Intervenciones para el tratamiento de la hemorragia posextracción
Resumen
Antecedentes
La hemorragia posextracción (HPE) es una complicación reconocida, que ocurre con frecuencia en la práctica dental y se define como una hemorragia que persiste por más de ocho a 12 horas después de la extracción dental. La incidencia de la hemorragia posextracción varía del 0% al 26%. Si la hemorragia posextracción no se controla, las complicaciones pueden variar desde hematomas de partes blandas hasta pérdida sanguínea grave. Las causas locales incluyen hemorragia de partes blandas y óseas. Las causas sistémicas incluyen problemas plaquetarios, trastornos de la coagulación o fibrinólisis excesiva, y problemas hereditarios o adquiridos (inducidos por fármacos). Hay una variedad amplia de técnicas que se indican para el tratamiento de la hemorragia posextracción, que incluyen intervenciones dirigidas a las causas locales y sistémicas. Esta es una actualización de una revisión publicada en junio de 2016.
Objetivos
Evaluar los efectos de las intervenciones para el tratamiento de diferentes tipos de hemorragia posextracción.
Métodos de búsqueda
El especialista en información del Grupo Cochrane de Salud Oral (Cochrane Oral Health's Information Specialist) buscó en las siguientes bases de datos: Registro Especializado de Ensayos Controlados del Grupo Cochrane de Salud Oral (Cochrane Oral Health) (hasta el 24 de enero de 2018), el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (the Cochrane Library, 2017, número 12), MEDLINE Ovid (1946 hasta el 24 de enero de 2018), Embase Ovid (1 de mayo de 2015 hasta el 24 de enero de 2018) y CINAHL EBSCO (1937 hasta el 24 de enero de 2018). Se hicieron búsquedas de ensayos en curso en el US National Institutes of Health Trials Registry (ClinicalTrials.gov) y en la World Health Organization International Clinical Trials Registry Platform. Se realizaron búsquedas en las listas de referencias de las revisiones sistemáticas pertinentes.
Criterios de selección
Se consideraron los ensayos controlados aleatorizados (ECA) que evaluaron cualquier intervención para tratar la HPE, con participantes masculinos o femeninos de cualquier edad, independientemente del tipo de diente (anterior o posterior, mandibular o maxilar). Los ensayos podrían comparar un tipo de intervención con otro, con placebo o con ningún tratamiento.
Obtención y análisis de los datos
Tres pares de autores de la revisión examinaron de forma independiente los registros obtenidos de la búsqueda. Se obtuvieron las copias completas de los ensayos potencialmente pertinentes. Si se hubieran extraído datos se habrían seguido los métodos descritos en el Manual Cochrane para revisiones sistemáticas de intervenciones para el análisis estadístico.
Resultados principales
No se encontraron ensayos controlados aleatorizados apropiados para inclusión en esta revisión.
Conclusiones de los autores
No fue posible identificar informes de ensayos controlados aleatorizados que evaluaran los efectos de diferentes intervenciones para el tratamiento de la hemorragia posextracción. Debido a la falta de evidencia fiable sobre este tema, los médicos deben utilizar su experiencia clínica para determinar las formas más apropiadas para tratar esta afección, en dependencia de los factores relacionados con los pacientes. Se necesitan ensayos clínicos bien diseñados y realizados de forma apropiada sobre este tema que se ajusten a la declaración CONSORT (www.consort‐statement.org/).
PICOs
Resumen en términos sencillos
Intervenciones para el control de la hemorragia después de la extracción de dientes
Pregunta de la revisión
Esta revisión se realizó para evaluar diferentes intervenciones para el tratamiento de la hemorragia después de la extracción de dientes.
Antecedentes
Después de la extracción de dientes es normal que el área sangre y luego coagule, generalmente a los pocos minutos. Es anormal si la hemorragia continúa sin que se forme el coágulo o persiste por más de ocho a 12 horas; este hecho se conoce como hemorragia posextracción (HPE). Dichos incidentes de hemorragia pueden causar dificultades a los pacientes, que pudieran necesitar consultas e intervenciones dentales de urgencia. Las causas de la HPE pueden ser locales, una enfermedad sistémica o un fármaco. Para controlar esta hemorragia se practican muchos métodos locales y sistémicos, según la pericia del médico. Para informar a los médicos acerca del mejor tratamiento se necesita evidencia de estudios que hayan asignado al azar a pacientes a uno de al menos dos grupos diferentes que reciban tratamientos diferentes o ningún tratamiento (es decir, "ensayos controlados aleatorizados" o ECA).
Características de los estudios
Los autores, que trabajan con el Grupo Cochrane de Salud Oral (Cochrane Oral Health Group), actualizaron esta revisión de ECA que evalua intervenciones para tratar la hemorragia después de la extracción de dientes. La revisión original se publicó en enero de 2016. Para esta versión, se buscó en la bibliografía médica y dental hasta el 24 de enero de 2018. No se encontraron ECA que cumplieran los criterios de inclusión para esta revisión.
Resultados clave y calidad de la evidencia
Esta revisión mostró que no existe evidencia de ECA de la efectividad de ninguna intervención disponible para la hemorragia posextracción. Se necesitan ECA de alta calidad para ayudar a los médicos y los pacientes a hacer elecciones fundamentadas acerca de las opciones de tratamiento.
Authors' conclusions
Summary of findings
| Interventions for treating post‐extraction bleeding | ||||||
| Patient or population: people with post‐extraction bleeding Settings: hospital or dental practice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bleeding, as measured by:
| No data are available as no RCTs have been conducted on interventions to treat post‐extraction bleeding | |||||
| Patient‐reported outcomes related to pain or discomfort during the procedure | ||||||
| Treatment‐associated average cost | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Tooth removal, or extraction, is one of the most common invasive oral surgical procedures carried out in routine dental practice (Van Galen 2014), and post‐extraction bleeding is a recognised, frequently encountered complication (McCormick 2014a). Immediately following the removal of a tooth, bleeding or oozing commonly occurs. This bleeding can be easily controlled in most cases (Amer 2014), and almost completely stops within eight hours of extraction. However, sometimes it may continue, resulting in a life‐threatening situation (Funayama 1994). It is important to distinguish between active bleeding from the surgical site and oozing. The active bleeding complication is commonly termed 'post‐extraction bleeding' (PEB) or 'post‐operative bleeding after extraction'. Amer 2014 has described PEB as "evidence of bleeding beyond the pressure pack". Lockhart 2003 has provided four criteria to define PEB, namely that it:
-
continues beyond 12 hours;
-
causes the patient to call or return to the dental practitioner, or go to the accident and emergency department;
-
results in the development of a large haematoma or ecchymosis within the oral soft tissues; or
-
requires a blood transfusion, hospitalisation, or both.
The rate of postoperative bleeding after extraction of mandibular third molars is 0.6% and after extraction of maxillary third molars is 0.4% (Chiapasco 1993). Jensen 1974 reviewed 103 cases of postoperative prolonged bleeding after oral surgery and reported that 75% of PEB occurred within eight hours of the surgery, and only four patients had coagulation deficiencies. He also reported that postoperative prolonged bleeding from the mandibular molars is more common (80%) than bleeding from the maxillary molars (20%) because of the highly vascular floor of the mouth. Wells 2000 reported that the risk of prolonged bleeding was 0.2% to 1.4% in cases of third molar removal surgery. Iwabuchi 2014 reported 2.77% clinically‐significant PEB in patients receiving warfarin therapy, and 0.39% in non‐warfarin groups, regardless of the type of teeth (95% CI 0.65% to 4.10%). Kataoka 2016 reported that the incidence of PEB ranged from 0 to 26% in their cohort study. Yagyuu 2017 reported that the risk of post‐extraction bleeding was similar for patients on direct oral anticoagulants and Vitamin K antagonist extractions.
Post‐extraction bleeding has been attributed to various factors that can be broadly classified as local and systemic (McDonnell 2013; Van Galen 2014). Post‐extraction bleeding can be caused locally, from soft tissue or bone bleeding. Soft tissue bleeding can be due to traumatic extraction, leading to laceration of blood vessels (arterial, venous or capillary). Bone or osseous bleeding can be from either the nutrient canals or from the central vessels. Inflammation at the site of extraction, the presence of infection, traumatic extraction, and failure of the patient to follow post‐extraction instructions have also been associated with PEB. Systemic factors include platelet problems, coagulation disorders or excessive fibrinolysis, and inherited or acquired problems (medication induced).
Post‐extraction bleeding can be categorised as primary prolonged bleeding, intermediate or reactionary prolonged bleeding, and secondary prolonged bleeding. Primary prolonged bleeding occurs during the extraction procedure, and may be due to traumatic extraction leading to laceration of blood vessels, infections, such as periapical granuloma, or injury to the bone. Patients with primary prolonged bleeding present with their mouth actively filling with blood immediately after removing the haemostatic dressing. Reactionary bleeding occurs a few hours post‐extraction and is more common in patients with systemic disorders or patients on anticoagulant therapy. Secondary bleeding (liver clots) usually occurs 7 to 10 days after extraction, and is a complication rarely encountered in dental practice (Malik 2008; Table 1). Abdullah 2014 has classified PEB as mild (oozing), moderate (bleeding persisting on the second day of extraction), and severe (any bleeding that needed hospitalisation).
| Normal bleeding | Post‐extraction bleeding | ||
| Primary | Reactionary | Secondary | |
|
|
|
|
|
In this review, we considered all the definitions and classifications described above as PEB.
Description of the intervention
The treatment of bleeding complications following a dental extraction is an essential skill for the dental practitioner (McCormick 2014b). Clinical decision making on how to control PEB depends on multiple factors, including the surgical location and site of bleeding, wound size, extent of bleeding, accessibility of the bleeding site, and timing of bleeding (Howe 2013). Furthermore, the selection of an intervention strategy to achieve haemostasis (blood clot formation at the site of vessel injury (Traver 2006)) also depends upon whether the patient is taking any medication or is suffering from any systemic disease, such as cirrhosis, that can affect bleeding and coagulation (McCormick 2014b).
Interventions for treating PEB can be broadly categorised into local and systemic interventions. Local interventions can be further subdivided into surgical interventions, non‐surgical interventions and a combination of both.
Local interventions
-
Surgical intervention mainly involves suturing the extraction or bleeding site (Bajkin 2014; Van Galen 2014).
-
Non‐surgical haemostatic measures, or styptics, encompass an array of pharmacotherapies, sealants, adhesives, absorbable agents, biologics, and combination products (Howe 2013). Common haemostatic agents used in oral surgery in extraction sites include the following (Al‐Belasy 2003; Mingarro‐de‐León A 2014): local pressure application with gauze, oxidised cellulose (Abdullah 2014), gel foam, thrombin, collagen fleeces (Baumann 2009), cyanoacrylate glue, acrylic or surgical splints (Anderson 2013), local antifibrinolytic solutions, such as tranexamic acid mouthwash (Carter 2003), fibrin glue or adhesive (Cocero 2015), resorbable gelatin sponge, collagen sponge, gauze soaked with tranexamic acid (Perdigão 2012), chlorhexidine bio‐adhesive gel, calcium alginate (Scarano 2014), Haemocoagulase (Joshi 2014), Ankaferd Blood Stopper (Amer 2014), green tea extract (Soltani 2014), Chitosan‐based dressings (Pippi 2015; Sharma 2017), and bone wax.
-
Various combinations of surgical and non‐surgical interventions have also been used, such as tranexamic acid mouthwash along with gelatin sponge and sutures, and fibrin glue with collagen fleece and sutures (Al‐Belasy 2003).
Systemic interventions
Systemic interventions are especially important in patients who have an associated systemic cause for bleeding. The role of local haemostatics is limited in these cases, because their use results in only temporary cessation of bleeding (Auluck 2004). Systemic interventions include administration of fresh frozen plasma (FFP), platelets, or both (Cocero 2015), factor replacement therapy, using recombinant or plasma‐derived anti‐haemophilic factor A (FVIII) or anti‐haemophilic factor B or Christmas factor (FIX) in the case of haemophilia, and plasma‐derived Von Willebrand factor (VWF)/FVIII concentrates in the case of Von Willebrand disease (Anderson 2013), intranasal desmopressin (Stanca 2010), intravenous synthetic vasopressin (Minkin 2015), oral or intravenous tranexamic acid (Morimoto 2004), oral or intravenous epsilon amino‐d‐caproic acid (Van Galen 2014). There are contradictory opinions on discontinuation of antithrombotic medications; for example, Aframian 2007 suggests the discontinuation of these medications, whereas Wahl 2016 suggests these medications for dental procedures should not be interrupted, as the prognosis of potential post‐extraction bleeding that could result from antithrombotic continuation is better than the prognosis of a potential stroke or heart attack that could follow antithrombotic interruption.
How the intervention might work
Haemostasis, or control of bleeding, in the oral cavity is dependent on the dynamic balance between fibrin formation and resolution and is influenced by the external environment, which contains both plasminogen and plasminogen activators (Carter 2003). It is a complex interaction between platelets, plasma proteins, and coagulation and fibrinolytic pathways. The clotting cascade involves the sequential activation of proenzymes in a stepwise response, which ultimately provides local generation of fibrin lattices that reinforce the platelet plug (Traver 2006). The coagulation process consists of three main phases: initiation, amplification, and propagation (Figure 1; Glick 2013). The initiation phase begins with injury to the endothelium and tissue factor release, ultimately leading to thrombin formation. Platelet aggregation and activation occur during the amplification phase (Glick 2013), and provide the initial haemostatic response (Traver 2006). Finally, fibrin formation and stabilisation of the platelet clot occur during the propagation phase (Glick 2013).
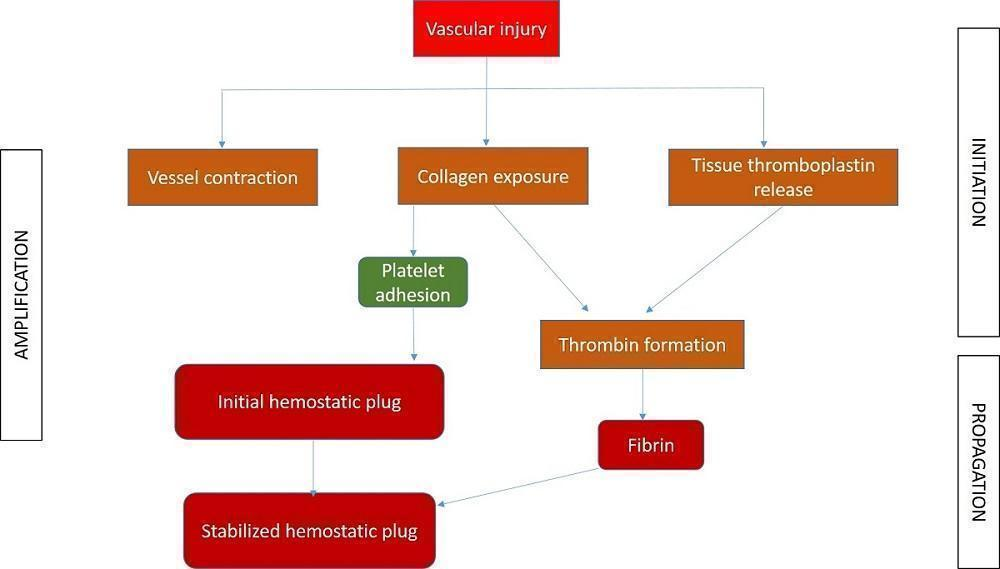
Different phases of coagulation
Different interventions to control PEB basically interfere with the clotting cascade at different levels, resulting in cessation of bleeding. The mechanism of action of various interventions can be broadly summarised, based on the different phases.
-
Initiation phase: pressure packs, suture, bone wax, cellulose, styptics, gel foam, tranexamic acid, aminocaproic acid, cryoprecipitate, desmopressin (DDAVP), factor VIII concentrate and prothrombin complex concentrate (PCC) act at different stages of this phase.
-
Amplification phase: ethamsylate, haemostatic collagen and Actcell® act during this phase.
-
Propagation phase: cryoprecipitate and recombinant factor (VIIa) act during this phase.
Local interventions work either mechanically or by augmenting the coagulation cascade. Haemostatic agents act to stop bleeding by causing vasoconstriction or promoting platelet aggregation, whereas tissue adhesives or sealants bind to and close defects in tissue (Traver 2006). Systemic interventions work by inhibiting fibrinolysis or promoting coagulation (Van Galen 2014).
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain in the Cochrane Library (Worthington 2015). This review was identified as a priority title by the oral and maxillofacial surgery expert panel (Cochrane OHG priority review portfolio).
A wide array of techniques are suggested for the treatment of PEB and different guidelines have been published (Higginson 2007; University of Cambridge). Until our first version of this review in June 2016, there had been no Cochrane review to summarise the effects of the various interventions available to treat PEB and provide evidence to guide clinical dental practice. Considering the different complex interventions addressing various outcome measures, it seems important to attempt to describe the components of interventions and to identify effective intervention strategies. A systematic review can inform the implementation of different approaches and trigger the development of new interventions on the basis of current best evidence. A systematic review on this topic is also needed since interventions of questionable effectiveness and unclear consequences might be in use.
Objectives
To assess the effects of interventions for treating different types of post‐extraction bleeding.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) evaluating any intervention for treating post‐extraction bleeding (PEB). We excluded quasi‐RCTs, cross‐over trials and preventive trials. We had planned to include split‐mouth studies, provided there was no possibility of contamination. Split‐mouth design is one of the self‐controlled study designs that is unique to dentistry. The design is characterised by subdividing the mouth of the subject into homogeneous within‐patient experimental units such as quadrants, sextants, contralateral or ipsilateral quadrants or sextants, or a symmetrical combination of these.
Types of participants
We considered trials with participants of any age and either gender, who reported PEB, regardless of the type of teeth (anterior or posterior, mandibular or maxillary).
Types of interventions
We considered any surgical or non‐surgical technique or material used for the treatment of PEB. We had planned to make the following comparisons.
-
Direct comparisons of different interventions
-
Intervention versus placebo or no treatment
Types of outcome measures
Primary outcomes
-
Bleeding ‐ measured by:
-
amount of blood loss;
-
complete cessation of bleeding, as assessed clinically by the investigator;
-
time required for the control of bleeding.
Secondary outcomes
-
Patient‐reported outcomes related to pain or discomfort during the procedure;
-
Treatment‐associated average cost;
-
Adverse effects.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
-
Cochrane Oral Health’s Trials Register (searched 24 January 2018) (Appendix 1);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 24 January 2018) (Appendix 2);
-
MEDLINE Ovid (1946 to 24 January 2018) (Appendix 3);
-
Embase Ovid (1 May 2015 to 24 January 2018) (Appendix 4);
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 24 January 2018) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Due to the Cochrane Centralised Search Project to identify all clinical trials in the database and add them to CENTRAL, only most recent months of the Embase database were searched at the review's inception, and this search was updated for this version of the review. See the searching page on the Cochrane Oral Health website for more information. No other restrictions were placed on the date of publication when searching the electronic databases.
Searching other resources
The following trial registries were searched for ongoing studies:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 24 January 2018) (Appendix 6);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 24 January 2018) (Appendix 7).
We searched the reference lists of relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions.
Data collection and analysis
Selection of studies
Three pairs of review authors (Ashok L (AL) and Prashanti Eachempati (PE), Himanshi Aggarwal (HA) and Kiran Kumar (KK), and Muthu MS (MMS) and Haszelini Hassan (HH)), independently and in duplicate, screened the titles and abstracts from the electronic searches to identify potentially eligible studies. The search was designed to be sensitive and include controlled clinical trials; these were filtered out early in the selection process if they were not randomised. We obtained full‐text copies of all eligible and potentially eligible studies, which were independently evaluated by two authors (Sumanth Kumbargere Nagraj (SKN) and PE) . We resolved disagreements by discussion. When resolution was not possible, we consulted an arbiter (Adinegara Lutfi Abas). We assessed articles in languages other than English after the abstracts had been translated (Table 1). We did not find any trials that fulfilled the inclusion criteria.
Data extraction and management
We had planned that two review authors (SKN, PE) would independently extract the data; and would not have been blinded to the authors. We would have resolved any disagreements by discussion between the two review authors, if necessary, consulting a third review author (PE) in order to reach consensus. We had planned to extract data using a customised data extraction form. All the items in the data extraction form were designed following guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have entered study details into the 'Characteristics of included studies' table in Review Manager (RevMan) software (RevMan 2014), recording the following details for each included trial:
-
publication details such as year of publication, language;
-
demographic details of the report;
-
inclusion and exclusion criteria;
-
type of trial, sample size, method of randomisation, allocation concealment, blinding, method of assessing the outcomes and drop‐outs, if any;
-
type of intervention;
-
details of the outcomes reported;
-
duration of follow‐up;
-
results of the intervention;
-
funding details.
We had planned to write, email or telephone the contact author of included studies when clarification of details or additional data were required.
Assessment of risk of bias in included studies
Two review authors (SKN and PE) had planned to independently assess the risk of bias in the included trials in seven domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective outcome reporting (reporting bias);
-
other biases.
For each of these components, we had planned to assign a judgment regarding the risk of bias as either high, low, or unclear based on guidance in Higgins 2011. We had planned to contact the trial authors if details were missing or unclear, and resolve disagreements through consensus. We had planned to record our judgements and justifications in 'Risk of bias' tables for each included study and generate a 'Risk of bias' summary graph and figure. We would have used these judgements to grade the overall quality of evidence for each comparison and outcome in the 'Summary of findings' tables.
We had planned to summarise the risk of bias according to Higgins 2011; see Table 2.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
We had expected our primary outcome to be expressed in dichotomous data. We would have expressed the effect estimate as a risk ratio (RR) with 95% confidence intervals (CI). We had expected our secondary outcomes to be presented as dichotomous data, continuous data, or ordinal scales. We had planned to use means and standard deviations to calculate mean differences and 95% CI for continuous data measured with the same scales and standardised mean differences if studies used different scales to measure the same outcome.
If data were expressed in ordinal scales, we had planned to explore the possibility of converting them to dichotomous outcomes. If outcomes were reported at baseline, trial endpoints, or follow‐up, we had planned to extract the mean change and standard deviation from baseline for each treatment group. We had intended to pool either end scores or change scores, but preferred end scores when available; we would have combined end and change scores where necessary.
We had planned to analyse data expressed as counts (number of bleeding incidents) in the same way as continuous data.
Unit of analysis issues
We expected two types of non‐standard study designs in this review:
-
multiple treatment groups;
-
split‐mouth design
We were expecting data related to repeated observation on participants for our secondary outcome of time required for the control of bleeding. In this case, we had planned to follow the method described in section 9.3.4 of the Cochrane Handbook (Higgins 2011).
In trials where adverse effects were described as counts, we wanted to follow the method described in section 9.2.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the case of dropouts, we had planned to use what the paper reports and deal with it in the 'Risk of bias' assessment. We were not expecting to find any cluster‐randomised trials for this condition.
Dealing with missing data
We had planned to contact study authors to obtain missing data. We would have used the methods outlined in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions to calculate missing standard deviations. If it had not been possible to calculate the SDs, we would have described the outcomes qualitatively (Higgins 2011).
Assessment of heterogeneity
If meta‐analyses were performed, we would have assessed heterogeneity using a Chi² test, where a P value < 0.1 indicated statistically significant heterogeneity. We would have quantified heterogeneity using the I² statistic as follows:
-
0% to 40% implies slight heterogeneity;
-
30% to 60% moderate heterogeneity;
-
50% to 90% substantial heterogeneity;
-
75% to 100% considerable heterogeneity.
If there was considerable heterogeneity (I² > 75%), which could not be explained by the subgroup analyses, we planned not to conduct meta‐analysis. We had planned to interpret I² values between 0% to 40% as possibly insignificant, 30% to 60% as possibly significant, 50% to 90% as possibly substantial, and 75% to 100% as possibly very substantial ('considerable'); depending on whether the inconsistency in results was due to differences in the direction of effect estimates between trials rather than due to differences in the magnitude of effect estimates favouring an intervention (Deeks 2011).
Assessment of reporting biases
If there were more than 10 studies included in a meta‐analysis, we had planned to assess the possible presence of reporting bias by testing for asymmetry in a funnel plot. If present, we would have carried out statistical analyses using the methods described by Egger 1997.
Data synthesis
We had planned to analyse the data using RevMan software (RevMan 2014). If the data available from the studies had similar comparisons and outcomes, we would have undertaken a meta‐analysis. Our general approach would have been to use a random‐effects model. With this approach, the CIs for the average intervention effect are wider than those obtained using a fixed‐effect approach, leading to a more conservative interpretation. We wanted to use all end scores or all change scores when available, but would have combined end and change scores where necessary, using the criteria in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We had planned to report the results from studies not included in a meta‐analysis in additional tables.
Subgroup analysis and investigation of heterogeneity
If there was significant heterogeneity, we had planned to explore the reasons by performing the following subgroup analyses based on different groups of patients. The subgroups were to be divided based on:
-
type of PEB (primary, reactionary, or secondary);
-
type of extraction (surgical or forceps);
-
type of underlying bleeding or clotting disorder (deficiency of factors, qualitative disorders, vessel disorders);
-
drug history (anticoagulant, antiplatelet, or combination);
-
type of teeth (deciduous or permanent, mobile or firm, maxillary or mandibular, anterior or posterior).
Sensitivity analysis
If there were sufficient included studies, we would have undertaken sensitivity analysis based on risk of bias, including only studies at low risk of bias.
Summarising findings and assessing the quality of the evidence
We had planned to use the GRADE approach to interpret findings (Schunemann 2008). We had planned to use GRADE Profiler software (GRADEpro GDT 2014), and import data from RevMan 2014 to create 'Summary of findings' tables for each comparison included in the review. These tables were to provide information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined, and the sum of available data on the primary and secondary outcomes. The GRADE approach considers ‘quality’ to be a judgment of the extent to which we can be confident that the estimates of effect are correct (Schunemann 2008). A body of evidence from randomised controlled studies is initially graded as high and downgraded by one or two levels on each of five domains, after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency of results, precision of the results, and the possibility of publication bias. A quality level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. 'Low' and 'very low' quality evidence limit our confidence in the effect estimate (Balshem 2011).
Results
Description of studies
We found no published or ongoing randomised controlled trials that evaluated interventions for treating post‐extraction bleeding.
Results of the search
Our search strategy identified 1916 titles and abstracts of studies up to 24 January 2018. These were independently assessed for relevance by three pairs of review authors (AL and PE; HA and KK; MMS and HH). We checked the reference lists of the excluded studies and added another 24 references. After removal of duplicates, we had a total of 1187 records. We rejected 1147 based on the abstracts. We obtained full texts for other 40 trials. Twenty‐eight of these were not RCTs (studies not in English that we translated are listed in Appendix 8). We excluded the other 12 trials for reasons described below. None of the trials met the inclusion criteria for our review (Figure 2).
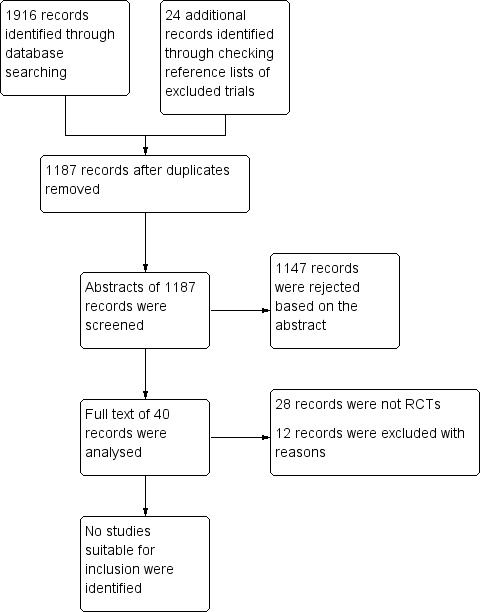
Study flow diagram
Included studies
We did not find any studies suitable for inclusion.
Excluded studies
We excluded 12 trials for the following reasons. Al‐Mubarak 2006 evaluated the safety of dental extractions in patients taking oral anticoagulants. The study evaluated the efficacy of suturing and the effect of warfarin on prevention of post‐extraction bleeding. Ak 2012; Carter 2003; Howard 1973; Henderson 1998; Pinsent 1986; CTRI/2017/09/009784; NCT02918045; NCT03108365 and Zhou 1985 were preventive trials. Kjellman 1973 assessed pain management after removal of impacted third molars. Medeiros 2011 assessed the amount of intraoperative and postoperative bleeding with acetylsalicylic acid versus acetylsalicylic acid therapy that was suspended seven days preoperatively.
Risk of bias in included studies
No trials were included.
Effects of interventions
No studies fulfilled our inclusion criteria.
Discussion
Post‐extraction bleeding (PEB) is one of the treatment complications of dental extraction that might make a patient panic and seek immediate dental consultation. With the increasing number of patients on anticoagulant therapy with aspirin, warfarin, and clopidogrel, the chance of encountering PEB is increasing. Usually, these people are aware of their medical condition and report their medical history. It is normal to use precautionary measures to prevent PEB in such patients. However, this may not be the situation in low‐ and middle‐income countries, where the majority of patients may not give a proper medical and drug history, and medical records may not be accessible. Hence, it is important to know how to control PEB in cases where no preventive measures were used.
Post‐extraction bleeding can also occur due to local or systemic problems that are not expected in routine dental extractions. We do not have any evidence‐based guidelines to manage such cases. The present review aimed to assess the effects of various interventions for the treatment of different types of PEB.
We did not find any suitable trials to include in our review. This is because most of the trials advocated preventive measures prior to and immediately after extraction, and reported either the incidence of PEB or tested preventive measures. Most of these trials reported the management of PEB based on clinician experience. Several trials tested whether anticoagulants, antiplatelets, or antifibrinolytics should be stopped before dental extraction, and reported varying incidence rates of PEB in control and intervention groups.
The majority of the preventive trials randomised extraction cases into intervention groups. An ideal trial for this review would randomise PEB (primary or reactionary or secondary) cases instead.
This topic seems to be an unexplored area of primary research. We found one observational trial in a German clinical trial register in which postoperative bleeding incidents after dental treatment in patients with and without anticoagulant therapy were studied (DRKS00009286). We found no non‐Cochrane systematic reviews on this topic. We identified two narrative reviews based on the authors' opinions (Leonard 1995; McCormick 2014a). We found two websites that have published guidelines to manage PEB (Emed handbook by Higginson 2007; University of Cambridge).
We observed the term 'post‐extraction bleeding' being used in different ways. Joshi 2014 used PEB terminology to describe the normal bleeding that happens after dental extraction in their study. Al‐Bahlani 2001 describes any type of bleeding after dental extraction as postoperative bleeding. This can create confusion and there is a need to standardise PEB terminology and its definition.

Different phases of coagulation
| Interventions for treating post‐extraction bleeding | ||||||
| Patient or population: people with post‐extraction bleeding Settings: hospital or dental practice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bleeding, as measured by:
| No data are available as no RCTs have been conducted on interventions to treat post‐extraction bleeding | |||||
| Patient‐reported outcomes related to pain or discomfort during the procedure | ||||||
| Treatment‐associated average cost | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Normal bleeding | Post‐extraction bleeding | ||
| Primary | Reactionary | Secondary | |
|
|
|
|
|
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |

