Estimulación magnética periférica repetitiva para el deterioro y la discapacidad en personas después de un accidente cerebrovascular
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only
#2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees
#3 MeSH descriptor: [Brain Ischemia] explode all trees
#4 MeSH descriptor: [Brain Infarction] this term only
#5 MeSH descriptor: [Brain Stem Infarctions] this term only
#6 MeSH descriptor: [Cerebral Infarction] this term only
#7 MeSH descriptor: [Infarction, Anterior Cerebral Artery] this term only
#8 MeSH descriptor: [Infarction, Middle Cerebral Artery] this term only
#9 MeSH descriptor: [Infarction, Posterior Cerebral Artery] this term only
#10 MeSH descriptor: [Ischemic Attack, Transient] this term only
#11 MeSH descriptor: [Carotid Artery Diseases] this term only
#12 MeSH descriptor: [Carotid Artery Thrombosis] this term only
#13 MeSH descriptor: [Carotid Stenosis] this term only
#14 MeSH descriptor: [Cerebral Arterial Diseases] this term only
#15 MeSH descriptor: [Intracranial Arteriosclerosis] this term only
#16 MeSH descriptor: [Intracranial Arteriovenous Malformations] explode all trees
#17 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
#18 MeSH descriptor: [Intracranial Hemorrhages] this term only
#19 MeSH descriptor: [Cerebral Hemorrhage] this term only
#20 MeSH descriptor: [Cerebral Intraventricular Hemorrhage] this term only
#21 MeSH descriptor: [Intracranial Hemorrhage, Hypertensive] this term only
#22 MeSH descriptor: [Subarachnoid Hemorrhage] this term only
#23 MeSH descriptor: [Stroke] this term only
#24 MeSH descriptor: [Hemorrhagic Stroke] this term only
#25 MeSH descriptor: [Ischemic Stroke] explode all trees
#26 MeSH descriptor: [Vasospasm, Intracranial] this term only
#27 MeSH descriptor: [Stroke Rehabilitation] this term only
#28 (stroke or poststroke or post‐stroke or cerebrovasc* or (cerebr* near/3 vasc*) or CVA* or apoplectic or apoplex* or (transient near/3 isch?emic near/3 attack) or tia* or SAH or AVM or ESUS or ICH or (cerebral small vessel near/3 disease*)):ti,ab,kw
#29 ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or ((anterior or posterior) near/3 circulat*) or lenticulostriate or ((basilar or brachial or vertebr*) near/3 arter*)) near/3 (disease or damage* or disorder* or disturbance or dissection or syndrome or arrest or accident or lesion or vasculopathy or insult or attack or injury or insufficiency or malformation or obstruct* or anomal*)):ti,ab,kw
#30 ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) near/3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) near/3 arter*) or space‐occupying or brain ventricle* or lacunar or cortical or ocular) near/3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* or vasospasm or obstruct* or vasoconstrict*)):ti,ab,kw
#31 ((cerebr* or cerebell* or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) near/3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) near/3 arter*) or space‐occupying or brain ventricle* or subarachnoid* or arachnoid*) near/3 (h?emorrhag* or h?ematom* or bleed*)):ti,ab,kw
#32 ((carotid or cerebr* or cerebell* or intracranial or ((basilar or brachial or vertebr*) near/3 arter*)) near/3 (aneurysm or malformation* or block* or dysplasia or disease* or bruit or injur* or narrow* or obstruct* or occlusion or constriction or presclerosis or scleros* or stenos* or atherosclero* or arteriosclero* or plaque* or thrombo* or embol* or arteriopathy)):ti,ab,kw
#33 MeSH descriptor: [Hemiplegia] this term only
#34 MeSH descriptor: [Paresis] this term only
#35 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees
#36 (hemipleg* or hemipar* or paresis or paraparesis or paretic):ti,ab,kw
#37 {or #1‐#36}
#38 MeSH descriptor: [Magnetic Field Therapy] this term only
#39 MeSH descriptor: [Magnetics] this term only
#40 MeSH descriptor: [Electromagnetic Fields] this term only
#41 MeSH descriptor: [Electromagnetic Phenomena] this term only
#42 MeSH descriptor: [Magnetic Fields] this term only
#43 ((magnet* or electromagnet* or electro‐magnet*) NEAR/5 (field* or coil* or induction)):ti,ab,kw
#44 ((peripheral or nerv* or musc* or spine or spinal) NEAR/5 (magnet* or electromagnet* or electro‐magnet*) NEAR/5 (stimulat* or neurostimulat*)):ti,ab,kw
#45 (PMS or rPMS or PrMS):ti,ab,kw
#46 {or #38‐#45}
#47 #37 AND #46
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or hemorrhagic stroke/ or exp ischemic stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. stroke rehabilitation/
3. (stroke or poststroke or post‐stroke or cerebrovasc$ or (cerebr$ adj3 vasc$) or CVA$ or apoplectic or apoplex$ or (transient adj3 isch?emic adj3 attack) or tia$ or SAH or AVM or ESUS or ICH or (cerebral small vessel adj3 disease$)).tw.
4. ((cerebr$ or cerebell$ or arteriovenous or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulat$) or lenticulostriate or ((basilar or brachial or vertebr$) adj3 arter$)) adj3 ((blood adj5 clot$) or disease$ or damage$ or disorder$ or disturbance or dissection or lesion or syndrome or arrest or accident or lesion or vasculopathy or insult or attack or injury or insufficiency or malformation or obstruct$ or anomal$)).tw.
5. ((cerebr$ or cerebell$ or arteriovenous or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulation) or basal ganglia or ((basilar or brachial or vertebr$) adj3 arter$) or space‐occupying or brain ventricle$ or lacunar or cortical or ocular) adj3 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$ or vasospasm or obstruct$ or vasoconstrict$)).tw.
6. ((cerebr$ or cerebell$ or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulation) or basal ganglia or ((basilar or brachial or vertebr$) adj3 arter$) or space‐occupying or brain ventricle$ or subarachnoid$ or arachnoid$) adj3 (h?emorrhag$ or h?ematom$ or bleed$)).tw.
7. hemiplegia/ or exp paresis/ or exp gait disorders, neurologic/
8. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
9. or/1‐8
10. magnetic field therapy/
11. magnetics/
12. electromagnetic fields/ or electromagnetic phenomena/ or magnetic fields/
13. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
14. ((peripheral or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
15. (PMS or rPMS or PrMS).tw.
16. or/10‐15
17. 9 and 16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. clinical trials as topic.sh.
23. randomly.ab.
24. trial.ti.
25. or/18‐24
26. exp animals/ not humans.sh.
27. 25 not 26
28. 17 and 27
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp basal ganglion haemorrhage/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or cerebral artery disease/ or exp carotid artery disease/ or brain atherosclerosis/ or stroke rehabilitation/ or exp intracranial aneurysm/ or occlusive cerebrovascular disease/ or basilar artery obstruction/ or exp cerebral sinus thrombosis/ or middle cerebral artery occlusion/ or vertebral artery stenosis/ or ocular ischemic syndrome/ or vertebrobasilar insufficiency/ or exp carotid artery/ or carotid artery surgery/ or carotid endarterectomy/
2. exp stroke patient/
3. (stroke or poststroke or post‐stroke or cerebrovasc$ or (cerebr$ adj3 vasc$) or CVA$ or apoplectic or apoplex$ or (transient adj3 isch?emic adj3 attack) or tia$ or SAH or AVM or (cerebral small vessel adj3 disease)).tw.
4. ((cerebr$ or cerebell$ or arteriovenous or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulat$) or lenticulostriate or ((basilar or brachial or vertebr$) adj3 arter$)) adj3 (disease or damage$ or disorder$ or disturbance or dissection or lesion or syndrome or arrest or accident or lesion or vasculopathy or insult or attack or injury or insufficiency or malformation or obstruct$ or anomal$)).tw.
5. ((cerebr$ or cerebell$ or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulation) or basal ganglia or ((basilar or brachial or vertebr$) adj3 arter$) or space‐occupying or brain ventricle$ or subarachnoid$ or arachnoid$) adj3 (h?emorrhage or h?ematoma or bleed$ or microh?emorrhage or microbleed or (encephalorrhagia or hematencephal$))).tw.
6. ((cerebr$ or cerebell$ or arteriovenous or vertebrobasil$ or interhemispheric or hemispher$ or intracran$ or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA$ or ((anterior or posterior) adj3 circulation) or basal ganglia or ((basilar or brachial or vertebr$) adj3 arter$) or space‐occupying or brain ventricle$ or lacunar or cortical or ocular) adj3 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$ or vasospasm or obstruct$ or vasculopathy or vasoconstrict$)).tw.
7. ((carotid or cerebr$ or cerebell$ or intracranial or basilar or brachial or vertebr$) adj3 (aneurysm or malformation$ or dysplasia or disease or bruit or injur$ or obstruct$ or occlusion or constriction or presclerosis or scleros$ or stenos$ or atherosclero$ or arteriosclero$ or plaque$ or thrombo$ or embol$ or arteriopathy)).tw.
8. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/
9. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
10. or/1‐9
11. magnetotherapy/
12. exp magnetic field/ or exp magnetism/
13. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
14. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
15. (PMS or rPMS or PrMS).tw.
16. or/11‐15
17. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
18. Randomization/
19. Controlled clinical trial/ or "controlled clinical trial (topic)"/
20. control group/ or controlled study/
21. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
22. Crossover Procedure/
23. Double Blind Procedure/
24. Single Blind Procedure/ or triple blind procedure/
25. placebo/ or placebo effect/
26. (random$ or RCT or RCTs).tw.
27. (controlled adj5 (trial$ or stud$)).tw.
28. (clinical$ adj5 trial$).tw.
29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
30. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
33. (cross‐over or cross over or crossover).tw.
34. (placebo$ or sham).tw.
35. trial.ti.
36. (assign$ or allocat$).tw.
37. controls.tw.
38. or/17‐37
39. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
40. 38 not 39
41. 10 and 16 and 40
Appendix 4. CINAHL (EBSCO) search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units")
S2 TI ( (stroke or poststroke or post‐stroke or cerebrovasc* or (cerebr* N3 vasc*) or CVA* or apoplectic or apoplex* or (transient N3 isch?emic N3 attack) or tia* or SAH or AVM or (cerebral small vessel N3 disease)) ) OR AB ( (stroke or poststroke or post‐stroke or cerebrovasc* or (cerebr* N3 vasc*) or CVA* or apoplectic or apoplex* or (transient N3 isch?emic N3 attack) or tia* or SAH or AVM or (cerebral small vessel N3 disease)) )
S3 TI ( ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulat*) or lenticulostriate or ((basilar or brachial or vertebr*) N3 arter*)) N3 (disease or damage* or disorder* or disturbance or dissection or lesion or syndrome or arrest or accident or lesion or vasculopathy or insult or attack or injury or insufficiency or malformation or obstruct* or anomal*)) ) OR AB ( ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulat*) or lenticulostriate or ((basilar or brachial or vertebr*) N3 arter*)) N3 (disease or damage* or disorder* or disturbance or dissection or lesion or syndrome or arrest or accident or lesion or vasculopathy or insult or attack or injury or insufficiency or malformation or obstruct* or anomal*)) )
S4 TI ( ((cerebr* or cerebell* or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) N3 arter*) or space‐occupying or brain ventricle* or subarachnoid* or arachnoid*) N3 (h?emorrhage or h?ematoma or bleed* or microh?emorrhage or microbleed or (encephalorrhagia or hematencephal*))) ) OR AB ( ((cerebr* or cerebell* or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) N3 arter*) or space‐occupying or brain ventricle* or subarachnoid* or arachnoid*) N3 (h?emorrhage or h?ematoma or bleed* or microh?emorrhage or microbleed or (encephalorrhagia or hematencephal*))) )
S5 TI ( ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) N3 arter*) or space‐occupying or brain ventricle* or lacunar or cortical or ocular) N3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* or vasospasm or obstruct* or vasculopathy or vasoconstrict*)) ) OR AB ( ((cerebr* or cerebell* or arteriovenous or vertebrobasil* or interhemispheric or hemispher* or intracran* or corpus callosum or intracerebral or intracortical or intraventricular or periventricular or posterior fossa or infratentorial or supratentorial or MCA* or ((anterior or posterior) N3 circulation) or basal ganglia or ((basilar or brachial or vertebr*) N3 arter*) or space‐occupying or brain ventricle* or lacunar or cortical or ocular) N3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* or vasospasm or obstruct* or vasculopathy or vasoconstrict*)) )
S6 TI ( ((carotid or cerebr* or cerebell* or intracranial or basilar or brachial or vertebr*) N3 (aneurysm or malformation* or dysplasia or disease or bruit or injur* or obstruct* or occlusion or constriction or presclerosis or scleros* or stenos* or atherosclero* or arteriosclero* or plaque* or thrombo* or embol* or arteriopathy)) ) OR AB ( ((carotid or cerebr* or cerebell* or intracranial or basilar or brachial or vertebr*) N3 (aneurysm or malformation* or dysplasia or disease or bruit or injur* or obstruct* or occlusion or constriction or presclerosis or scleros* or stenos* or atherosclero* or arteriosclero* or plaque* or thrombo* or embol* or arteriopathy)) )
S7 TI (hemipleg* or hemipar* or paresis or paraparesis or paretic) OR AB (hemipleg* or hemipar* or paresis or paraparesis or paretic)
S8 (MH "Hemiplegia")
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
S10 (MH "Magnetics+") OR (MH "Magnet Therapy+") OR (MH "Magnets")
S11 ( TI (magnet* or electromagnet* or electro‐magnet*) N5 therap* ) OR ( AB (magnet* or electromagnet* or electro‐magnet*) N5 therap* )
S12 ( TI ((magnet* or electromagnet* or electro‐magnet*) N5 (field* or coil* or induction)) ) OR ( AB ((magnet* or electromagnet* or electro‐magnet*) N5 (field* or coil* or induction)) )
S13 ( TI ((peripher* or nerv* or musc* or spine or spinal) N5 (magnet* or electromagnet* or electro‐magnet*) N5 (stimulat* or neurostimulat*)) ) OR ( AB ((peripher* or nerv* or musc* or spine or spinal) N5 (magnet* or electromagnet* or electro‐magnet*) N5 (stimulat* or neurostimulat*)) )
S14 S10 OR S11 OR S12 OR S13
S15 (MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S16 (MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S17 (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S18 (MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
S19 (MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
S20 PT (clinical trial or randomized controlled trial)
S21 TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S22 TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S23 TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S24 TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S25 ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S26 TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S27 TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
S28 TI (placebo* or sham) or AB (placebo* or sham)
S29 TI trial
S30 TI (assign* or allocat*) or AB (assign* or allocat*)
S31 TI controls or AB controls
S32 TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S33 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32
S34 S9 AND S14 AND S33
Appendix 5. PsycINFO (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. or/1‐6
8. exp magnetism/
9. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
10. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
11. (PMS or rPMS or PrMS).tw.
12. or/8‐11
13. clinical trials/ or treatment effectiveness evaluation/ or placebo/
14. (random$ or RCT or RCTs).tw.
15. (controlled adj5 (trial$ or stud$)).tw.
16. (clinical$ adj5 trial$).tw.
17. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw
18. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
19. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
20. (cross‐over or cross over or crossover).tw.
21. (placebo$ or sham).tw.
22. trial.ti.
23. (assign$ or allocat$).tw.
24. controls.tw.
25. or/13‐24
26. 7 and 12 and 25
Appendix 6. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ or brain injuries/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw.
7. or/1‐6
8. exp magnetics/
9. exp electromagnetics/ or exp electromagnetic fields/
10. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
11. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
12. (PMS or rPMS or PrMS).tw.
13. or/8‐12
14. clinical trials/ or randomized controlled trials/ or random allocation/
15. research design/ or comparative study/
16. double blind method/ or single blind method/
17. placebos/
18. (random$ or RCT or RCTs).tw.
19. (controlled adj5 (trial$ or stud$)).tw.
20. (clinical$ adj5 trial$).tw.
21. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
22. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
23. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
24. (cross‐over or cross over or crossover).tw.
25. (placebo$ or sham).tw.
26. trial.ti.
27. (assign$ or allocat$).tw.
28. controls.tw.
29. or/14‐28
30. 7 and 13 and 29
Appendix 7. OTseeker: Occupational Therapy Systematic Evaluation of Evidence search strategy
[Any Field] like 'stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH' AND [Any Field] like 'magnet* or electromagnet* or electro‐magnet*' AND [Method] like 'Randomised controlled trial'
Appendix 8. PEDro (Physiotherapy Evidence Database) search strategy
Therapy: electrotherapies, heat, cold
Subdiscipline: neurology
Method: clinical trial
Match all search terms (AND)
Appendix 9. Ichushi‐Web (Japanese medical database) search strategy
(脳卒中/AL or 脳梗塞/AL or 脳出血/AL or クモ膜下出血/AL or 脳血管障害/AL) and (磁気/AL) and (臨床試験/AL or 比較試験/AL or ランダム化比較試験/AL or 準ランダム化比較試験/AL or 第I相試験/AL or 第II相試験/AL or 第III相試験/AL or 第IV相試験/AL or 盲検/AL or ランダム/AL or プラセボ/AL or 対照群/AL or コントロール群/AL)
(We used Japanese characters in the search.)
Appendix 10. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov
( peripheral AND ( magnetic OR electromagnetic OR electro‐magnetic ) OR PMS OR rPMS OR PrMS ) AND AREA[StudyType] EXPAND[Term] COVER[FullMatch] "Interventional" AND AREA[ConditionSearch] ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke )
Appendix 11. ISRCTN registry
(cerebrovascular OR stroke OR TIA OR SAH OR "transient ischemic attack" OR (cerebral AND (ischemia OR ischemia OR embolism OR infarction OR haematoma OR hematoma OR haemorrhage OR hemorrhage))) AND magnet*
Appendix 12. Stroke Trials Registry
Intervention ; Clinical Trials:“Magnetic”
Appendix 13. World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
stroke or poststroke or apoplex or cerebral vasc or brain vasc or cerebrovasc or transient ischemic or tia or cva or SAH – Title AND magnetic OR electromagnetic OR electro‐magnetic OR PMS OR rPMS OR PrMS ‐ Intervention
Appendix 14. Japanese UMIN Clinical Trials Registry (UMIN‐CTR)
Study type: Intervention:“Magnetic”
Appendix 15. Japan Registry of Clinical Trials (jRCT)
Intervention: “Magnetic”
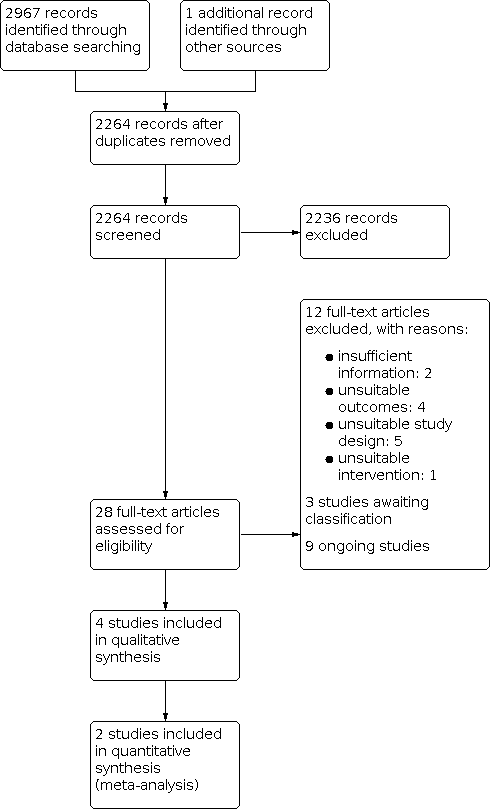
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: rPMS versus sham, Outcome 1: Muscle strength at the end of treatment

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 1: Activities of daily living at the end of treatment

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 2: Activities of daily living at the end of follow‐up

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 3: Upper limb function at the end of treatment

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 4: Upper limb function at the end of follow‐up

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 5: Spasticity of the elbow at the end of treatment

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 6: Spasticity of the elbow at the end of follow‐up

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 7: Spasticity of the wrist at the end of treatment

Comparison 2: rPMS plus rehabilitation versus sham plus rehabilitation, Outcome 8: Spasticity of the wrist at the end of follow‐up
| Active rPMS only compared with sham rPMS in stroke | ||||||
| Patient or population: people with stroke Setting: not reported Intervention: active rPMS Comparison: sham rPMS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with sham rPMS | Risk with rPMS | |||||
| Activities of daily living (ADLs) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Upper limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Lower limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (elbow) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (wrist) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Muscle strength | Mean muscle strength 10.44 kg | MD 3 kg higher | ‐ | 18 | ⊕⊕⊝⊝ |
|
| Death | ‐ | ‐ | See comment | ‐ | ‐ | No trials reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; rPMS: repetitive peripheral magnetic stimulation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne study with small sample size; 95% CI overlaps zero. | ||||||
| Active rPMS only compared with no intervention in stroke | ||||||
| Patient or population: people with stroke Setting: not available Intervention: active rPMS Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no intervention | Risk with rPMS | |||||
| Activities of daily living (ADLs) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Upper limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Lower limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (elbow) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (wrist) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Muscle strength | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Death | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; rPMS: repetitive peripheral magnetic stimulation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Active rPMS plus rehabilitation compared with sham rPMS plus rehabilitation in stroke | ||||||
| Patient or population: people with stroke Setting: neurological rehabilitation hospital Intervention: active rPMS plus rehabilitation Comparison: sham rPMS plus rehabilitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with sham rPMS plus rehabilitation | Risk with active rPMS plus rehabilitation | |||||
| Activities of daily living (ADLs) | Mean ADL score 50 | MD 3 lower (16.35 lower to 10.35 higher) | ‐ | 63 | ⊕⊕⊝⊝ |
|
| Upper limb function | Mean upper limb function score 13 | MD 2 higher | ‐ | 63 | ⊕⊕⊝⊝ |
|
| Lower limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (elbow) | Mean spasticity (elbow) score 1.41 | MD 0.41 lower | ‐ | 63 | ⊕⊕⊝⊝ |
|
| Spasticity (wrist) | Mean spasticity (wrist) score 2.13 | MD 0.2 lower | ‐ | 63 | ⊕⊕⊝⊝ |
|
| Muscle strength | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Death | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; rPMS: repetitive peripheral magnetic stimulation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne study with small sample size; 95% CI overlaps zero. | ||||||
| Active rPMS plus rehabilitation compared with rehabilitation only in stroke | ||||||
| Patient or population: people with stroke Setting: not available Intervention: active rPMS plus rehabilitation Comparison: rehabilitation only | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with rehabilitation only | Risk with active rPMS plus rehabilitation | |||||
| Activities of daily living (ADLs) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Upper limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Lower limb function | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (elbow) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Spasticity (wrist) | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Muscle strength | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| Death | ‐ | ‐ | See comment | ‐ | ‐ | No trials measured this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; rPMS: repetitive peripheral magnetic stimulation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Muscle strength at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Activities of daily living at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2 Activities of daily living at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3 Upper limb function at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4 Upper limb function at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5 Spasticity of the elbow at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6 Spasticity of the elbow at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7 Spasticity of the wrist at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.8 Spasticity of the wrist at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

