Revascularización completa versus revascularización del vaso culpable en infarto de miocardio con elevación del ST con enfermedad de múltiples vasos
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 7 centres in the UK. | |
| Participants | Inclusion criteria: suspected or confirmed acute MI, significant ST elevation or LBBB on ECG (in cases of LBBB, angiographic confirmation of culprit coronary occlusion was required), < 12 hours of symptom onset, scheduled for P‐PCI for clinical reasons, provision of verbal assent followed by written informed consent, MVD, the non‐culprit vessel had to be a major (> 2 mm) epicardial coronary artery or branch (> 2 mm) and be suitable for stent implantation. Exclusion criteria: any exclusion criteria for P‐PCI; aged < 18 years; clear indication for, or contraindication to, multi‐vessel P‐PCI according to operator judgement; previous Q‐wave MI; people with prior CABG, cardiogenic shock, ventricular septal defect, or moderate/severe mitral regurgitation; chronic kidney disease (Cr > 200 μmol/L or eGFR < 30 mL/minute/1.73 m2); suspected or confirmed thrombosis of a previously stented artery; where the only significant non‐IRA lesion is a chronic total occlusion. Diagnostic criteria MVD: culprit vessel plus at least 1 non‐culprit coronary artery with at least 1 lesion deemed angiographically significant. Significant stenosis: > 70% diameter stenosis in 1e plane or > 50% in 2 planes. Sample size: complete revascularisation n = 150 and culprit‐only revascularisation n = 146. | |
| Interventions | Complete revascularisation: complete revascularisation at the same procedure, unless operator decided, for clinical reasons, that the procedure needed to be staged, in the cases of staged intervention, it was mandated that the non‐culprit lesions be treated during the index admission. Culprit‐only revascularisation: intervention on the culprit artery unless participant needed revascularisation based on ischaemic symptoms or significant ischaemia evidenced in imaging tests. | |
| Outcomes | Primary: composite of all‐cause mortality, recurrent MI, heart failure, and ischaemia‐driven revascularisation within 12 months after index procedure. Secondary: cardiovascular death, individual components of the primary endpoint, stroke, major bleeding, and contrast‐induced nephropathy. | |
| Notes | Protocol ID: ISRCTN70913605. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice‐response program was utilised to randomise participants. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed immediately after the angiography and before the intervention of the culprit artery via a centralised 24/7 telephone randomisation service. |
| Blinding of participants and personnel (performance bias) | High risk | Study was open label for the participants. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes adjudicator clinicians were blinded to the group allocation. |
| Incomplete outcome data (attrition bias) | High risk | Similar dropout rate in both groups, 7.3% culprit‐only vs 5.5% complete revascularisation group; however, given the small number of events, the result may be affected by attrition bias. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.isrctn.com ISRCTN70913605. |
| Other bias | Low risk | Study was funded by the national funding institution British Heart Foundation and Medical Research Council (MRC)/National Institutes of Health Research (NIHR) (UK) and the funding institution was not involved in the study other than economically. |
| Methods | RCT. Randomisation ratio: 2:1. Number of study centres: 1 centre in the Netherlands. | |
| Participants | Inclusion criteria: MVD with successful P‐PCI for STEMI. Exclusion criteria: urgent indication for additional revascularisation, aged > 80 years, chronic occlusion of 1 of the non‐culprit artery(ies), prior CABG, left main stenosis of ≥ 50%, restenotic lesions in non‐culprit artery(ies), chronic atrial fibrillation, limited life‐expectancy, or other factors that made complete follow‐up unlikely. Diagnostic criteria MVD: ≥ 1 significant stenosis in at least 2 major epicardial coronary arteries or the combination of a side branch and a main epicardial vessel provided that they supplied different territories. Significant stenosis: diameter ≥ 50% in luminal diameter in at least 1 view. FFR < 0.75 defined ischaemic stenosis and those were intervened only, and > 90% stenosis were intervened without FFR measurement. Sample size: complete revascularisation n = 80 and culprit‐only revascularisation n = 41. | |
| Interventions | Complete revascularisation: staged intervention on significant stenotic non‐culprit lesions compatible with ischaemia (FFR < 0.75) with plain angioplasty, BMS, or DES. Culprit‐only revascularisation: medical management after P‐PCI of culprit artery only unless ischaemic symptoms were elicited with exercise testing, dobutamine stress echocardiography, or myocardial scintigraphy, in those cases ischaemia‐guided revascularisation was performed. | |
| Outcomes | Primary: EF at 6 months. Secondary: change in EF, wall motion score, left ventricle end‐systolic and end‐diastolic volume, and MACE. | |
| Notes | Early termination because of slow enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Only mentioned in the study that echocardiographic and radionucleotide data were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | In the complete revascularisation group 1.3% dropped out, vs 2.4% in the culprit‐only group. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | High risk | Study had early termination because of slow enrolment. Unclear source of funding. |
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 2 centres in Denmark. | |
| Participants | Inclusion criteria: chest pain < 12 hours' duration and ST elevation > 0.1 mV in at least 2 contiguous leads and with diameter stenosis of > 50% in ≥ non‐culprit artery(ies). Exclusion criteria: intolerance to contrast media, anticoagulant, antithrombotic drugs, unconsciousness or cardiogenic shock, stent thrombosis, indications for CABG, or increased bleeding risk. Diagnostic criteria MVD: significant stenosis in ≥ 1 of the non‐culprit artery(ies) or their major side branches in addition to that in the culprit artery. Significant stenosis: > 50% stenosis visually in arteries > 2 mm diameter and FFR ≤ 0.8 or > 90% stenosis visually regardless FFR measurement. Sample size: complete revascularisation n = 314 and culprit‐only revascularisation n = 313. | |
| Interventions | Complete revascularisation: PCI of culprit and in a second intervention 48 hours after P‐PCI and before discharge, FFR‐guided PCI in all non‐culprit significant stenotic lesions and > 90% stenotic despite FFR measurement. Culprit‐only revascularisation: Intervention on the culprit‐only. | |
| Outcomes | Primary: composite of all‐cause mortality, non‐fatal MI, and ischaemia‐driven (subjective or objective) revascularisation of lesions in non‐culprit artery(ies) 1 year' follow‐up. Secondary: all‐cause mortality, non‐fatal MI, cardiac death, urgent or non‐urgent PCI of lesions in non‐culprit artery(ies). | |
| Notes | Protocol ID: NCT01960933. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed electronically via a centralised web‐based system. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) | Low risk | Even though the study was open label there was an independent events committee that adjudicated all events. |
| Incomplete outcome data (attrition bias) | Low risk | In the complete revascularisation group around 0.3% dropped out, while in the culprit‐only group all participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.ClinicalTrial.gov NCT01960933. |
| Other bias | Low risk | Study was funded by national funding institution (Danish Agency of Science, Technology and Innovation and Danish Council for Strategic Research) and the funding institution was not involved in the study other than economically. |
| Methods | RCT. Randomisation ratio: 1:1. Number of study centres: not described in abstract. | |
| Participants | Inclusion criteria: people with STEMI and MVD. Exclusion criteria: not described in abstract. Diagnostic criteria MVD: not described in abstract. Significant stenosis: not described in abstract. Sample size: complete revascularisation n = 100 and culprit‐only revascularisation n = 99. | |
| Interventions | Invasive: staged complete intervention after P‐PCI. Conservative: intervention of culprit‐only, unless participants had residual ischaemia based on stress echocardiogram, these participants would go for staged intervention. | |
| Outcomes | Primary: composite of cardiovascular death, non‐fatal MI, revascularisation of any vessel, or admission due to heart failure. Secondary: not described in abstract. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
| Methods | RCT multi‐centre. Randomisation ratio: 3:1. Number of study centres: not described in the article. | |
| Participants | Inclusion criteria: ischaemic chest pain started < 12 hours before hospital admission with or without ST‐segment elevation of ≥ 1 mm in ≥ 2 contiguous electrocardiographic leads (peripheral leads) or 2 mm in the precordial leads. MVD amenable to angioplasty of at least 2 lesions (culprit artery and ≥ 1 (maximum 3) lesions in a major non‐culprit coronary artery(ies)). Exclusion criteria: presence of significant lesions in vein grafts or arterial conduits or in segments previously treated with angioplasty or stent, recent thrombolysis (< 1 week), cardiogenic shock, defined as hypotension with systolic blood pressure < 90 mmHg and tachycardia > 100 beats/minute, not due to hypovolaemia or requiring inotropic support or balloon counter pulsation. Single‐vessel disease, left main stenosis of ≥ 50%, intention to treat > 1 totally occluded major epicardial vessel, diffuse calcification or severe tortuosity in the culprit and non‐culprit arteries preventing the implantation of the study stents. A sided branch > 2 mm which required being covered by the stent, unless the operator was willing and technically able to maintain patency of this side branch with either further balloon angioplasty or stent placement. Diagnostic criteria MVD: not defined in the article. Significant stenosis: not defined in the article. Sample size: complete revascularisation n = 53 and culprit‐only revascularisation n = 17. | |
| Interventions | Complete revascularisation: PCI of all, culprit and non‐culprit coronary artery lesions suitable to intervention with a heparin‐coated stent. Culprit‐only revascularisation: PCI of culprit artery only and intervention on non‐culprit artery(ies) was performed at discretion of the investigator, based on clinical status (persistent or recurrent angina), evidence of ischaemia in non‐invasive tests (perfusion scintigraphy or stress echo), angiographic severity of non‐culprit lesions and clinical relevance of the affected vessels as well as organisation standards of the participating centres. | |
| Outcomes | Primary: 12‐month revascularisation. Secondary: in‐hospital revascularisation, reinfarction, and death. Procedural in‐hospital and total hospital cost, 12 months' follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
| Methods | RCT. Number of study centres: not described. | |
| Participants | Inclusion criteria: people with prolonged (> 30 minutes) chest pain, started < 12 hours before hospital arrival and ST elevation of ≥ 1 mm in ≥ 2 contiguous limb electrocardiographic leads or 2 mm in precordial leads. Exclusion criteria: cardiogenic shock at presentation (systolic blood pressure ≤ 90 mmHg despite drug therapy), left main coronary disease (≥ 50% diameter stenosis), previous CABG surgery, severe valvular heart disease, and unsuccessful procedures. Diagnostic criteria MVD: stenosis of ≥ 2 epicardial coronary arteries or their major branches by visual estimation. Significant stenosis: > 70% diameter. Sample size: complete revascularisation n = 130 (65 staged and 65 at index procedure complete revascularisation) and culprit‐only revascularisation n = 84. | |
| Interventions | Complete revascularisation: revascularisation of all, culprit and non‐culprit significant stenosis at the index procedure or staged. Culprit‐only revascularisation: intervention on the culprit vessel only. | |
| Outcomes | Primary: MACE, cardiac or non‐cardiac death, in‐hospital death, re‐infarction, re‐hospitalisation for acute coronary syndrome, and revascularisation. Secondary: not mentioned. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Lack of information regarding how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts were not reported in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 6 centres in Czech Republic. | |
| Participants | Inclusion criteria: people with STEMI, angiographically successful primary PCI of culprit artery (TIMI flow grades II or III), ≥ 1 other significant stenoses of non‐culprit artery(ies) found by coronary angiography (diameter of artery ≥ 2.5 mm), enrolment ≥ 48 hours following onset of symptoms. Exclusion criteria: stenosis of the left main of left coronary artery ≥ 50%, haemodynamically significant valvular disease, people in cardiogenic shock during STEMI, haemodynamic instability, angina pectoris > grade 2 CCS lasting 1 month prior to STEMI. Diagnostic criteria MVD: ≥ 1 vessel, beside of the culprit vessel, with significant stenosis. Significant stenosis: > 70% stenosis of non‐culprit artery(ies). Sample size: complete revascularisation n = 106 and culprit‐only revascularisation n = 108. | |
| Interventions | Complete revascularisation: PCI of the culprit artery and staged intervention for the non‐culprit artery(ies) between days 3 and 40 after the index procedure. Culprit‐only revascularisation: intervention on the culprit artery only. | |
| Outcomes | Primary: composite endpoint of death, non‐fatal acute MI, and stroke. Secondary: cardiovascular death, recurrent MI, target vessel failure, progression of studied stenosis of non‐culprit artery, stroke, hospitalisation for heart failure, changes in left ventricular EF, hospitalisation for unstable angina pectoris, outcomes of questionnaire regarding angina pectoris, target vessel revascularisation, non‐culprit target lesion revascularisation. | |
| Notes | Only abstract published. Protocol ID: NCT01332591. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the abstract or in protocol posted on www.ClinicalTrial.gov. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the abstract or in protocol posted on www.ClinicalTrial.gov. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Open‐label study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts not reported in the abstract. |
| Selective reporting (reporting bias) | Low risk | Abstract reported the primary outcomes indicated in the published protocol in www.ClinicalTrial.gov NCT01332591. |
| Other bias | Low risk | Study was funded by a Research Grant from the Czech Ministry of Health and by The International Clinical Research Center of St. Anne's University Hospital Brno (FNUSA‐ICRC) which is funded by the European Union. |
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 5 centres in the UK. | |
| Participants | Inclusion criteria: people of any age with STEMI and MVD detected at the time of angiography. Exclusion criteria: cardiogenic shock, unable to provide consent, previous CABG, non‐infarct artery stenosis of͵≥ 50% in the left main stem or the ostial branch of both the left anterior descending and circumflex arteries (because these are indications for CABG), or if the only non‐infarct stenosis was a chronic total occlusion (because it was felt that PCI in such circumstances was contraindicated owing to a low success rate). Diagnostic criteria MVD: presence of significant stenosis in ≥ 1 coronary artery other than the culprit vessel. Significant stenosis: stenosis ≥ 50%. Sample size: complete revascularisation n = 234 and culprit‐only revascularisation n = 231. | |
| Interventions | Complete revascularisation: intervention on all, culprit and non‐culprit arteries with stenosis of ≥ 50%. Culprit‐only revascularisation: PCI of culprit vessel only, except in people with refractory angina with objective evidence of ischaemia which may require staged intervention. | |
| Outcomes | Primary: composite of death from cardiac causes, non‐fatal MI, or refractory angina. Secondary: death from non‐cardiac causes and revascularisation procedure. | |
| Notes | Study was stopped earlier because of a highly significant difference between groups, favouring the complete revascularisation group. The study was funded by Bart's and the London Trust (BLT) Charitable Foundation (UK). Protocol ID: ISRCTN73028481. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) | High risk | Study was open label for the participant. |
| Blinding of outcome assessment (detection bias) | Low risk | Independent cardiologist and cardiac surgeon who were not notified about study‐group assignments examined specified primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Similar dropout rate in both groups, 4.3% in the complete vs 3.5% in the culprit‐only intervention group; however, given the small number of events, the results may be susceptible to attrition bias. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.isrctn.com ISRCTN73028481. |
| Other bias | High risk | Early termination of the study may overestimate/underestimate certain differences. |
| Methods | RCT. Randomisation ratio: 1:1. Number of study centres: not mentioned in the study. | |
| Participants | Inclusion criteria: people with STEMI, non‐culprit artery(ies) with significant stenosis, blood vessel > 2.5 mm and suitable for PCI. Exclusion criteria: cardiogenic shock, CABG, undetermined culprit vessel, person refused PCI, non‐culprit vessel occlusion is chronic, blood vessel diameter < 2.5 mm, lesions non‐suitable for PCI, non‐culprit vessel stenosis > 90%. Diagnostic criteria MVD: non‐culprit vessel significant stenosis. Significant stenosis: between 75% and 90%. Sample size: complete revascularisation n = 215 and culprit‐only revascularisation n = 213. | |
| Interventions | Complete revascularisation: PCI of the culprit vessel and staged intervention for the non‐culprit lesions between days 7 and 10 after the index procedure. Culprit‐only revascularisation: PCI of culprit vessel only and intervention on non‐culprit vessels was performed if participant had evidence of ischaemia (symptoms, ECG changes, or nuclear study consistent with ischaemia). | |
| Outcomes | All‐cause mortality, MACE (MI and cardiac death), hospitalisation due to cardiac reasons (angina, heart failure, re‐hospitalisation for PCI), total hospitalisation time, stent number and hospital cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article the method for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article the method for randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
BMS: bare‐metal stent; CABG: coronary artery bypass graft; CCS: Canadian Cardiovascular Society; Cr: creatinine; DES: drug‐eluting stent; ECG: electrocardiogram; EF: ejection fraction; eGFR: estimated glomerular filtration rate; FFR: fractional flow reserve; LBBB: left bundle branch block; MACE: major adverse cardiovascular event; MI: myocardial infarction; MVD: multi‐vessel disease; n: number of participants; non‐IRA: non‐infarct related artery; PCI: percutaneous coronary intervention; P‐PCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; STEMI: ST elevated myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong outcomes. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong participant population. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong comparator. | |
| Wrong comparator. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. | |
| Wrong study design. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Revascularisation Strategies for STEMI; The CMR Endpoint Study. |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD as evidenced by ≥ 1 significant (≥ 70% by visual assessment or FFR < 0.80 for 50% to 70% stenosis) stenosis in non‐culprit artery(ies). |
| Interventions | 1 time primary PCI of the culprit and non‐culprit lesions vs intervention of the culprit vessel only. |
| Outcomes | Primary: infarct size by CMR. Secondary: MACE rate at 12 months. |
| Starting date | April 2014. |
| Contact information | Shahar Lavi [email protected]. |
| Notes |
| Trial name or title | Complete Lesion Versus Culprit Lesion Revascularisation (COCUA). |
| Methods | Not described. |
| Participants | People with acute STEMI and MVD. |
| Interventions | 1 time primary PCI of the culprit and non‐culprit lesions vs intervention of the culprit vessel only. |
| Outcomes | Not described. |
| Starting date | July 2011. |
| Contact information | Seung Woon Rha [email protected]. |
| Notes | www.ClinicalTrial.gov NCT01180218. |
| Trial name or title | Comparison Between FFR Guided Revascularisation Versus Conventional Strategy in Acute STEMI Patients with MVD (CompareAcute). |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | FFR‐guided revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: composite endpoint of all‐cause mortality, non‐fatal myocardial infarction, any revascularisation, and cerebrovascular events (MACCE) at 12 months between groups. Secondary: composite endpoint of cardiac death, myocardial infarction, revascularisation, stroke and major bleeding, composite of hospitalisation for heart failure and unstable angina pectoris, all‐cause mortality, stent thrombosis, bleeding, treatment costs, and each component of the primary endpoint. |
| Starting date | May 2011. |
| Contact information | Steffen Helqvist. |
| Notes | www.ClinicalTrial.gov NCT01399736. |
| Trial name or title | Complete vs. Culprit‐only Revascularisation to Treat Multi‐vessel Disease After Primary PCI for STEMI (COMPLETE). |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Staged complete revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: composite of cardiovascular death or new myocardial infarction. Secondary: composite of cardiovascular death, new myocardial infarction, ischaemia‐driven revascularisation, or hospitalisation for unstable angina or heart failure. |
| Starting date | December 2012. |
| Contact information | Shamir Mehta [email protected]. |
| Notes | www.ClinicalTrial.gov NCT01740479. |
| Trial name or title | Strategies of Revascularisation in Patients with ST‐segment Elevation Myocardial Infarction (STEMI) and Multivessel Disease. |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Staged complete revascularisation strategy vs culprit vessel only intervention and stress echocardiography and revascularisation if required. |
| Outcomes | Primary: combined event of cardiovascular death/re‐myocardial infarction/revascularisation of any vessel/admission due to heart failure. |
| Starting date | September 2010. |
| Contact information | Rodrigo Estevez‐Loureiro, MD. |
| Notes |
| Trial name or title | FIT (Fast Infarction Treatment): Complete Revascularisation During Primary Percutaneous Coronary Intervention (PCI) Can be Achieved Safely With an Improved Clinical Outcome During the Indexed Hospitalisation. |
| Methods | Double‐blind RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Complete revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: death at 30 days, stent thrombosis, target vessel failure and re‐acute myocardial infarction. Secondary: bleeding, TIMI frame count and vascular site access complications. |
| Starting date | July 2010. |
| Contact information | Azienda Ospedaliera San Camillo Forlanini. |
| Notes |
CMR: cardiac magnetic resonance; FFR: fractional flow reserve; MACE: major adverse cardiovascular event; MVD: multi‐vessel disease; RCT: randomised controlled trial; STEMI: ST elevated myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term all‐cause mortality Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 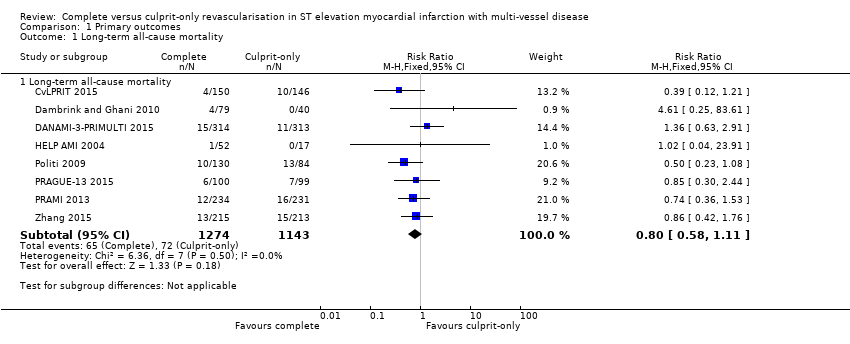 Comparison 1 Primary outcomes, Outcome 1 Long‐term all‐cause mortality. | ||||
| 1.1 Long‐term all‐cause mortality | 8 | 2417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.58, 1.11] |
| 2 Long‐term cardiovascular mortality Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 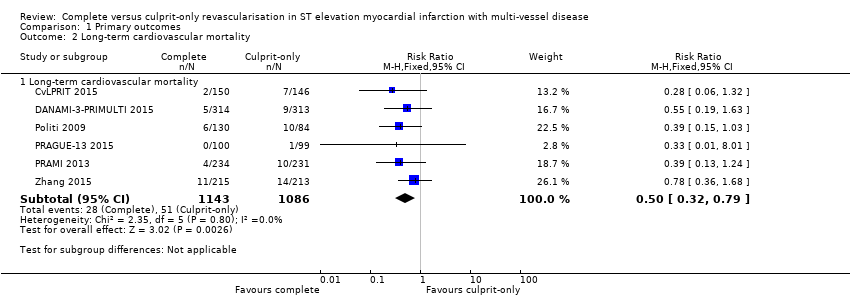 Comparison 1 Primary outcomes, Outcome 2 Long‐term cardiovascular mortality. | ||||
| 2.1 Long‐term cardiovascular mortality | 6 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.79] |
| 3 Long‐term non‐fatal myocardial infarction Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 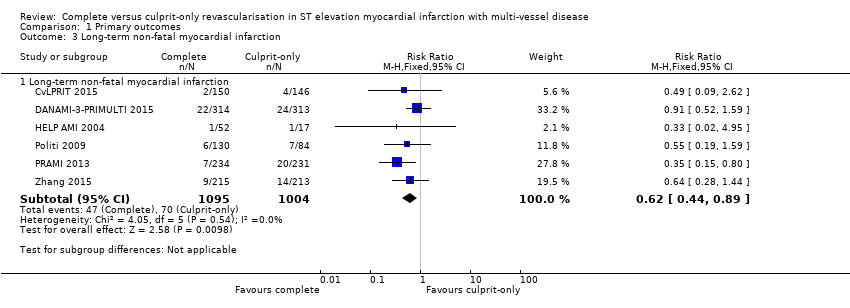 Comparison 1 Primary outcomes, Outcome 3 Long‐term non‐fatal myocardial infarction. | ||||
| 3.1 Long‐term non‐fatal myocardial infarction | 6 | 2099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.89] |
| 4 Acute kidney injury Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 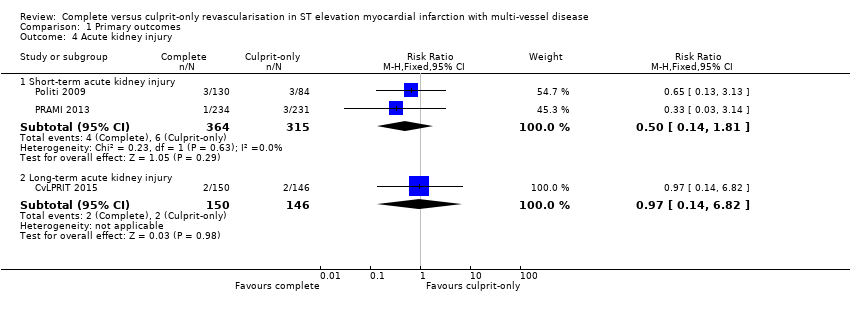 Comparison 1 Primary outcomes, Outcome 4 Acute kidney injury. | ||||
| 4.1 Short‐term acute kidney injury | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.81] |
| 4.2 Long‐term acute kidney injury | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.82] |
| 5 Stroke Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Primary outcomes, Outcome 5 Stroke. | ||||
| 5.1 Short‐term stroke | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [0.24, 102.26] |
| 5.2 Long‐term stroke | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.01] |
| 6 Bleeding Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Primary outcomes, Outcome 6 Bleeding. | ||||
| 6.1 Short‐term bleeding | 3 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.86] |
| 6.2 Long‐term bleeding | 2 | 923 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term all‐cause mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Secondary outcomes, Outcome 1 Short‐term all‐cause mortality. | ||||
| 1.1 Short‐term all‐cause mortality | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.18, 2.37] |
| 2 Short‐term cardiovascular mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Secondary outcomes, Outcome 2 Short‐term cardiovascular mortality. | ||||
| 2.1 Short‐term cardiovascular mortality | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.18] |
| 3 Short‐term non‐fatal myocardial infarction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 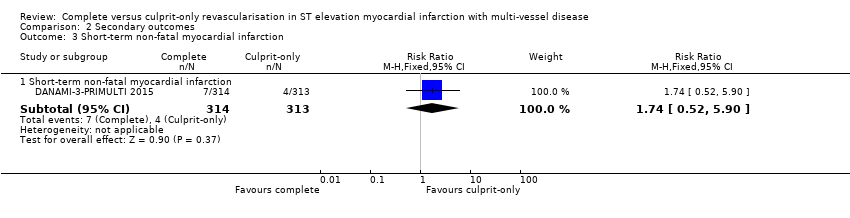 Comparison 2 Secondary outcomes, Outcome 3 Short‐term non‐fatal myocardial infarction. | ||||
| 3.1 Short‐term non‐fatal myocardial infarction | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.52, 5.90] |
| 4 Revascularisation Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 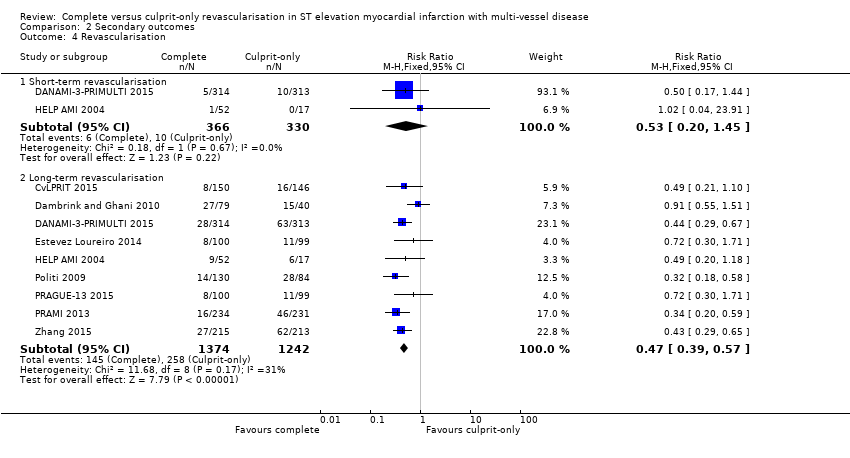 Comparison 2 Secondary outcomes, Outcome 4 Revascularisation. | ||||
| 4.1 Short‐term revascularisation | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.45] |
| 4.2 Long‐term revascularisation | 9 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.39, 0.57] |
| 5 Cost ≥ 1 year Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1948.0 [‐9171.85, 5275.85] |
| Analysis 2.5  Comparison 2 Secondary outcomes, Outcome 5 Cost ≥ 1 year. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term all‐cause mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from proportion event in control (Pc) group of 6.3% with an alpha of 2% and beta of 10%.
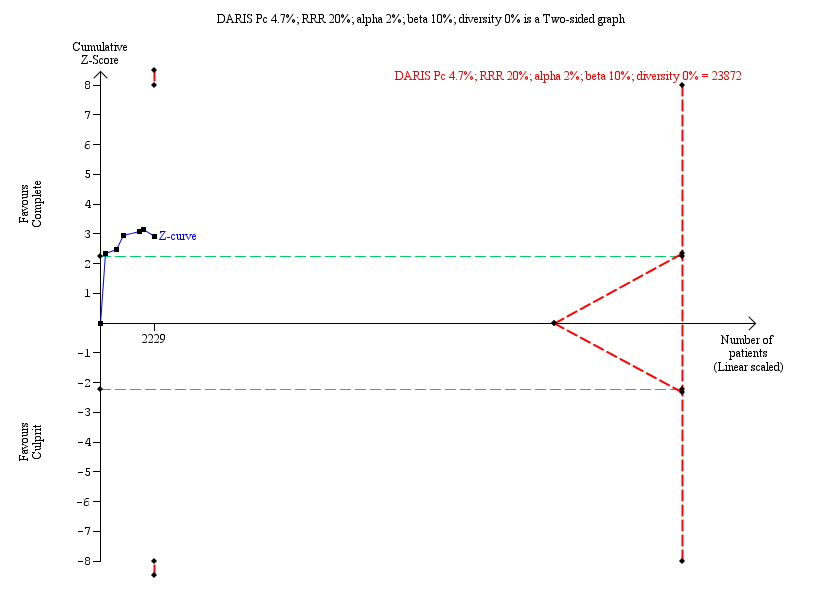
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term cardiovascular mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 4.7% with an alpha of 2% and beta of 10%.

Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term non‐fatal myocardial infarction. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 7.0% with an alpha of 2% and beta of 10%.
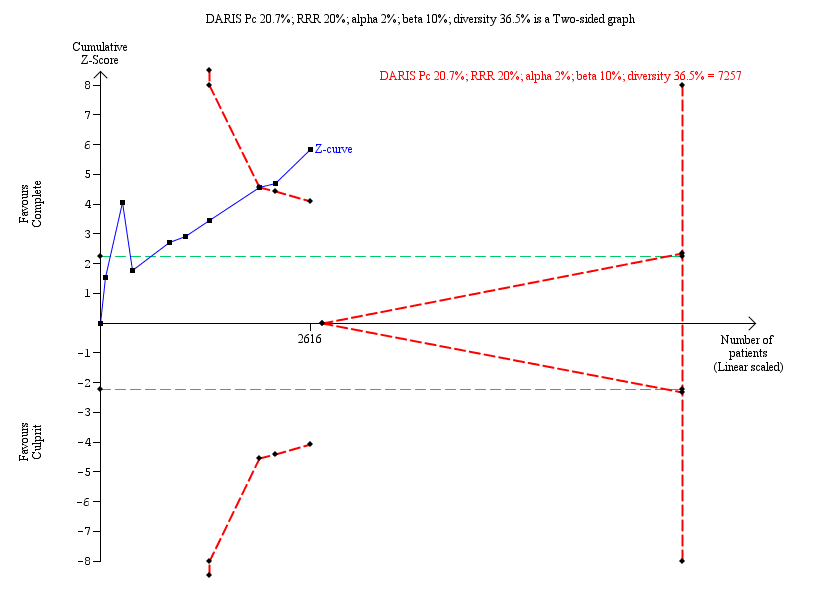
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term revascularisation. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 20.7% with an alpha of 2% and beta of 10%.

Comparison 1 Primary outcomes, Outcome 1 Long‐term all‐cause mortality.
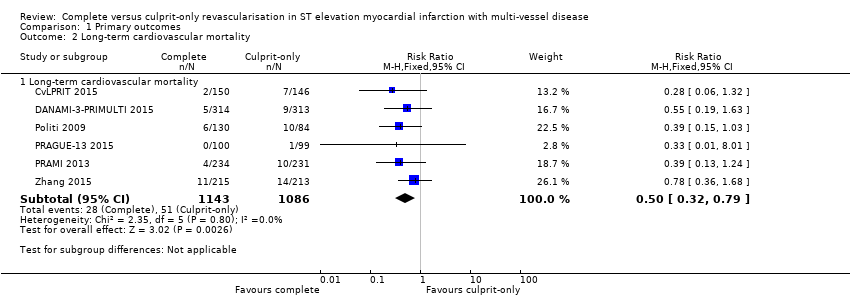
Comparison 1 Primary outcomes, Outcome 2 Long‐term cardiovascular mortality.

Comparison 1 Primary outcomes, Outcome 3 Long‐term non‐fatal myocardial infarction.
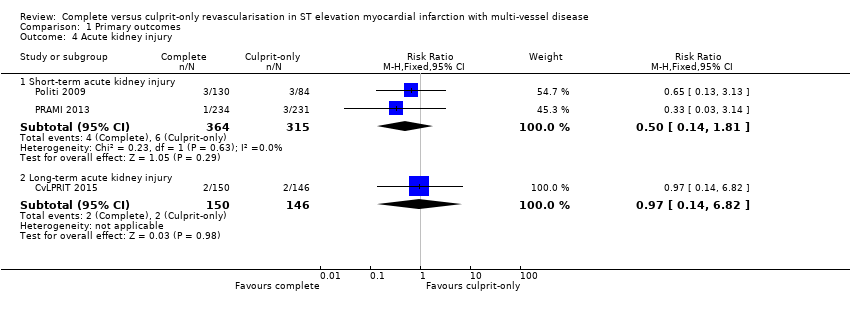
Comparison 1 Primary outcomes, Outcome 4 Acute kidney injury.
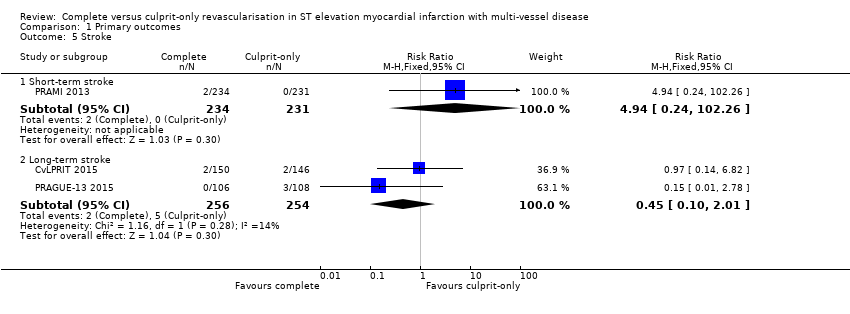
Comparison 1 Primary outcomes, Outcome 5 Stroke.

Comparison 1 Primary outcomes, Outcome 6 Bleeding.
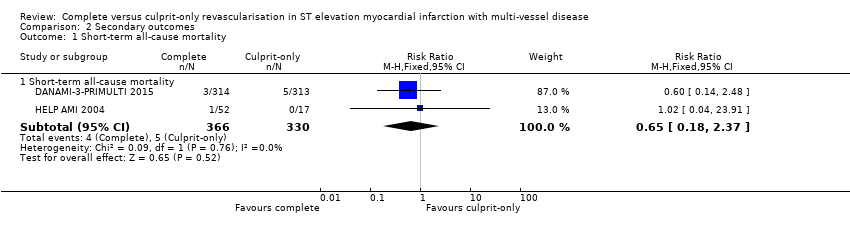
Comparison 2 Secondary outcomes, Outcome 1 Short‐term all‐cause mortality.
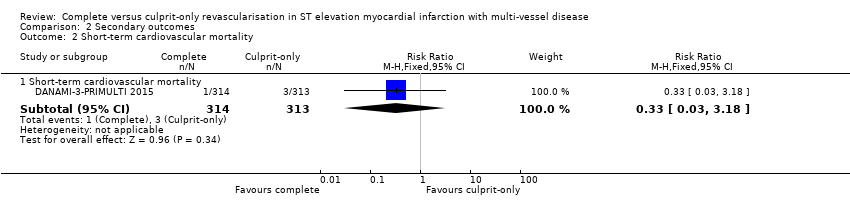
Comparison 2 Secondary outcomes, Outcome 2 Short‐term cardiovascular mortality.

Comparison 2 Secondary outcomes, Outcome 3 Short‐term non‐fatal myocardial infarction.
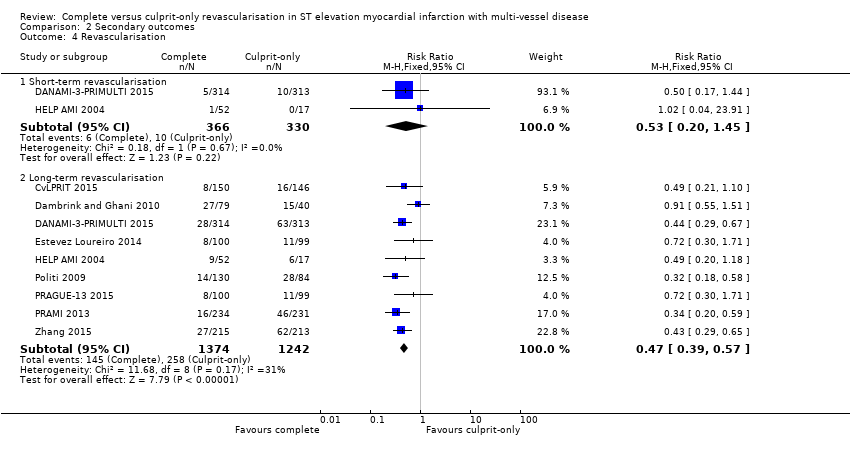
Comparison 2 Secondary outcomes, Outcome 4 Revascularisation.

Comparison 2 Secondary outcomes, Outcome 5 Cost ≥ 1 year.
| Complete revascularisation compared to culprit‐only revascularisation in ST elevated myocardial infarction with multi‐vessel disease | ||||||
| Patient or population: people with STEMI and MVD. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with culprit only | Risk with complete revascularisation | |||||
| Long‐term all‐cause mortality (≥ 1 year after the intervention) | Study population | RR 0.80 | 2417 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 63 per 1000 | 50 per 1000 | |||||
| Long‐term cardiovascular mortality (≥ 1 year after the intervention) | Study population | RR 0.50 | 2229 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 47 per 1000 | 23 per 1000 | |||||
| Long‐term myocardial infarction (≥ 1 year after the intervention) | Study population | RR 0.62 | 2099 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 70 per 1000 | 43 per 1000 | |||||
| Overall adverse events (pooled short and long term) | Study population | OR 0.84 | 4086 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 29 per 1000 | 24 per 1000 | |||||
| Short‐term all‐cause mortality (within the first 30 days after the intervention) | Study population | RR 0.65 | 696 | ⊕⊝⊝⊝ | HELP‐AMI trial did not describe in detail their methodology to analyse for bias. | |
| 15 per 1000 | 10 per 1000 | |||||
| Long‐term revascularisation (≥ 1 year after the intervention) | Study population | RR 0.47 | 2616 | ⊕⊝⊝⊝ | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 208 per 1000 | 98 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MVD: multi‐vessel disease; RCT: randomised controlled trial; RR: risk ratio; STEMI: ST elevated myocardial infarction. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to publication (reporting) bias. 2 Downgraded due to study limitations (largely risk of attrition bias and selection bias). 3 Downgraded because of indirectness: black and Hispanic people, as well as women were under‐represented. 4 Downgraded due to imprecision. | ||||||
| Study | Dates | Complete revascularisation (staged vs 1 time) | Intervention criteria in non‐culprit vessel | Mean follow‐up (years) | Description multi‐vessel disease | Country | Number of centres |
| May 2011 to May 2013 | At index procedure or before discharge. 65% of participants in invasive group had at index procedure. | > 70% diameter stenosis in 1 plane or > 50% in 2 planes. | 2.5 | Culprit vessel plus ≥ 1 non‐infarct‐related epicardial artery with ≥ 1 lesion deemed angiographically significant (> 70% stenosis in 1 plane or > 50% in 2 planes). | UK | 7 | |
| June 2004 to February 2007. | Staged 7.5 days after P‐PCI. | FFR < 0.75 and in stenosis > 90%, PCI was performed without FFR measurement. PCI was with BMS or DES. | 3 | ≥ 1 significant stenosis (> 50% stenosis in ≥ 1 view) in ≥ 2 major epicardial coronary arteries, or the combination of a side branch and a main epicardial vessel provided that they supplied different territories. | The Netherlands | 1 | |
| March 2011 to February 2014 | Staged 2 days after P‐PCI. | FFR < 0.8 and those > 90% stenotic arteries visually. | 2.2 | Significant stenosis (> 50% stenosis visually in arteries > 2 mm diameter) in ≥ 1 of the non‐culprit epicardial coronary arteries or their major side branches in addition to the infarct‐related artery. | Denmark | 2 | |
| 2010 to 2013 | Staged. | Complete. Criteria not described in study. | 1 | NR. | Spain | NR | |
| NR | Index procedure. | Not described. | 1 | NR. | Not described | NR | |
| January 2003 to December 2007 | At index procedure or staged mean 56 days after P‐PCI. 50% participants of complete revascularisation had at intervention of the non‐culprit lesions at index procedure. | > 70% diameter stenosis. | 2.5 | > 70% diameter stenosis of ≥ 2 epicardial coronary arteries or their major branches by visual estimation. | Not described | NR | |
| September 2008 to December 2014 | Staged between 3 and 40 days after P‐PCI. | > 70% stenosis of non‐culprit coronary artery. | 3 | ≥ 1 vessel, beside the culprit vessel, with significant stenosis (> 70% stenosis). | Czech Republic | 6 | |
| April 2008 to January 2013 | At index procedure. | Stenosis ≥ 50%. | 2 | The presence of stenosis ≥ 50% in ≥ 1 coronary artery other than the culprit vessel. | UK | 5 | |
| January 2009 to June 2012 | Staged between 7 and 10 days after P‐PCI. | 75% to 90%. | 2 | Non‐culprit vessel with significant stenosis (75% to 90% stenosis). | China | NR | |
| BMS: bare‐metal stent; DES: drug‐eluting stent; FFR: fractional flow reserve; NR: not reported in the article; PCI: percutaneous coronary intervention; P‐PCI: primary percutaneous coronary intervention. | |||||||
| Study | Group | Sample size (n) | Participants (n (%)) | Dropouts (n (%)) | % Male | Mean age (years) | % HTN | % DM | % HLD | % Prior MI | % Anterior STEMI |
| Complete | 150 | 139 (92.7) | 11 (7.3) | 85.3 | 64.6 | 36 | 12.7 | 27.3 | 4.7 | 36 | |
| Culprit‐only | 146 | 139 (95.2) | 8 (5.5) | 76.7 | 65.3 | 35 | 13.7 | 23.3 | 3.4 | 35.6 | |
| Complete | 80 | 71 (88.8) | 1 (1.3) | 80 | 62 | 26.3 | 6.3 | 15 | 6.3 | 21.3 | |
| Culprit‐only | 41 | 41 (100) | 1 (2.4) | 80.5 | 61 | 42.5 | 5 | 30 | 4.9 | 23.3 | |
| Complete | 314 | 294 (93.6) | 1 (0.3) | 80 | 64 | 41.4 | 9.2 | NR | 5.4 | 33.4 | |
| Culprit‐only | 313 | 313 (100) | 0 | 81.5 | 63 | 46.6 | 13.4 | NR | 8.6 | 35.8 | |
| Complete | 100 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | 99 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Complete | 52 | NR | NR | 88.5 | 63.5 | 36.5 | 11.5 | 41.2 | NR | 52 | |
| Culprit‐only | 17 | NR | NR | 82.4 | 65.3 | 58.8 | 41.2 | 53 | NR | 59 | |
| Complete | 130 | NR | NR | 78.5 | 64 | 57 | 16.2 | NR | NR | 45.4 | |
| Culprit‐only | 84 | NR | NR | 76.2 | 66.5 | 60 | 23.8 | NR | NR | 41.7 | |
| Complete | 106 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | 108 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Complete | 234 | 223 (95.3) | 10 (4.3) | 75.6 | 62 | 40.2 | 15 | NR | 8.1 | 28.6 | |
| Culprit‐only | 231 | 229 (99) | 8 (3.5) | 80.5 | 62 | 40.3 | 20.8 | NR | 7 | 38.5 | |
| Complete | 215 | NR | NR | 61 | 62.3 | 64.2 | 36.7 | 35.3 | NR | 36.7 | |
| Culprit‐only | 213 | NR | NR | 67.1 | 62 | 61 | 35.2 | 36.6 | NR | 40 | |
| DM: diabetes mellitus; HLD: hyperlipidaemia; HTN: hypertension; MI: myocardial infarction; n: number of participants; NR: not reported in the article; STEMI: ST elevated myocardial infarction. | |||||||||||
| Study | Group | Symptoms to PCI time (minute) | PCI without stenting (n (%)) | DES (n (%)) | BMS (n (%)) | 2‐Vessel disease (n (%)) | 3‐Vessel disease (n (%)) | Received PCI non‐culprit (n (%)) | DAPT | DAPT duration |
| Complete | 182 | NR | 141 (94) | NR | 119 (79.3) | 31 (20.7) | 139 (92.7) | Yes | NR | |
| Culprit‐only | 159 | NR | 127 (87) | NR | 110 (75.3) | 36 (24.7) | 0 | |||
| Complete | NR | 6 (7.5) | 18 (22.5) | 56 (70) | 60 (75) | 20 (25) | 48 (60) | Yes | 1 month | |
| Culprit‐only | NR | 7 (17.1) | 7 (7.1) | 27 (66) | 33 (80.5) | 8 (19.5) | 0 | |||
| Complete | NR | 12 (3.8) | 298 (95) | 0 | NR | 97 (31) | 193 (61.5) | Yes | 1 year | |
| Culprit‐only | NR | 18 (5.8) | 290 (92.7) | 0 | NR | 100 (32) | 0 | |||
| Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| Complete | 210 | 0 | 52 (100) | 0 | 36 (69) | 16 (30.8) | NR | Yes | 1 month | |
| Culprit‐only | 236 | 0 | 17 (100) | 0 | 9 (53) | 8 (47) | NR | |||
| Complete | NR | NR | 11 (8.5) | NR | NR | 48 (37) | NR | NR | NR | |
| Culprit‐only | NR | NR | 10 (12) | NR | NR | 21 (25) | NR | |||
| Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| Complete | NR | 1 (< 1) | 147 (63) | 86 (37) | 143 (61.1) | 91 (39) | 223 (95.3) | Yes | 1 month | |
| Culprit‐only | NR | 0 | 135 (58) | 96 (42) | 155 (67.1) | 76 (33) | 2 (1) | |||
| Complete | 214 | 0 | 215 (100) | 0 | NR | NR | NR | NR | NR | |
| Culprit‐only | 227 | 0 | 213 (100) | 0 | NR | NR | NR | |||
| BMS: bare‐metal stent; DAPT: dual antiplatelet therapy; DES: drug‐eluting stent; n: number of participants; NR: not reported in the article; PCI: percutaneous coronary intervention. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term all‐cause mortality Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Long‐term all‐cause mortality | 8 | 2417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.58, 1.11] |
| 2 Long‐term cardiovascular mortality Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Long‐term cardiovascular mortality | 6 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.79] |
| 3 Long‐term non‐fatal myocardial infarction Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Long‐term non‐fatal myocardial infarction | 6 | 2099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.89] |
| 4 Acute kidney injury Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term acute kidney injury | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.81] |
| 4.2 Long‐term acute kidney injury | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.82] |
| 5 Stroke Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Short‐term stroke | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [0.24, 102.26] |
| 5.2 Long‐term stroke | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.01] |
| 6 Bleeding Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short‐term bleeding | 3 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.86] |
| 6.2 Long‐term bleeding | 2 | 923 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term all‐cause mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term all‐cause mortality | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.18, 2.37] |
| 2 Short‐term cardiovascular mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Short‐term cardiovascular mortality | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.18] |
| 3 Short‐term non‐fatal myocardial infarction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term non‐fatal myocardial infarction | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.52, 5.90] |
| 4 Revascularisation Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term revascularisation | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.45] |
| 4.2 Long‐term revascularisation | 9 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.39, 0.57] |
| 5 Cost ≥ 1 year Show forest plot | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1948.0 [‐9171.85, 5275.85] |

