Rola cyklicznej obwodowej stymulacji magnetycznej (ang. rPMS ‐ repetitive Periferal Magnetic Stimulation) u osób po udarze mózgu w poprawie codziennych czynności i funkcjonowania.
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Study grouping: parallel group | |
| Participants | Inclusion criteria: chronic unilateral, first‐ever stroke more than 12 months before the start of the study. Participants with stroke presented with paretic ankle muscles with spasticity (medical records), had a CT or MRI scan taken within the previous 5 years, and were able to walk independently (i.e. with no physical assistance) more than 10 m with or without an assistive device Exclusion criteria: use of antispastic medication; past vertebral surgery; major circulatory, respiratory, or cardiac disease; neurological disease/deficit other than stroke; severe lower limb orthopaedic condition; or cognitive disorder Baseline characteristics rPMS (n = 9)
Sham (n = 9)
Baseline comparability between 2 groups: rPMS group was earlier from onset than sham group Loss of follow‐up: 0% | |
| Interventions | Intervention characteristics rPMS
Sham
Sham stimulation was applied using the same parameters but at a very low intensity | |
| Outcomes | Muscle strength: dorsiflexion strength (kg)
| |
| Identification | Sponsorship source: Canadian Foundation for Innovation (CS) and studentships from the Fondsde la Recherche en Sante du Quebec (LDB, HMA) and the Canadian Institutes for Health Research (LDB, HMA) Country: Canada Setting: n/a Authors' names: Louis‐David Beaulieu, Hugo Masse‐Alarie, Brenda Brouwer, Cyril Schneider Institution: Laboratoire de Neurostimulation et Neurosciences Cliniques Email: [email protected] Address: Centre de recherche du CHU de Quebec, Axe Neurosciences RC‐9800, 2705 Boulevard Laurier, Quebec, QC G1V 4G2, Canada | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | To ensure blinding, all participants were informed at enrolment that they could receive real rPMS or sham stimulation over the paretic lower limb, but they were not provided with information about the location of the coil or sensations induced by stimulation |
| Blinding of outcome assessment (detection bias) | Low risk | Experimenters performing pre‐ and post‐intervention measures and analysis had to leave the room during the intervention and remained blind to group allocation during the experiments and to times of measurement during analysis (i.e. pre‐ or post‐intervention) until completion of analyses |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: 0% |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
| Methods | Study design: RCT Study grouping: parallel group | |
| Participants | Inclusion criteria: hemiparesis caused by stroke or traumatic brain injury; spasticity of an upper extremity, with a score of 1 to 3 on the Tardieu scale; ages between 18 and 75 years Exclusion criteria: metal implant in the head or within the stimulation area; medically implanted device (cardiac pacemaker, cochlear implant, or medication pump); pregnancy; comorbidity with other neurodegenerative disorders or other neurological or orthopaedic disorders; increased intracranial pressure; unstable fracture of the paretic upper extremity Baseline characteristics rPMS (n = 31)
Sham (n = 32)
Baseline comparability between 2 groups: only rPMS groups included traumatic brain injury; rPMS group earlier from onset than sham group Loss to follow‐up: 0.05%; ITT analysis was performed | |
| Interventions | Intervention characteristics rPMS
Sham
| |
| Outcomes | Activities of daily living: Barthel Index (scores range from 0 to 100)
Upper limb function: Fugl‐Meyer Assessment (scores range from 0 to 66)
Spasticity: Modified Tardieu Scale of elbow and wrist (scores range from 0 to 5)
| |
| Identification | Sponsorship source: Cambridge Electronic Design Limited, Unit 4, Science Park, Milton Rd, Cambridge, CB4 0FE, UK. MAG&More GmbH,Geisenhausenerstrasse 11A, 81379 Munich, Germany Country: Germany Setting: neurological rehabilitation hospital Authors' names: Carmen Krewer, Sandra Hartl, Friedemann Muller, Eberhard Koenig Institution: Schoen Klinik Bad Aibling, Motor Research Department, Bad Aibling, Germany Email: CKrewer@schoen‐kliniken.de Address: Schoen Klinik Bad Aibling, Kolbermoorer Strasse 72, D‐83043 Bad Aibling, Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Randomised allocation was done by an individual not involved in any other part of the study |
| Blinding of participants and personnel (performance bias) | Low risk | Active coil makes typical discharge noises. Blinding of participants and personnel was enough |
| Blinding of outcome assessment (detection bias) | Low risk | Trained therapists, blinded for treatment allocation, assessed each participant |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: 5%; no differences in reasons why outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
| Methods | Study design: cross‐over trial | |
| Participants | Inclusion criteria: single history of CNS lesion due to stroke or traumatic brain injury; lesion interval > 12 months; increased muscle tone, i.e. 1, 2, 3, or 4 in the Modified Ashworth Score (0‐5) in affected wrist or finger joints; no volitional distal motor function of the affected arm, except for mass flexion; no metal implants or open wounds in the stimulation area; no deep vein thrombosis; no relevant oedema; no pacemaker; no preceding botulinum toxin injection within previous 6 months; signed written informed consent (approved by local ethics committee) Exclusion criteria: n/a Baseline characteristics Group 1 (rPMS‐sham) (n = 20)
Group 2 (sham‐rPMS) (n = 20)
Baseline comparability between 2 groups: group 1 was younger than group 2 Loss to follow‐up: 0% | |
| Interventions | Intervention characteristics rPMS
Sham
| |
| Outcomes | Spasticity: Modified Ashworth Score of wrist and finger (scores range from 0 to 4)
| |
| Identification | Sponsorship source: n/a Country: Germany Setting: n/a Comments: The Verein zur Förderung der Hirnforschung und Rehabilitation, e.V., Berlin Authors' names: Werner C, Schrader M, Wernicke S, Bryl B, Hesse S Institution: Medical Park Berlin Humboldtmühle, Neurological Rehabilitation, Charité, University Medicine Berlin, Germany Email: [email protected] Address: Medical Park Berlin Charité – University Medicine Berlin An der Mühle 2‐9, Berlin 13507, Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was conducted with the help of a computer‐generated lot (www.randomizer.at) |
| Allocation concealment (selection bias) | Low risk | Before start of therapy, the sub‐investigator of the study attached the rPMS or sham coil according to group assignment |
| Blinding of participants and personnel (performance bias) | Low risk | This study used a sham coil delivered with an atypical clicking sound. Therapists who applied stimulation and muscle stretch were not aware of whether the coil used was the one intended for rPMS or sham |
| Blinding of outcome assessment (detection bias) | Low risk | A rater, blinded to treatment allocation, assessed participants |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: 0% |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
CT: computed tomography
ITT: intention‐to‐treat
MRI: magnetic resonance imaging
RCT: randomised controlled trial
rPMS: repetitive peripheral magnetic stimulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Unsuitable study design | |
| Unsuitable outcomes | |
| Unsuitable outcomes | |
| Unsuitable outcomes | |
| Unsuitable intervention | |
| Unsuitable study design | |
| Unsuitable study design |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | n/a |
| Participants | Participants with stroke |
| Interventions | Low‐frequency magnetic fields |
| Outcomes | Spasticity |
| Notes |
| Methods | Comparative study |
| Participants | 42 participants with stroke (mean age 64 ± 1.0 years) |
| Interventions | 10 daily sessions of 1 Hz repetitive transcranial magnetic stimulation and repetitive peripheral magnetic stimulation |
| Outcomes | Motor Club Assessment Scale |
| Notes |
| Methods | Comparative study |
| Participants | 121 participants with ischaemic stroke in the acute period |
| Interventions | Technique of frequency‐modulated magnetolaser therapy |
| Outcomes | n/a |
| Notes |
| Methods | Study design: RCT |
| Participants | 18 participants with stroke and spastic hemiparesis (mean age 60.8 years; 9 females, 9 males; 3 to 12 months after stroke) |
| Interventions | Daily (5 times a week) session with repetitive peripheral magnetic stimulation over a period of 4 weeks, consisting of 12 repetitions of 4000 stimuli with 12‐second breaks between serials |
| Outcomes | Range of motion of wrist, Action Reach Arm Test, Bard and Hirschberg score, Ashworth Score, Gerstenbrand Spasticity Rating Scale |
| Notes |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effect of pairing peripheral and transcranial magnetic stimulations triggered by actual movement on motor plasticity |
| Methods | Cross‐over trial |
| Participants | Inclusion criteria: people with chronic stroke (more than 3 months after onset) who were inpatients and outpatients of Tohoku University Hospital |
| Interventions | Subthreshold peripheral and transcranial magnetic stimulations with actual movement |
| Outcomes | Direction of transcranial magnetic stimulation‐induced upper limb movement of the paretic side, excitability of corticospinal tract |
| Starting date | 1 October 2015 |
| Contact information | Akihiko Asao, Tohoku University Graduate School of Medicine, Department of Physical Medicine and Rehabilitation, 2‐1 Seiryo‐machi, Aoba‐ku, Sendai, Miyagi, Japan |
| Notes | UMIN000019106 |
| Trial name or title | The effect of repetitive peripheral magnetic stimulation in stroke‐rehabilitation: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: subacute stroke (occurred no longer than 6 months previously), spastic hemiparesis of the upper limb (at least modified Ashworth Scale 1); Exclusion criteria: epilepsy, implanted metal in the stimulation area, implanted medical devices, dysfunctional speech comprehension, pregnancy |
| Interventions | Stimulation intensity is adjusted individually for each participant, so that a joint movement results from the muscle contraction. Muscles of the upper arm and forearm are stimulated with a butterfly coil; the participant takes a sitting position with raised feet in the wheelchair or on a chair with backrest; the arm is placed to be stimulated or maintained by the therapist |
| Outcomes | Primary outcome is group difference in the Fugl‐Meyer‐Score 3 weeks post stimulation. Secondary outcome is group difference in the Katz Index of Independence Activities of Daily Living scale (ADL) score after 6 months |
| Starting date | 23 September 2014 |
| Contact information | Kristin Pohl, Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse‐Straße 46 07639 Bad Klosterlausnitz Germany |
| Notes | DRK00007722 |
| Trial name or title | The effects of repetitive peripheral magnetic stimulation in patient with spastic hemiparesis after stroke: a randomised‐controlled study |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: subacute stroke (occurred no longer than 6 months previously); spastic hemiparesis of the upper limb (at least modified Ashworth Scale 1); Exclusion criteria: epilepsy, implanted metal in the stimulation area, implanted medical devices, dysfunctional speech comprehension, pregnancy |
| Interventions | Stimulation is 15 minutes daily for 3 weeks for a total of 15 sessions. Stimulation intensity is adjusted individually for each participant, so that a joint movement results from the muscle contraction. Muscles of the upper arm and forearm are stimulated with a butterfly coil. Participant takes a sitting position with raised feet in a wheelchair or on a chair with a backrest. The arm is then placed to be stimulated or maintained by the therapist |
| Outcomes | Primary outcome is the Fugl‐Meyer Test of the upper extremity ‐ a test that evaluated the function of the affected upper extremity. This test will be performed directly after the end of the 3 weeks of intervention/control intervention. Secondary outcome is determined with a questionnaire ‐ the Katz Index of Independence Activities of Daily Living. That questionnaire aims to identify dependence on performance of activities of daily living and will be performed 6 months after the end of the intervention/control intervention |
| Starting date | 15 April 2015 |
| Contact information | Kristin Pohl, Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse‐Straße 46 07639 Bad Klosterlausnitz, Germany |
| Notes | DRKS00007899 |
| Trial name or title | Repetitive peripheral magnetic stimulation for patients with hemiplegia |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: cerebral hemisphere damage, people who could walk independently, modified Rankin Scale between 0 and 2 before onset |
| Interventions | Repetitive peripheral magnetic stimulation + standard physical therapy |
| Outcomes | Knee extension strength, evaluation time: at the time of starting physical therapy, 1 week later, 2 weeks later, 1 month later, 2 months later Stroke Impairment Assessment Set, 10 meter walking speed, Functional Independence Measure, quadriceps muscle thickness, acceleration during walking, Berg Balance Scale, Timed Up and Go Test, biochemical blood test, number of days until gait reacquisition, hospitalisation |
| Starting date | 1 October 2015 |
| Contact information | Keita Suzuki, Kawasaki University of Medical Welfare, Department of Rehabilitation, 288 Matsushima, Kurashiki, Okayama, Japan |
| Notes | UMIN000018750 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Muscle strength at the end of treatment Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.44, 8.44] |
| Analysis 1.1  Comparison 1 rPMS versus sham, Outcome 1 Muscle strength at the end of treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living at the end of treatment Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐16.35, 10.35] |
| Analysis 2.1  Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 1 Activities of daily living at the end of treatment. | ||||
| 2 Activities of daily living at the end of follow‐up Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.86, 10.86] |
| Analysis 2.2  Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 2 Activities of daily living at the end of follow‐up. | ||||
| 3 Upper limb function at the end of treatment Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.91, 8.91] |
| Analysis 2.3  Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 3 Upper limb function at the end of treatment. | ||||
| 4 Upper limb function at the end of follow‐up Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐2.92, 10.92] |
| Analysis 2.4 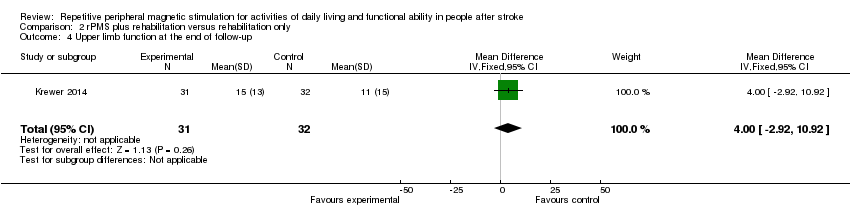 Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 4 Upper limb function at the end of follow‐up. | ||||
| 5 Spasticity at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 5 Spasticity at the end of treatment. | ||||
| 5.1 Spasticity at the end of treatment (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.89, 0.07] |
| 5.2 Spasticity at the end of treatment (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.76, 0.36] |
| 6 Spasticity at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6 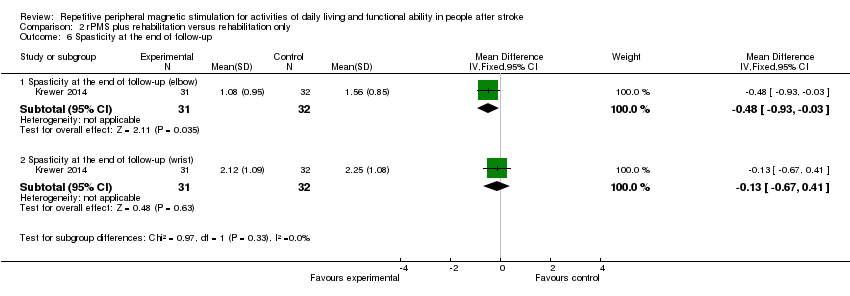 Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 6 Spasticity at the end of follow‐up. | ||||
| 6.1 Spasticity at the end of follow‐up (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.93, ‐0.03] |
| 6.2 Spasticity at the end of follow‐up (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.67, 0.41] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
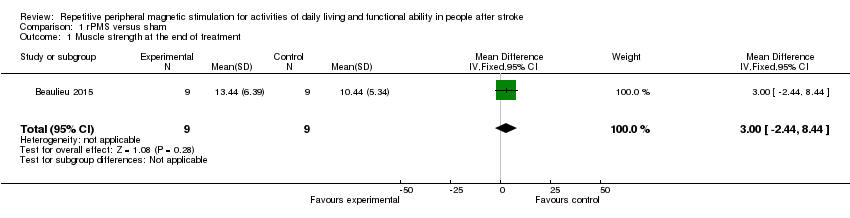
Comparison 1 rPMS versus sham, Outcome 1 Muscle strength at the end of treatment.

Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 1 Activities of daily living at the end of treatment.
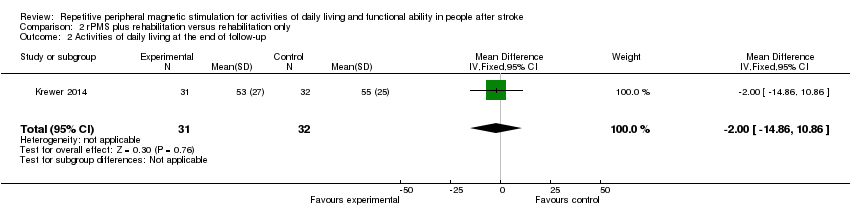
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 2 Activities of daily living at the end of follow‐up.

Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 3 Upper limb function at the end of treatment.

Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 4 Upper limb function at the end of follow‐up.

Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 5 Spasticity at the end of treatment.

Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 6 Spasticity at the end of follow‐up.
| rPMS compared with any type of control intervention in stroke | ||||||
| Patient or population: people with stroke Setting: Germany and Canada | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with any type of control intervention | Risk with rPMS | |||||
| Activities of daily living (ADLs) | Mean activities of daily living score was 50 | MD 3 lower | ‐ | 63 | ⊕⊕⊝⊝ | |
| Upper limb function | Mean upper limb function score was 13 | MD 2 higher | ‐ | 63 | ⊕⊕⊝⊝ | |
| Lower limb function ‐ not measured | ‐ | ‐ | See comments | ‐ | ‐ | No trials measured this outcome |
| Spasticity (elbow) | Mean spasticity (elbow) score was 1.41 | MD 0.41 lower | ‐ | 63 | ⊕⊕⊝⊝ | |
| Spasticity (wrist) | Mean spasticity (wrist) score was 2.13 | MD 0.2 lower | ‐ | 63 | ⊕⊕⊝⊝ | |
| Muscle strength | Mean muscle strength was 10.44 kg | MD 3 kg higher | ‐ | 18 | ⊕⊕⊝⊝ | |
| Death ‐ not reported | ‐ | ‐ | See comments | ‐ | ‐ | No trials reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne study with small sample size; 95% CI overlaps zero | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Muscle strength at the end of treatment Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.44, 8.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living at the end of treatment Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐16.35, 10.35] |
| 2 Activities of daily living at the end of follow‐up Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.86, 10.86] |
| 3 Upper limb function at the end of treatment Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.91, 8.91] |
| 4 Upper limb function at the end of follow‐up Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐2.92, 10.92] |
| 5 Spasticity at the end of treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Spasticity at the end of treatment (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.89, 0.07] |
| 5.2 Spasticity at the end of treatment (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.76, 0.36] |
| 6 Spasticity at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Spasticity at the end of follow‐up (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.93, ‐0.03] |
| 6.2 Spasticity at the end of follow‐up (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.67, 0.41] |

