Intervenciones para el tratamiento de la hemorragia posextracción
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
-
((tooth or teeth or molar* or bicuspid* or cuspid* or incisor*):ti,ab) AND (INREGISTER)
-
((extract* or remov* or surgery):ti,ab) AND (INREGISTER)
-
(#1 and #2) AND (INREGISTER)
-
((bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* or haemorrag*):ti,ab) AND (INREGISTER)
-
(#3 and #4) AND (INREGISTER)
Appendix 2. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh ^"Tooth extraction"]
#2 [mh Tooth]
#3 (tooth or teeth or molar* or bicuspid* or cuspid* or incisor*)
#4 [mh ^"Tooth, impacted"]
#5 {or #2‐#4}
#6 (extract* or remov* or surgery)
#7 #5 and #6
#8 #1 or #7
#9 [mh ^"Blood loss, surgical"]
#10 [mh ^"Postoperative hemorrhage"]
#11 [mh ^"Hemorrhagic disorders"]
#12 [mh ^"Oral hemorrhage"]
#13 (bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* haemorrag*)
#14 {or #9‐#13}
#15 #8 and #14
Appendix 3. MEDLINE Ovid search strategy
1. Tooth extraction/
2. exp Tooth/
3. (tooth or teeth or molar$ or bicuspid$ or cuspid$ or incisor$).ti,ab.
4. Tooth, impacted/
5. or/2‐4
6. (extract$ or remov$ or surgery).ti,ab.
7. 5 and 6
8. 1 or 7
9. Blood loss, surgical/
10. Postoperative hemorrhage/
11. Hemorrhagic disorders/
12. Oral hemorrhage/
13. (bleed$ or "blood loss" or hemorrhag$ or haemorrhag$ or hemorrag$ or haemorrag$).ti,ab.
14. or/9‐13
15. 8 and 14
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Tooth extraction/
2. exp Tooth/
3. (tooth or teeth or molar$ or bicuspid$ or cuspid$ or incisor$).ti,ab.
4. 2 or 3
5. (extract$ or remov$ or surgery).ti,ab.
6. 4 and 5
7. 1 or 6
8. Bleeding/
9. Bleeding disorder/
10. Oral bleeding/
11. (bleed$ or "blood loss" or hemorrhag$ or haemorrhag$ or hemorrag$ or haemorrag$).ti,ab.
12. or/8‐11
13. 7 and 12
This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/
2. Controlled clinical study/
3. Random$.ti,ab.
4. randomization/
5. intermethod comparison/
6. placebo.ti,ab.
7. (compare or compared or comparison).ti.
8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
9. (open adj label).ti,ab.
10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
11. double blind procedure/
12. parallel group$1.ti,ab.
13. (crossover or cross over).ti,ab.
14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
15. (assigned or allocated).ti,ab.
16. (controlled adj7 (study or design or trial)).ti,ab.
17. (volunteer or volunteers).ti,ab.
18. trial.ti.
19. or/1‐18
20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
21. 19 not 20
Appendix 5. CINAHL EBSCO search strategy
S13 S8 and S12
S12 S9 or S10 or S11
S11 (bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* haemorrag*)
S10 (MH "Postoperative Hemorrhage")
S9 (MH "Blood Loss, Surgical")
S8 S1 or S7
S7 S5 and S6
S6 (extract* or remov* or surgery)
S5 S2 or S3 or S4
S4 (MH "Tooth, Impacted")
S3 (tooth or teeth or molar* or bicuspid* or cuspid* or incisor*)
S2 (MH "Tooth+")
S1 (MH "Tooth Extraction")
This subject search was linked to Cochrane Oral Health’s filter for CINAHL EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design
S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study")
S3 TI random* or AB random*
S4 AB "latin square" or TI "latin square"
S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over)
S6 MH Placebos
S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*)
S8 TI blind* or AB mask* or AB blind* or TI mask*
S9 S7 and S8
S10 TI Placebo* or AB Placebo* or SU Placebo*
S11 MH Clinical Trials
S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. US National Institutes of Health Trials Registry (ClinicalTrials.gov) search strategy
“oral surgery” and bleed
“oral surgery” and hemorrhage
tooth and extraction and bleed
tooth and extraction and hemorrhage
Appendix 7. The WHO International Clinical Trials Registry Platform search strategy
Oral surgery and bleed
Oral surgery and hemorrhage
Oral surgery and haemorrhage
Tooth extraction and bleed
Tooth extraction and hemorrhage
Tooth extraction and haemorrhage
Appendix 8. Studies we translated and rejected as ineligible for inclusion
| Trial identification | Title | Language | Translator/s | Reasons for rejection |
| The use of 5‐oxytryptamine in post‐extraction haemorrhages | Italian | Giovanni Lodi | 1. The study is not randomised. Randomisation is never mentioned. The author stated that "the patients have been divided as follows" (page 1386). 2. Patients were at risk of bleeding, and not with post‐extraction bleeding. | |
| Evaluation of the haemostatic effect of Dicynon in dentoalveolar surgery | Czech | Andrea Pokorna | The study does not fulfil criteria as it describes application of Dicynone in four groups of patients – no randomisation, no detailed description of the patient groups. | |
| Stomatological haemorrhages; haemostasis with GRF (gelatin‐resorcin‐formol) | Italian | Maddalena Manfredi | This study is a description of a topical haemostasis technique without any report about patients. | |
| Therapy of post‐extraction haemorrhages in haemophiliac patients with epsilon‐aminocaproic acid (EACA). | Italian | Maddalena Manfredi | This is a case series. | |
| Use of "reptilase" in postoperative haemorrhage of the dental apparatus | German | Ubai Alsharif | This is a case series of 14 patients who were treated with Raptilase, and does not fulfil the inclusion criteria. | |
| Prevention and treatment of haemorrhage after extraction of teeth by using the pulvis of cibotium barometz‐alum burn | Chinese | Dr Liyuan Ma, Professors Chengge Hua, Zongdao Shi and Mr.Loh Zheng Tao | The trial is a RCT with two arms; however, the randomisation method is not reported. This is a preventive study for reducing post‐extraction complications including PEB, instead of managing post‐extraction bleeding. | |
| Cepevit‐K preparation in controlling haemorrhages and bleeding following tooth extraction | Polish | Joanna Zajac and Malgorzata Bala | Not a randomised controlled trial. They used Cepevit‐K: ‐ after extraction for 22 patients (for 17, bleeding was stopped within eight minutes; chirurgical treatment was provided for the others). ‐ two days before extraction in 18 patients with low coagulation time (for four patients in this group, additional chirurgical treatment was needed). The only comparison is 20 other people with bleeding after tooth extraction, where the author used other techniques (other than chirurgical treatment); it only worked for eight patients, so the other 12 were given Cepevit‐K. | |
| Clinical evaluation of the results of treatment of post‐extraction bleeding with new drugs E.A.C.A., styptanon | Polish | Joanna Zajac and Malgorzata Bala | Not a randomised controlled trial. Study was based on observation of 69 patients, all with blood coagulation problems: some after extraction with bleeding, some before extraction. For all blood analyses were done and according to results: ‐ for some: epsilon aminocaproic acid (EACA) was used ‐ for others: Styptanon was used ‐ for others: EACA and Styptanon together | |
| Use of aminocaproic acid for stopping the haemorrhage after tooth extraction | Russian | Lilia Ziganshina and Anna Misyail Abdul Rashid | This is an abstract describing 100% success of 5% aminocapronic acid in 135 patients. This was not a trial; there was no comparison. | |
| Therapy of haemorrhage following extractions | German | Karin Rau and Anette Bluemle | This is not a randomised control trial. In this publication, the interventions for post‐extraction bleeding are explained in detail, but not within the scope of a clinical study. | |
| The use of new haemostatic drug in dental practice | Russian | Lilia Ziganshina and Anna Misyail Abdul Rashid | This is a single case report. | |
| Hemorrhage in the dental office. Study of local haemostatic treatment | French | Paul Tramini | This paper is just a recommendation for practitioners and students in case of PEB problems. No data are available. | |
| Application of epsilon‐aminocaproic acid for oral mucosal bleeding in haemophiliacs | Polish | Joanna Zajac and Malgorzata Bala | This article is not a RCT. All participants (13 children with haemophilia: nine type A and four type B) received epsilon‐aminocaproic (20% solution) acid 24 hours before tooth extraction. Intolerance developed in some children, so the dose was changed (from 0.1 g/kg of body weight to 0.05g/kg), or the drug was administered intravenously. |
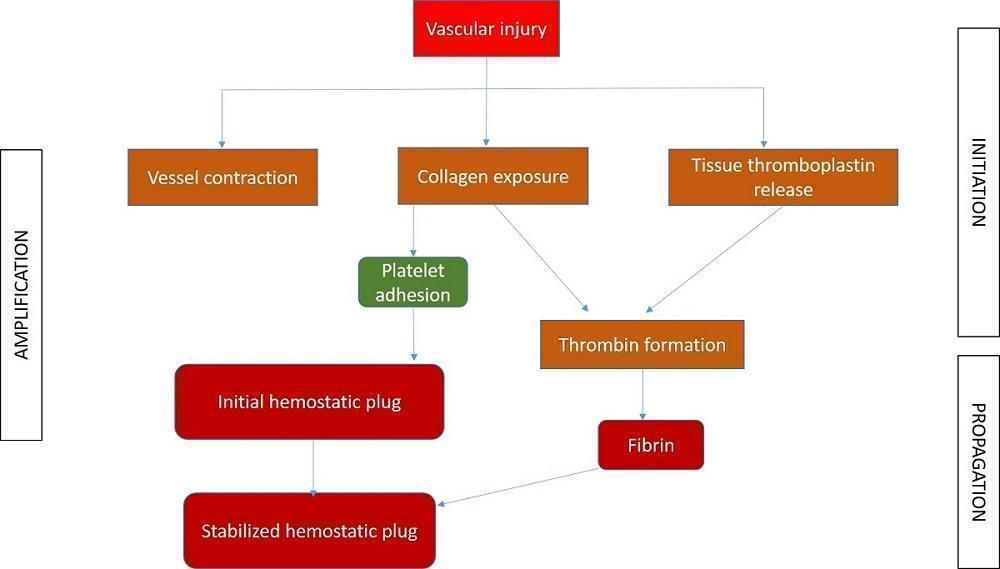
Different phases of coagulation

Study flow diagram
| Interventions for treating post‐extraction bleeding | ||||||
| Patient or population: people with post‐extraction bleeding Settings: hospital or dental practice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bleeding, as measured by:
| No data are available as no RCTs have been conducted on interventions to treat post‐extraction bleeding | |||||
| Patient‐reported outcomes related to pain or discomfort during the procedure | ||||||
| Treatment‐associated average cost | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Normal bleeding | Post‐extraction bleeding | ||
| Primary | Reactionary | Secondary | |
|
|
|
|
|
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |

