonide).ab,ti. 32 24 or 25 or 26 or 29 or 30 or 31 33 23 and 32 |
| Ovid EMBASE | Trial registries (via CRS) |
| 1 exp sinusitis/ or paranasal sinus disease/ 2 atrophic rhinitis/ or chronic rhinitis/ or rhinosinusitis/ or vasomotor rhinitis/ 3 exp paranasal sinus/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).tw. 5 (kartagener* adj3 syndrome*).tw. 6 (inflamm* adj5 sinus*).tw. 7 ((maxilla* or frontal*) adj3 sinus*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp recurrent disease/ 11 (chronic or persis* or recurrent*).tw. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.tw. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).tw. 16 13 or 14 or 15 17 exp nose polyp/ 18 exp nose disease/ or exp nose/ 19 exp polyp/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).tw. 22 (rhinopolyp* or CRSwNP).tw. 23 16 or 17or 20 or 21 or 22 24 exp *corticosteroid/ 25 exp steroid/ 26 exp antiinflammatory agent/ 27 exp nonsteroid antiinflammatory agent/ 28 26 not 27 29 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* or beclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).tw. 30 24 or 28 or 29 31 23 and 30 | ClinicalTrials.gov Condition: rhinitis OR sinusitis OR rhinosinusitis OR (nose AND polyp*) OR (nasal AND polyp*) OR CRSsNP OR CRSwNP OR CRS ICTRP Title: rhinitis OR sinusitis OR rhinosinusitis OR CRSsNP OR CRSwNP OR CR OR All: (nose AND polyp*) OR (nasal AND polyp*) NB These searches were run from 1 March 2015 to 11 August 2015, when these terms were last searched to populate the Cochrane ENT trials register in CRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 | ||
| No. dropped out (no follow‐up data for any outcome available) | ||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 | ||
| 1This should be the people who received the treatment and were therefore not considered 'drop‐outs' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants | Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] |
| Interventions | Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes | Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: | |
| Allocation concealment (selection bias) | Quote: "…" Comment: | |
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: | |
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: | |
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: | |
| Selective reporting (reporting bias) | Quote: "…" Comment: | |
| Other bias (see section 8.15) Insensitive/non‐validated instrument? | Quote: "…" Comment: | |
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: | |||||||
| Generic HRQL (instrument name/range) Time point: | |||||||
| Symptom score (overall) (instrument name/range) Time point: | |||||||
| Added total ‐ if scores reported separately for each symptom (range) Time point: | |||||||
| Nasal blockage/obstruction/congestion (instrument name/range) | |||||||
| Nasal discharge (instrument name/range) | |||||||
| Facial pain/pressure (instrument name/range) | |||||||
| Smell (reduction) (instrument name/range) | |||||||
| Headache (instrument name/range) | |||||||
| Cough (in children) (instrument name/range) | |||||||
| Polyp size (instrument name/range) | |||||||
| CT score (instrument name/range) | |||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Epistaxis/nose bleed | INCS Saline irrigation | |||||
| Local irritation (sore throat, oral thrush, discomfort) | INCS Saline irrigation | |||||
| Osteoporosis (minimum 6 months) | INCS | |||||
| Stunted growth (children, minimum 6 months) | INCS | Can also be measured as average height | ||||
| Mood disturbances | OCS | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) | OCS Antibiotics | |||||
| Insomnia | OCS | |||||
| Osteoporosis (minimum 6 months) | INCS OCS | |||||
| Discomfort | Saline irrigation | |||||
| Suspected allergic reaction (rash or skin irritation | Antibiotics | |||||
| Anaphylaxis or other serious allergic reactions such as Stevens‐Johnson | Antibiotics | |||||
| Comments: | ||||||
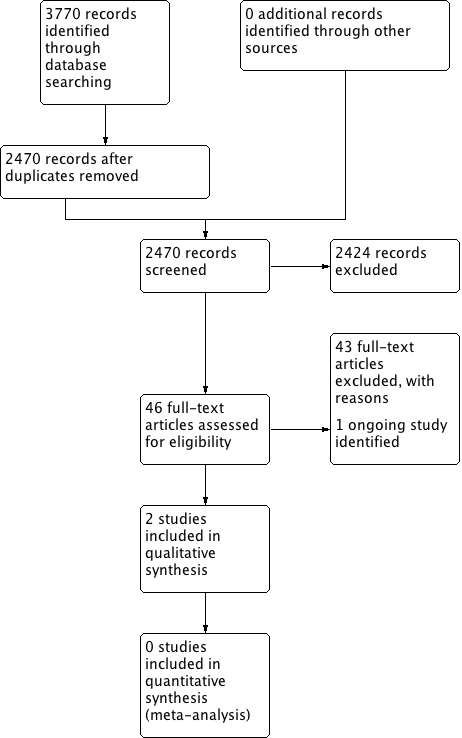
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
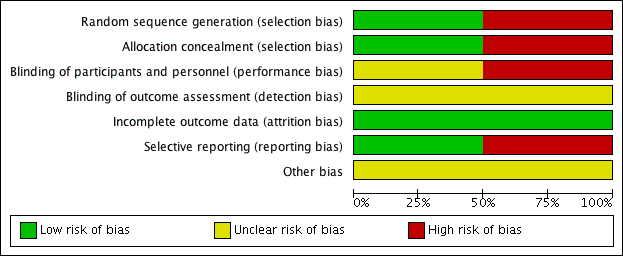
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
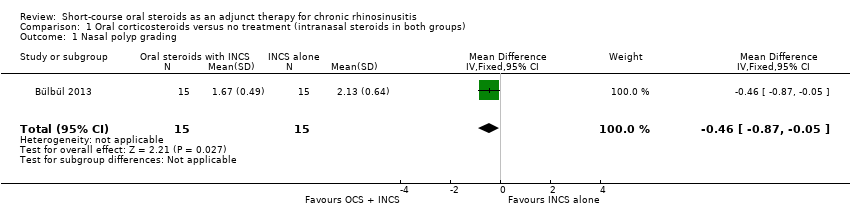
Comparison 1 Oral corticosteroids versus no treatment (intranasal steroids in both groups), Outcome 1 Nasal polyp grading.
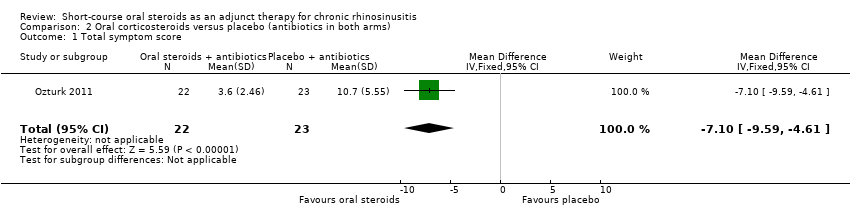
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 1 Total symptom score.
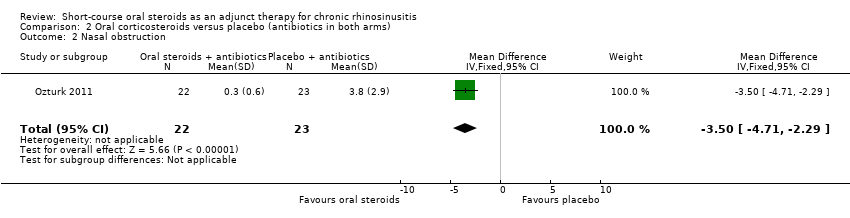
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 2 Nasal obstruction.
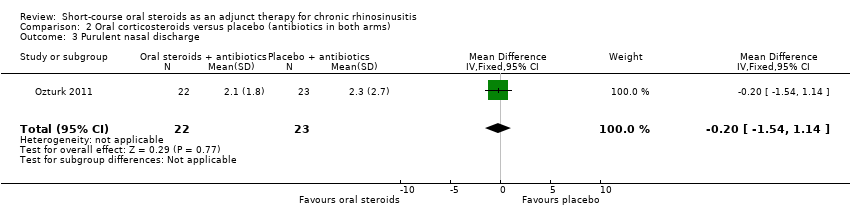
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 3 Purulent nasal discharge.
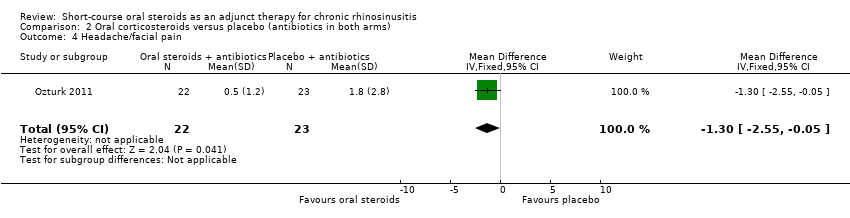
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 4 Headache/facial pain.
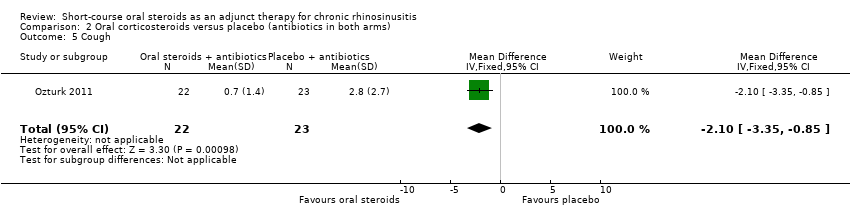
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 5 Cough.
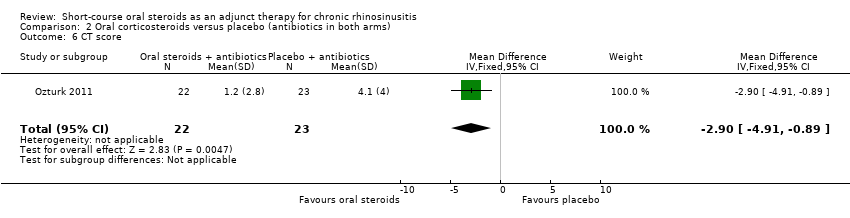
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 6 CT score.
| Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both arms) for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis | ||||||
| Outcomes No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life | — | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score | — | No RCT reported this outcome | ||||
| Adverse effect: mood or behavioural disturbances | — | No RCT reported this outcome | ||||
| Health‐related quality of life | — | No RCT reported this outcome | ||||
| Adverse effect: insomnia | — | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances ‐ not measured | — | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Short‐course oral corticosteroids compared to placebo (antibiotics in both groups) for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis | |||||
| Outcomes No of participants (studies) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | |||
| Disease‐specific health‐related quality of life | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score, assessed with: 4 individual symptoms measured on 0 to 10 visual analogue scale summed to provide a range of 0 to 40 No. of participants: 45 | The mean disease severity score without oral steroids was 15.2 | The mean disease severity score with oral steroids was 3.6 | The mean disease severity score in the intervention group was 7.10 lower (9.59 lower to 4.61 lower) | ⊕⊕⊝⊝ | A lower score indicates less severe symptoms. The results relate to a standardised mean difference of 1.61 standard deviations lower (‐2.29 to 0.93 lower), corresponding to a large difference. |
| Adverse effect: mood or behavioural disturbances | No RCT reported this outcome | ||||
| Health‐related quality of life | No RCT reported this outcome | ||||
| Adverse effect: insomnia | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Study completed only in children (mean age 8 years old). Study follow‐up time was less than 3 months (1 month). Scales were not validated and were completed by "parents and children". 2Symptoms included in this score were: purulent nasal discharge, nasal obstruction, cough and facial pain/headache. | |||||
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare; appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent, occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
| References: Da Silva 2006; Naber 1996; Stanbury 1998 NSAIDs: non‐steroidal anti‐inflammatory drugs | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nasal polyp grading Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total symptom score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.59, ‐4.61] |
| 2 Nasal obstruction Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐4.71, ‐2.29] |
| 3 Purulent nasal discharge Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.54, 1.14] |
| 4 Headache/facial pain Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐2.55, ‐0.05] |
| 5 Cough Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.35, ‐0.85] |
| 6 CT score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.91, ‐0.89] |

