Lentes intraoculares con filtro para luz azul (LIO) para la protección de la salud macular
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011977.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Review: LED drafted the initial review, with substantial input, discussion and editing from LB and PRK. All of the statistical analyses were confirmed by LB. All authors provided final approval of the review.
Protocol: LED drafted the initial protocol, with substantial input, discussion and editing from LB and PRK. All authors provided final approval of the protocol.
LED is the guarantor of the review.
Sources of support
Internal sources
-
The University of Melbourne, Australia.
-
Australian Catholic University, Australia.
External sources
-
National Health and Medical Research Council (NHMRC), Australia.
This review is undertaken as part of a 2015 NHMRC Translating Research Into Practice (TRIP) Fellowship (APP1091833, CIA: Dr Laura Downie).
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
Laura Downie: none known
Ljoudmila Busija: none known
Peter Keller: none known
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. The methods section of this protocol includes some text from a standard template prepared by CEV. We thank Jennifer Evans and Anupa Shah for their assistance during the writing of the review. We thank Sharon Bentley, Catey Bunce, John Lawrenson, Ruth Hogg and Ana Quartilho for comments on the protocol, review or both. We thank the following translators: Covadonga Bascarán for translating a Spanish study (Cristobal 2005), Justin Wormald for translating a Russian study (Shpak 2012), Wu Taixiang and Hsin‐Wen Wu for translating the following Chinese studies (Chen 2013; Sun 2007; Wen 2012), Alex Schuster for translating the following German studies (Mayer 2006; Mester 2008b), Piotr Kanclerz for translating the following Polish papers (Lak 2007; Stopyra 2012).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 22 | Blue‐light filtering intraocular lenses (IOLs) for protecting macular health | Review | Laura E Downie, Ljoudmila Busija, Peter R Keller | |
| 2015 Nov 27 | Blue‐light filtering intraocular lenses (IOLs) for protecting macular health | Protocol | Laura E Downie, Ljoudmila Busija, Peter R Keller | |
Differences between protocol and review
After discussion with the editorial base it was decided to redefine the scope of the search strategy to fulfil the stated objectives of the review. Therefore the search strategy has been re‐designed to reflect this amendment. Medline now contains Epub Ahead of Print records so PubMed is not being searched.
Contrast sensitivity has been expressed as log Contrast Sensitivity, as reported by the study authors, rather than log Contrast Threshold (%).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Cataract Extraction [adverse effects, statistics & numerical data];
- *Lenses, Intraocular;
- *Light;
- Color;
- Contrast Sensitivity;
- Filtration [*instrumentation];
- Macula Lutea [*radiation effects];
- Macular Degeneration [*prevention & control];
- Postoperative Complications [*prevention & control];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
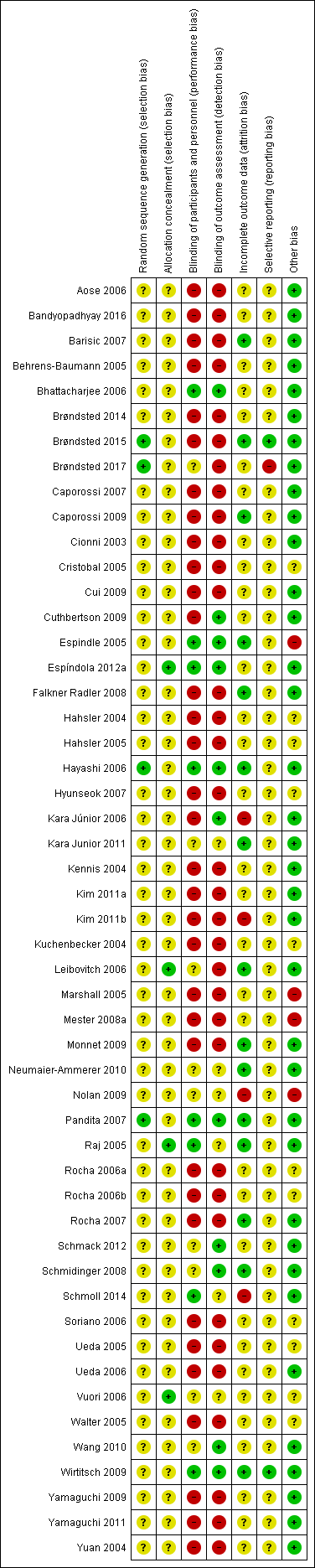
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.1 Change in distance best‐corrected visual acuity (BCVA), between baseline and 12 months (accepting measures for 6‐18 months' follow‐up. If change in distance BCVA not reported, we have utilised data reported at the end of the follow‐up period).

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period.

Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.2 Effect on photopic contrast sensitivity function, measured in log Contrast Threshold (%) using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (with an acceptable follow‐up range of 3‐9 months).
![Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months) [logCT].](/es/cdsr/doi/10.1002/14651858.CD011977.pub2/media/CDSR/CD011977/image_n/nCD011977-AFig-FIG07.png)
Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months) [logCT].
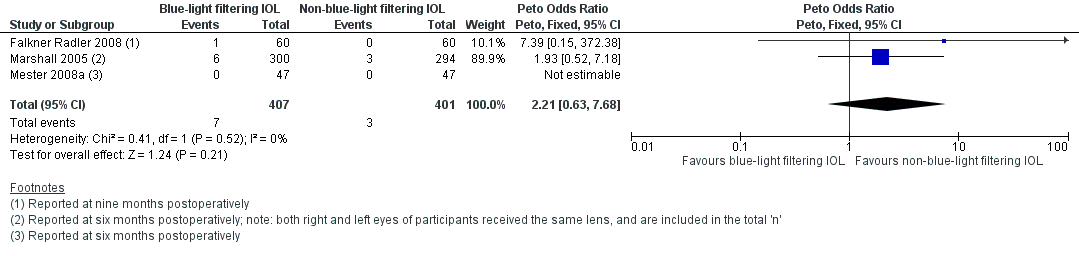
Forest plot of comparison 1. Blue‐light filtering IOL vs non‐blue‐light filtering IOL, outcome: 1.5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or optical coherence tomography or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months).

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period..

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period..

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months).

Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT).
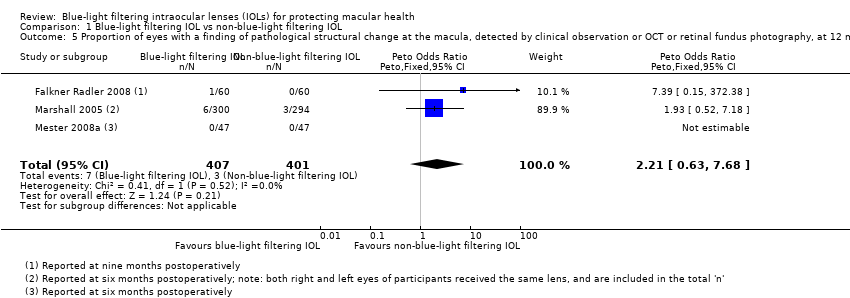
Comparison 1 Blue‐light filtering IOL vs non‐blue‐light filtering IOL, Outcome 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months).
| Blue‐light filtering IOL compared to non‐blue‐light filtering IOL for protecting macular health | ||||||
| Patient or population: adults undergoing cataract surgery with IOL implantation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐blue‐light filtering IOL | Risk with blue‐light filtering IOL | |||||
| Change in distance BCVA, between baseline and 12 months | Mean change in distance BCVA between baseline and 12 months was 0 logMAR | MD 0.01 logMAR lower | ‐ | 131 | ⊕⊕⊕⊝ | A lower BCVA (in logMAR) indicates a higher level of visual acuity. Studies in this analysis reported data at the end of the follow‐up period rather than change from baseline. |
| Distance BCVA, considered as a dichotomous outcome (being the proportion of eyes that experienced loss of 15 or more letters from baseline BCVA), at six months | See comments | Not estimable | 63 | ⊕⊝⊝⊝ | There were no eyes, in either intervention group that had a loss of 15 or more letters from baseline BCVA. | |
| Contrast sensitivity function, measured in log Contrast Sensitivity at six months | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Colour discrimination, measured as the proportion of eyes that had a measurable loss from baseline using Farnsworth‐Munsell 100‐hue colour test score under photopic conditions at six months | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Proportion of participants with adverse events with a probable causal link with the study interventions at six months follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | No relevant combinable data available for this outcome |
| Proportion of eyes that developed late‐stage AMD, being CNV and/or GA, at three years of follow‐up | See comments | Not estimable | 50 | ⊕⊝⊝⊝ | In the 1 trial (Kara Junior 2011) there were no eyes, in either intervention group that developed late‐stage AMD at five years of follow‐up. | |
| Proportion of eyes that developed any stage of AMD at 12 months | See comments | Not estimable | 144 | ⊕⊝⊝⊝ | In both studies, there were no eyes, in either intervention group that developed any stage of AMD over the nominated follow‐up period. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded for risk of bias (‐1). Data derive from two relatively small studies, with one (Caporossi 2009) judged to have a high risk of bias in both masking domains, and the other (Vuori 2006) having an unclear risk of bias in most domains. | ||||||
| Study | Blue‐light filtering IOL name(s)a (Manufacturer) | Non‐blue‐light filtering IOL name(s)a |
| YA‐60BB (Hoya) | AcrySof SA60AT (Alcon) or AcrySof MA60BM (Alcon) or VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) or PC4406 (Optech) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural (Alcon) | AcrySof MA60BM (Alcon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| Not reported | Not reported | |
| AcrySof IQ SN60WF (Alcon) | AMO ZCBOO (Abbott Medical Optics) | |
| AcrySof IQ SN60WF (Alcon) | AMO ZCBOO (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Sensar AR40e (Abbott Medical Optics) or Tecnis Z9000 (Abbott Medical Optics) or Sofport L161AO (Bausch & Lomb) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Sensar AR40e (Abbott Medical Optics) or Tecnis Z9000 (Abbott Medical Optics) | |
| AcrySof SB30AL (Alcon) | AcrySof SA30AL (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Tecnis Z9001 (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) or AcrySof IQ SN60WF (Alcon) | Tecnis Z9000 (Abbott Medical Optics) or Cee On Edge (Abbott Medical Optics) or Akreos AO (Bausch & Lomb) or Akreos Adapt (Bausch & Lomb) | |
| AcrySof Natural (Alcon) | AcrySof single‐piece (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | Akreos Fit (Bausch & Lomb) or Akreos AO (Bausch & Lomb) or AcrySof SA60AT as described in the methods (but appears to be inadvertently described as the MA60AC in the reporting of the results) | |
| AcrySof Natural (Alcon) or AF‐1 UY (Hoya) | AcrySof single‐piece (Alcon) or AF‐1 UV (Hoya) | |
| SN60 (Alcon) | SA60 (Alcon) | |
| YA (Hoya) or SN60 (Alcon) | VA (Hoya) or SA60 (Alcon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | Tecnis ZA9003 (Abbott Medical Optics) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof MA30AC (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | Tecnis Z9000 (Pfizer) or Opti‐Edge (Abbott Medical Optics) | |
| AcrySof IQ SN60WF (Alcon) | OII Biovue3 (BioVue) or YA60BBR (Hoya) | |
| AcrySof IQ SN60WF (Alcon) | Tecnis Z9003 (Abbott Medical Optics) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof single‐piece SA60AT (Alcon) | |
| AcrySof Natural IOL SB30AL (Alcon) (the current marketed version of this lens is the SN60AT) | AcrySof SA30AL (Alcon) | |
| AF‐1 UY (Hoya) | AF‐1 UV (Hoya) | |
| AcrySof SN60AT (Alcon) | AcrySof MA60AC (Alcon) or AcrySof SA60AT (Alcon) | |
| AF1 UY (Hoya) or AcrySof SN60AT (Alcon) | AF1 UV (Hoya) or AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof SN60AT (Alcon) or AcrySof SN60WF (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AcrySof IQ (Alcon) or AcrySof Natural (Alcon) | Sensar (Abbott Medical Optics) | |
| AcrySof IQ (Alcon) or AcrySof Natural (Alcon) | Sensar (Abbott Medical Optics) | |
| AcrySof IQ (Alcon) or AcrySof SN60AT (Alcon) | Sensar AR40 (Allergan) | |
| Oculaid PC 440Y Orange Series (Ophtec BV) | Oculaid PC 430Y Elite Series (Ophtec BV) | |
| AF‐1 UY (Hoya) | AF‐1 UV (Hoya) | |
| AcrySof SN60WF (Alcon) | Tecnis ZCB (Abbott Medical Optics) | |
| AcrySof SN60AT (Alcon) or AcrySof SN60WF (Alcon) | Sensar (Abbott Medical Optics) | |
| ENV‐13 (Menicon) | ES‐13 (Menicon) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof Natural SN60AT (Alcon) | AcrySof SA60AT (Alcon) | |
| AF‐1 YA‐60BB (Hoya) | AF‐1 UV‐60BB (Hoya) | |
| AY‐1 UY (Hoya) or Arium Matrix Model 4000 (Medennium) | MC611MI (HumanOptics) | |
| YA‐60BB (Hoya) | VA‐60BB (Hoya) | |
| AcrySof SN60WF (Alcon) or AcrySof SN60AT (Alcon) or Py60AD (Hoya) | Tecnis Z9003 (Abbott Medical Optics) | |
| AcrySof SN60WF (Alcon) or AcrySof SN60AT (Alcon) or Py60AD (Hoya) | Tecnis Z9003 (Abbott Medical Optics) | |
| Not reported (Hoya) | Not reported (not reported) | |
| aDetails of the interventions are provided as per the details available in the included studies. | ||
| Study | Study population: number of participants (number of eyes) | Number of intraoperative complication(s) | Details of intraoperative complication(s) |
| Blue‐light filtering IOLs: n = 65 (65); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 33 (33) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (60) Non‐blue‐light filtering IOL: n = 30 (60) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 75 (150); this group combined individuals assigned to 3 different non‐blue‐light filtering IOLs | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 39 (41) Non‐blue‐light filtering IOL: n = 18 (20) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Overall: n = 31 (31) | Not reported | One participant developed endophthalmitis and was removed from the trial, being replaced (after re‐randomisation) by another individual. There was one anterior capsular rim tear and one posterior capsule tear without vitreous loss. No details were provided in relation to which group(s) the adverse events occurred in. | |
| Blue‐light filtering IOL: n = 27 (27) Non‐blue‐light filtering IOL: n = 52 (77) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = ? (42) Non‐blue‐light filtering IOL: n = ? (26) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 9 (9) Non‐blue‐light filtering IOL: n = 10 (10) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 150 (300) Non‐blue‐light filtering IOL: n = 147 (294) | Blue‐light filtering IOL: n = 1 Non‐blue‐light filtering IOL: none | There was one case of lens dislocation during surgery in the blue‐light filtering IOL group, in a case in which a posterior capsule rupture had occurred during cataract extraction. | |
| Blue‐light filtering IOL: n = 19 (19) Non‐blue‐light filtering IOL: n = 40 (40); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: n = 2 Non‐blue‐light filtering IOL: none | In the blue‐light filtering IOL lens group "two IOLs were placed with 1 haptic in the capsular bag and 1 haptic outside the capsular bag; these 2 patients were also excluded from the final statistical analysis." | |
| Blue‐light filtering IOL: n = 73 (73); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 36 (36) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 31 (31) Non‐blue‐light filtering IOL: n = 31 (31) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| Blue‐light filtering IOL: n = 19 (25) Non‐blue‐light filtering IOL: n = 18 (27) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Not applicable | |
| IOL: intraocular lens | |||
| Study | Study population: number of participants (number of eyes) | Number of postoperative complication(s) | Details of postoperative complication(s) |
| Blue‐light filtering IOLs: n = 65 (65); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 33 (33) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at four weeks of follow‐up | |
| Blue‐light filtering IOL: n = 30 (60) Non‐blue‐light filtering IOL: n = 30 (60) | Blue‐light filtering IOL: n = 3 eyes Non‐blue‐light filtering IOL: n = 4 eyes | Although the study authors reported that "there were no postoperative complications", n = 3 eyes from the blue‐light filtering IOL group and n = 4 eyes from the non‐blue‐light filtering IOL group required Nd:YAG capsulotomy at six months of follow‐up. | |
| Blue‐light filtering IOL: n = 38 (38) Non‐blue‐light filtering IOL: n = 35 (35) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at six months of follow‐up. | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 75 (150); this group combined individuals assigned to three different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at two months of follow‐up | |
| Blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different blue‐light filtering IOLs Non‐blue‐light filtering IOL: n = 50 (100); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs | Blue‐light filtering IOL: n = 1 for Nd:YAG capsulotomy Non‐blue‐light filtering IOL: n = 1 for Nd:YAG capsulotomy | This study reports the two‐year follow‐up data for a subset of participants from Caporossi 2007. The study authors reported that "patients who underwent a capsulotomy before two‐year follow‐up (two patients) were excluded as it was not possible to perform aberrometric analysis. Two Nd:YAG laser capsulotomies were required in two patients who had AcrySof SN60AT IOL (blue‐light filtering IOL) and Tecnis Z9000 IOL (non‐blue‐light filtering IOL) implantation." | |
| Blue‐light filtering IOL: n = 39 (41); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 18 (20) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at two months of follow‐up | |
| Blue‐light filtering IOL: n = 27 (27) Non‐blue‐light filtering IOL: n = 52 (77) | Blue‐light filtering IOL: none for glaucoma or Nd:YAG capsulotomy Non‐blue‐light filtering IOL: none for glaucoma or Nd:YAG capsulotomy | The study authors reported that "at two years after surgery, all lenses were well centered and there was no evidence of posterior capsule opacity or glaucoma." | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | Blue‐light filtering IOL: n = 3 eyes Non‐blue‐light filtering IOL: n = 1 eye | Blue‐light filtering IOL: n = 1 postoperative iris capture, n = 1 spontaneously reabsorbed vitreous haemorrhage, n = 1 cystoid macular oedema at nine months after surgery Non‐blue‐light filtering IOL: n = 1 postoperative iris capture | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at eight weeks of follow‐up | |
| Blue‐light filtering IOL: n = 38 (38) Non‐blue‐light filtering IOL: n = 36 (36) (In both groups, data were obtained from both eyes and averaged) | Glare symptoms Blue‐light filtering IOL: n = 3 participants Non‐blue‐light filtering IOL: n = 2 participants Cyanopsia Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Postoperative complications (as measured by participant report of glare symptoms and cyanopsia) at three months after surgery One participant was reported to have a clinically significant epiretinal membrane in the macula (it is unclear which group the participant belonged to) | |
| Blue‐light filtering IOL: n = 30 (30) Non‐blue‐light filtering IOL: n = 30 (30) | "Three patients required neodymium:YAG laser capsulotomy for posterior capsule opacification," however group allocations were not specified. | Three participants (although this was not distinguished by the IOL intervention) at five years of follow‐up | |
| Blue‐light filtering IOL: n = 23 (23) Non‐blue‐light filtering IOL: n = 16 (16) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at eight weeks of follow‐up | |
| Blue‐light filtering IOL: n = 9 (9) Non‐blue‐light filtering IOL: n = 10 (10) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at six months of follow‐up | |
| Blue‐light filtering IOL: n = 150 (300) Non‐blue‐light filtering IOL: n = 147 (294) | Blue‐light filtering IOL: n = 12 Non‐blue‐light filtering IOL: n = 6 | At six months of follow‐up, in the blue‐light filtering IOL group, six eyes developed cystoid macula oedema and six eyes required secondary surgical intervention; none of the occurrences were considered IOL‐related. At six months of follow‐up, in the non‐blue‐light filtering IOL group, three eyes developed cystoid macula oedema and three eyes required secondary surgical intervention; none of the occurrences were considered IOL‐related. The study authors stated that "no Nd:YAG capsulotomy was performed in the first‐eye subgroup in either the test or control groups throughout the study period." | |
| Blue‐light filtering IOL: n = 19 (19) Non‐blue‐light filtering IOL: n = 40 (40); this group combined individuals assigned to 2 different non‐blue‐light filtering IOLs. | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at three months of follow‐up | |
| Blue‐light filtering IOL: n = 31 (31) Non‐blue‐light filtering IOL: n = 31 (31) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at three months of follow‐up | |
| Blue‐light filtering IOL: n = 80 (80); this group combined individuals assigned to 2 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = 38 (38) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | None at 3 months of follow‐up | |
| Blue‐light filtering IOL: n = 19 (25) Non‐blue‐light filtering IOL: n = 18 (27) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | The study authors stated that "in the biomicroscopic examination (at one month postoperatively) the posterior capsule appeared clear in all test eyes." | |
| Blue‐light filtering IOL: n = ? (120); this group combined individuals assigned to 3 different blue‐light filtering IOLs. Non‐blue‐light filtering IOL: n = ? (30) | Blue‐light filtering IOL: none Non‐blue‐light filtering IOL: none | Reported at one month of follow‐up | |
| IOL: intraocular lens | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in distance best‐corrected visual acuity (BCVA) for single‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months' follow‐up). If studies did not report change in distance BCVA, we utilised data reported at the end of the follow‐up period. Show forest plot | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 2 Change in distance best‐corrected visual acuity (BCVA) for paired‐eye trials, between baseline and 12 months (accepted measures for 6‐18 months follow‐up. If change in distance BCVA was not reported, we utilised data reported at the end of the follow‐up period. Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Contrast sensitivity, measured in log Contrast Sensitivity at 6 months (acceptable follow‐up range of 3‐9 months) Show forest plot | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.14, 0.14] |
| 4 Contrast sensitivity function, measured in log Contrast Sensitivity, using the mid‐range of the available spatial frequencies (between 6‐12 cycles/deg) at 6 months (acceptable follow‐up range of 3‐9 months; logCT) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Proportion of eyes with a finding of pathological structural change at the macula, detected by clinical observation or OCT or retinal fundus photography, at 12 months (acceptable follow‐up range of 6‐18 months) Show forest plot | 3 | 808 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [0.63, 7.68] |

