مقایسه اومکلیدینیوم بروماید در برابر دارونما در درمان افراد مبتلا به بیماری مزمن انسدادی ریه (COPD)
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase 3a study Number of study centres and locations: 153 centres in 14 countries (United States, Germany, Hungary, Netherlands, Estonia, Japan, Norway, Philippines, Denmark, Slovakia, Sweden, France, Ukraine, and Belgium) Study setting: multi‐centre | |
| Participants | Number screened: 2114 Intention‐to‐treat population: 1489 Severity of condition: moderate to very severe COPD Diagnostic criteria: ATS/ERS criteria Baseline mean post‐albuterol % predicted FEV1: 48.8 (UME), 48.5 (VI), 47.7 (UME/VI), 47.6 (placebo) Baseline mean smoking pack‐years: 44.0 (UME), 42.8 (VI), 45.4 (UME/VI), 43.6 (placebo) Current/former smoker, %: 53/47 (UME), 52/48 (VI), 50/50 (UME/VI), 52/48 (placebo) ≥ 40 years of age with a history of COPD, current or former smoker with a smoking history ≥ 10 pack‐years, post‐albuterol (salbutamol) FEV1 /FVC ratio < 0.70, FEV1 ≤ 70% predicted normal, and a score ≥ 2 on the modified Medical Research Council dyspnoea scale at screening Exclusion criteria: Current diagnosis of asthma or other known respiratory disorders, any clinically significant uncontrolled disease, an abnormal and significant ECG or 24‐hour Holter finding or significantly abnormal clinical laboratory findings Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Interventions: UME 125 μg, VI 25 μg, UME/VI 125/25 μg once daily via DPI in the morning Comparison: matching placebo once daily via DPI in the morning Concomitant medications: albuterol rescue medication and regular use of ICS at a stable dose (≤ 1000 μg/d fluticasone propionate or equivalent) were allowed Concomitant ICS users during study period, % of participants: 48 (UME), 46 (VI), 44 (UME/VI), 50 (placebo) Previous treatment with LABA, % of participants: 51 (UME), 51 (VI), 48 (UME/VI), 52 (placebo) Previous treatment with LAMA, % of participants: 37 (UME), 34 (VI), 35 (UME/VI), 32 (placebo) Previous treatment with ICS, % of participants: 48 (UME), 49 (VI), 44 (UME/VI), 52 (placebo) | |
| Outcomes | Primary outcome: Change from baseline in pre‐dose trough FEV1 on day 169 Secondary outcomes: Mean TDI focal score at day 168 Change from baseline in weighted mean FEV1 over 0 to 6 hours post dose at day 168 Other outcome measures: Change from baseline in mean SOBDA score for week 24 Rescue albuterol use (recorded daily using an electronic diary) HRQoL as measured by the SGRQ Time to first COPD exacerbation Adverse events | |
| Notes | Full‐text publication Study authors reported and declared possible conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from clinical study report): "the randomisation code was generated by GSK using a validated computerised system RandAll version 2.5" |
| Allocation concealment (selection bias) | Low risk | Quote (from clinical study report): "subjects were randomised using RAMOS, an Interactive Voice Response System (IVRS), a telephone based system used by the investigator or designee" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (from clinical study report): "double‐blind study; neither the subject nor the study physician knew which study drug the subject was receiving" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: double‐blind including outcomes assessor Quote (from clinical study report): "the interviewer was blinded for BDI/TDI" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Numbers of withdrawals and reasons were clearly stated for both intervention and placebo arms. Dropout was relatively balanced (UME/VI 19%, VI 26%, umeclidinium 23%, and placebo 33%) with similar reasons across groups. However, rates were high for a short duration study Primary analyses were performed on the intent‐to‐treat population, defined as all randomised participants who had received at least 1 dose of study medication Quote (from clinical study report): "missing data were not explicitly imputed in the primary MMRM analysis, although there was an underlying assumption that data were missing at random. The derived treatment differences were adjusted to take into account missing data. Sensitivity analyses used multiple imputation methods such as Missing at Random (MAR) approach, Copy Differences from Control (CDC) approach, and Last Mean Carried Forward (LMCF) approach." |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes in the protocol were reported in the published article, as well as in the clinical study report. Results for all outcomes were made available to the public on the website |
| Other bias | Low risk | No apparent source of bias was observed |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase 3 study Number of study centres and locations: 163 centres in 13 countries (United States, Bulgaria, Canada, Chile, Czech Republic, Greece, Japan, Mexico, Poland, Russia, South Africa, Spain, and Thailand) Study setting: multi‐centre | |
| Participants | Number screened: 2210 Intention‐to‐treat population: 1532 Severity of condition: moderate to severe COPD Diagnostic criteria: ATS/ERS criteria Baseline mean post‐albuterol % predicted FEV1: 46.8 (UME), 48.2 (VI), 47.8 (UME/VI), 46.7 (placebo) Baseline mean smoking pack‐years: 46.8 (UME), 44.7 (VI), 46.5 (UME/VI), 47.2 (placebo) Current/former smoker, %: 50/50 (UME), 47/53 (VI), 49/51 (UME/VI), 54/46 (placebo) ≥ 40 years of age with a history of COPD, current or former smoker with a smoking history ≥ 10 pack‐years, post‐salbutamol FEV1/FVC ratio < 0.70, post‐salbutamol FEV1 ≤ 70% of predicted normal, and a score ≥ 2 on the modified Medical Research Council dyspnoea scale at screening Exclusion criteria: Current diagnosis of asthma or other known respiratory disorders, including alpha‐1 antitrypsin deficiency, active tuberculosis, bronchiectasis, sarcoidosis, lung fibrosis, pulmonary hypertension, interstitial lung disease, any clinically significant uncontrolled disease (including cardiovascular‐related disease) as determined by study investigators, abnormal and clinically significant ECG or 24‐hour Holter ECG (if conducted), or significantly abnormal clinical laboratory finding Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Interventions: UME 62.5 μg, VI 25 μg, UME/VI 62.5/25 μg once daily via DPI in the morning Comparison: matching placebo once daily via DPI in the morning Concomitant medications: inhaled salbutamol (albuterol) as rescue medication and regular use of ICS at a stable dose (≤ 1000 μg/d of fluticasone propionate or equivalent) 30 days before screening was allowed Concomitant ICS users during study period, % of participants: 50 (UME), 49 (VI), 50 (UME/VI), 47 (placebo) Previous treatment with LABA, % of participants: 42 (UME), 38 (VI), 38 (UME/VI), 45 (placebo) Previous treatment with LAMA, % of participants: 18 (UME), 16 (VI), 16 (UME/VI), 21 (placebo) Previous treatment with ICS, % of participants: 55 (UME), 52 (VI), 53 (UME/VI), 51 (placebo) | |
| Outcomes | Primary outcome: Change from baseline in pre‐dose trough FEV1 on day 169 Secondary outcomes: Mean TDI focal score at day 168 Change from baseline in weighted mean (WM) FEV1 over 0 to 6 hours post dose at day 168 Other outcome measures: Change from baseline in mean SOBDA score for week 24 Rescue albuterol use (recorded daily using an electronic diary) HRQoL as measured by the SGRQ Time to first COPD exacerbation Adverse events | |
| Notes | Full‐text publication Study authors reported and declared possible conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from text): "A central randomisation schedule was generated using a validated computerised system (RandAll)" |
| Allocation concealment (selection bias) | Low risk | Quote (from text): "Patients were randomised using an automated, interactive telephone based system that registered and randomised medication assignment" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (from clinical study report): "double‐blind study; neither the subject nor the study physician knew which study drug the subject was receiving" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: double‐blind including outcomes assessor Quote (from clinical study report): "the interviewer was blinded for BDI/TDI" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Numbers of withdrawals and reasons were clearly stated for both intervention and placebo arms. Withdrawal rates and reasons were similar between groups although relatively high for short duration (UME/VI 20%, VI 24%, umeclidinium 22%, and placebo 27%) Primary analyses were performed on the intent‐to‐treat population, defined as all randomised participants who had received at least 1 dose of the double‐blind study medication Quote (from clinical study report): "missing data were not explicitly imputed in the primary MMRM analysis, although there was an underlying assumption that data were missing at random. The derived treatment differences were adjusted to take into account missing data. Sensitivity analyses used multiple imputation methods such as Missing at Random (MAR) approach, Copy Differences from Control (CDC) approach and Last Mean Carried Forward (LMCF) approach" |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes in the protocol were reported in the published article, as well as in the clinical study report. Results for all outcomes were made available to the public on the website |
| Other bias | Low risk | No apparent source of bias was observed |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase 3a study Number of study centres and locations: 53 centres in 6 countries (United States (28%), Romania (26%), Russian Federation (21%), South Africa (14%), Chile (7%), and Slovakia (4%)) Study setting: multi‐centre | |
| Participants | Number screened: 893 Intention‐to‐treat population: 562 Severity of condition: moderate to severe COPD Diagnostic criteria: ATS/ERS criteria Baseline mean post‐salbutamol % predicted FEV1: 54.2 (UME), 55.0 (UME/VI), 55.1 (placebo) Baseline mean smoking pack‐years: 39.2 (UME), 43.7 (UME/VI), 42.8 (placebo) Current/former smoker, %: 65/35 (UME), 60/40 (UME/VI), 65/35 (placebo) Current or former smokers ≥ 40 years of age, with smoking history ≥ 10 pack‐years and an established clinical history of COPD, as defined by ATS/ERS criteria, having a post‐salbutamol FEV1/FVC ratio < 0.70 and a post‐salbutamol FEV1 ≥ 35% and ≤ 80% of predicted values (as determined by Nutrition Health and Examination Survey III reference equations) Exclusion criteria: Current diagnosis of asthma or other respiratory disorders (including pulmonary hypertension and interstitial lung disease); historical/current evidence of clinically significant, uncontrolled cardiovascular, neurological, psychiatric, renal, hepatic, immunological, endocrine, or hematological abnormalities; hospitalisation for COPD/pneumonia within 12 weeks before first visit or lung resection in the 12 months before screening; hypersensitivity to any anticholinergic drug or beta2‐agonist; inability to withhold salbutamol and/or ipratropium bromide use for the 4‐hour period before spirometry; known or suspected history of alcohol or drug abuse; participation in the acute phase of a pulmonary rehabilitation programme; abnormal and significant findings from ECG monitoring, 24‐hour Holter monitoring, chest X‐rays, clinical chemistry, or haematology tests Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Interventions: UME 125 μg, UME/VI 125/25 μg once daily via DPI in the morning Comparison: matching placebo once daily via DPI in the morning Concomitant medications: salbutamol and/or ipratropium bromide as rescue medication via metered‐dose inhaler or nebules were permitted Concomitant ICS users during study period, % of participants: 32 (UME), 37 (UME/VI), 37 (placebo) Previous treatment with LABA, % of participants: 19 (UME), 20 (UME/VI), 21 (placebo) Previous treatment with LAMA, % of participants: 6 (UME), 7 (UME/VI), 7 (placebo) Previous treatment with ICS, % of participants: 33 (UME), 37 (UME/VI), 39 (placebo) | |
| Outcomes | Primary outcome: Number of participants with any AE or any SAE Secondary outcomes: Number of participants with at least 1 COPD exacerbation over the course of the 52‐week treatment period Time to first on‐treatment COPD exacerbation Change from baseline in alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), creatine kinase (CK), gamma glutamyl transferase (GGT), albumin, total protein, haemoglobin, calcium, carbon dioxide (CO2) content/bicarbonate, chloride, glucose, inorganic phosphorus (IP), potassium, sodium, urea/blood urea nitrogen (BUN), creatinine, direct bilirubin, indirect bilirubin, total bilirubin, and uric acid at months 3, 6, 9, and 12 Change from baseline in percentages of basophils, eosinophils, lymphocytes, monocytes, and segmented neutrophils in blood at months 3, 6, 9, and 12 Change from baseline in eosinophil count, platelet count, white blood cell (WBC) count, and hematocrit at months 3, 6, 9, and 12 Change from baseline to maximum SBP and change from baseline to minimum DBP over the 52‐week treatment period Maximum change from baseline in pulse rate, ECG parameters of QT interval corrected for heart rate by Bazett’s formula (QTcB), QT interval corrected for heart rate by Fridericia’s formula (QTcF), PR interval, and ECG parameter of heart rate over the 52‐week treatment period Number of participants with indicated ECG result interpretations at any time post baseline Number of participants with indicated change from screening to any time post baseline in Holter ECG interpretation Change from baseline in mean number of puffs of rescue medication (salbutamol and/or ipratropium bromide) per day over the 52‐week treatment period Change from baseline in percentage of rescue‐free days over the 52‐week treatment period Change from baseline in trough FEV1 and FVC at months 1, 3, 6, 9, and 12 | |
| Notes | Full‐text publication Study authors reported and declared possible conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from text): "Patients were randomised using codes generated by RandAll version 2.5, a validated computerised system" |
| Allocation concealment (selection bias) | Low risk | Quote (from text): "Patients were randomised using RAMOS (Randomisation and Medication Ordering System), an Interactive Voice Response System (IVRS), which is a telephone‐based randomisation system" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (from text): "Study drug was double‐blind. Neither the subjects nor the study site personnel knew the treatment assignments" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: double‐blind study Quote (from clinical study report): "ECG and Holter data were electronically transmitted to an independent cardiologist, blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Numbers of withdrawals and reasons were clearly stated for both intervention and placebo arms. Dropout rates were high but balanced across groups (UME/VI 37%, umeclidinium 41%, placebo 39%) The primary study population for all data presentations and analyses was the ITT population, defined as all participants randomised to treatment who received at least 1 dose of study drug |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes in the protocol were reported in the published article, as well as in the clinical study report. Results for all outcomes were made available to the public on the website |
| Other bias | Low risk | No apparent source of bias was observed |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase 3 study Number of study centres and locations: 27 centres in the United States, Germany, and Japan Study setting: multi‐centre | |
| Participants | Number screened: 246 Intention‐to‐treat population: 206 Severity of condition: moderate to severe COPD Diagnostic criteria: ATS/ERS criteria Baseline mean post‐salbutamol % predicted FEV1: 44.5 (UME 62.5), 47.9 (UME 125), 47.0 (placebo) Baseline mean smoking pack‐years: 45.2 (UME 62.5), 47.5 (UME 125), 52.3 (placebo) Current/former smoker, %: 54/46 (UME 62.5), 57/43 (UME 125), 53/47 (placebo) Inclusion criteria: ≥ 40 years of age with a clinical history of COPD, current or former (smoking‐free ≥ 6 months) cigarette smokers with a smoking history ≥ 10 pack‐years, post‐salbutamol FEV1/FVC ratio < 0.70 and post‐salbutamol FEV1 ≤ 70% predicted, and score ≥ 2 on the modified Medical Research Council dyspnoea scale at first visit Exclusion criteria: Current diagnosis of asthma or other clinically significant respiratory disorders other than COPD; any unstable, clinically significant disease or hospitalisation for COPD or pneumonia within 12 weeks of screening; use of systemic, oral, or parenteral corticosteroids within 6 weeks of screening or ICS > 1000 mg/d of fluticasone propionate or equivalent within 30 days of screening Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Interventions: UME 62.5 μg, UME 125 μg once daily via DPI in the morning Comparison: matching placebo once daily via identical DPI in the morning Concomitant medications: Inhaled salbutamol was permitted as needed but was withheld for 4 hours before and during study visits. ICS at a stable dose was allowed during run‐in and treatment periods. All inhaled bronchodilators were discontinued before screening (LABA at least 48 hours; tiotropium at least 14 days) Concomitant ICS users during study period, % of participants: 22 (UME 62.5), 23 (UME 125), 26 (placebo) Previous treatment with LABA, % of participants: 39 (UME 62.5), 42 (UME 125), 46 (placebo) Previous treatment with LAMA, % of participants: 35 (UME 62.5), 22 (UME 125), 32 (placebo) Previous treatment with ICS, % of participants: 23 (UME 62.5), 23 (UME 125), 26 (placebo) | |
| Outcomes | Primary outcome: Change from baseline in pre‐dose trough FEV1 on day 85 Secondary outcomes: Change from baseline in weighted mean (WM) FEV1 over 0 to 6 hours post dose on days 1, 28, and 84 Change from baseline in serial FEV1 at 1, 3, 6, 23, and 24 hours post dose on days 1 and 84 Other outcome measures: TDI focal score Proportion of participants with TDI score improvement ≥ 1 unit Trough FVC, WM FVC, serial FVC Time to onset (increase ≥ 100 mL above baseline in FEV1) Rescue salbutamol use (percentage of rescue‐free days and mean number of puffs per day) HRQoL assessed by the SGRQ COPD exacerbation Adverse events | |
| Notes | Full‐text publication Study authors reported and declared possible conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from text): "Treatment assignment was determined by a validated, computerised system (RandAll; GlaxoSmithKline, Slough, UK) and an automated, interactive telephone‐based system (GlaxoSmithKline Registration and Medication Ordering System (RAMOS), GlaxoSmithKline, Harlow, UK)" |
| Allocation concealment (selection bias) | Low risk | Quote (from text): "Treatment assignment was determined by a validated, computerised system (RandAll; GlaxoSmithKline, Slough, UK) and an automated, interactive telephone‐based system (GlaxoSmithKline Registration and Medication Ordering System (RAMOS), GlaxoSmithKline, Harlow, UK)" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (from text): "Patients and investigators were blinded to treatment assignment" and "patients were randomised 1:1:1 to receive UMEC 62.5 μg or 125 μg, or placebo once daily via identically appearing dry powder inhalers" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: double‐blind including outcomes assessor |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Numbers of withdrawals and reasons were clearly stated for both intervention and placebo arms. Withdrawal rate was relatively balanced with similar reasons between umeclidinium and placebo groups (umeclidinium 62.5 μg 10%, umeclidinium 125 μg 19%, and placebo 26%) Quote (from clinical study report): "missing data were not explicitly imputed in the primary MMRM analysis, although there was an underlying assumption that data were missing at random. The derived treatment differences were adjusted to take into account missing data. Sensitivity analyses used multiple imputation methods such as Missing at Random (MAR) approach, Copy Differences from Control (CDC) approach and Last Mean Carried Forward (LMCF) approach" |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes in the protocol were reported in the published article, as well as in the clinical study report. Results for all outcomes were made available to the public on the website |
| Other bias | Low risk | No apparent source of bias was observed |
AEs: adverse events; ATS: American Thoracic Society: BDI: Baseline Dyspnoea Index; COPD: chronic obstructive pulmonary disease: DBP: diastolic blood pressure; DPI: dry powder inhaler; ECG: electrocardiogram; ERS: Eurpoean Respiratory Society; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HRQoL: health‐related quality of life; ICSs: inhaled corticosteroids; ITT: intention‐to‐treat analysis; LABA: long‐acting beta2‐agonist; LAMA: long‐acting muscarinic antagonist; SAEs: serious adverse events; SBP: systolic blood pressure; SGRQ: St George's Respiratory Questionnaire; SOBDA: Shortness of Breath with Daily Activities; TDI: Transitional Dyspnoea Index; UME: umeclidinium bromide; VI: vilanterol.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study of umeclidinium bromide/vilanterol vs placebo with no umeclidinium monotherapy arm | |
| Cross‐over study of umeclidinium/vilanterol/fluticasone in healthy people | |
| Cross‐over study of umeclidinium/vilanterol/fluticasone in healthy people | |
| Study of umeclidinium in healthy adults | |
| Study of umeclidinium in healthy adults | |
| Cross‐over study | |
| Study duration of 28 days | |
| Study of umeclidinium/vilanterol with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium/vilanterol with no placebo arm | |
| Cross‐over study | |
| Study of umeclidinium/vilanterol vs fluticasone/salmeterol combination with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium/vilanterol vs fluticasone/salmeterol combination with no umeclidinium monotherapy and placebo arms | |
| Cross‐over study of umeclidinium/vilanterol with no placebo arm | |
| Cross‐over study of umeclidinium/vilanterol with no placebo arm | |
| Study of umeclidinium/vilanterol vs placebo with no umeclidinium monotherapy arm | |
| Study of umeclidinium vs tiotropium with no placebo arm | |
| Cross‐over study in healthy people | |
| Study of umeclidinium/vilanterol vs tiotropium plus indacaterol with no umeclidinium monotherapy and placebo arms | |
| Study duration of 7 days | |
| Cross‐over study in healthy adults | |
| Study of umeclidinium in healthy adults | |
| Cross‐over study in healthy people | |
| Study of umeclidinium/vilanterol vs tiotropium with no umeclidinium monotherapy and placebo arms | |
| Study of fluticasone/umeclidinium/vilanterol vs budesonide/formoterol with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium/vilanterol vs tiotropium with no umeclidinium monotherapy and placebo arms | |
| Cross‐over study | |
| Cross‐over study | |
| Cross‐over study | |
| Study of umeclidinium in healthy adults | |
| Cross‐over study in healthy people | |
| Non‐randomised study in healthy people | |
| Study of umeclidinium/vilanterol with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium in healthy adults | |
| Cross‐over study in healthy adults | |
| Cross‐over study of umeclidinium/vilanterol with no placebo arm | |
| Cross‐over study in healthy adults | |
| Study of umeclidinium/vilanterol vs placebo with no umeclidinium monotherapy arm | |
| Cross‐over study in healthy adults | |
| Study of umeclidinium/vilanterol vs indacaterol/tiotropium with no umeclidinium monotherapy and placebo arms | |
| Cross‐over study of umeclidinium/vilanterol with no umeclidinium monotherapy arm | |
| Cross‐over study of umeclidinium/vilanterol and indacaterol/glycopyrronium with no umeclidinium monotherapy and placebo arms | |
| Cross‐over study of umeclidinium/vilanterol and indacaterol/glycopyrronium with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium/vilanterol vs batefenterol with no umeclidinium monotherapy arm | |
| Study of ‘closed’ triple therapy (fluticasone/umeclidinium/vilanterol) vs 'open' triple therapy (fluticasone/vilanterol + umeclidinium) with no umeclidinium monotherapy and placebo arms | |
| Four‐week study of 'closed' triple therapy (fluticasone/umeclidinium/vilanterol) and 'open' triple therapy (fluticasone/vilanterol + umeclidinium) vs dual therapy (fluticasone/vilanterol) with no umeclidinium monotherapy arm | |
| Study of umeclidinium/vilanterol vs tiotropium/olodaterol with no umeclidinium monotherapy and placebo arms | |
| Study of fluticasone furoate/umeclidinium bromide/vilanterol in healthy participants | |
| Study of umeclidinium/vilanterol, umeclidinium, and salmeterol with no placebo arm | |
| Study of fixed‐dose triple combination umeclidinium/vilanterol/fluticasone with no umeclidinium monotherapy and placebo arms | |
| Study of umeclidinium vs glycopyrronium with no placebo arm | |
| Study of addition of umeclidinium to fluticasone/vilanterol with no umeclidinium monotherapy arm | |
| Study of addition of umeclidinium to fluticasone/vilanterol with no umeclidinium monotherapy arm | |
| Study of addition of umeclidinium to fluticasone/salmeterol with no umeclidinium monotherapy arm | |
| Study of addition of umeclidinium to fluticasone/salmeterol with no umeclidinium monotherapy arm | |
| Study of umeclidinium/vilanterol vs placebo with no umeclidinium monotherapy arm | |
| Study of umeclidinium/vilanterol vs fluticasone/salmeterol combination with no umeclidinium monotherapy and placebo arms | |
| Study of addition of umeclidinium to inhaled corticosteroid (ICS)/long‐acting beta2‐agonist (LABA) therapy with no umeclidinium monotherapy arm | |
| Cross‐over study of umeclidinium/vilanterol vs umeclidinium with no placebo arm | |
| Open‐label, single‐arm study of umeclidinium with no placebo arm | |
| Study of umeclidinium/vilanterol vs placebo with no umeclidinium monotherapy arm |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A 24‐week randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of 62.5 μg umeclidinium inhalation powder delivered once daily via a novel DPI in people with COPD |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase 3 study |
| Participants | Estimated enrolment: 454 Inclusion criteria: males and females 40 years of age and older; Asian ancestry; current or former smokers with at least 10 pack‐years of smoking; diagnosis of stable COPD according to ATS/ERS criteria with post‐bronchodilator FEV1 < 70% predicted and FEV1/FVC ratio < 0.70 at first visit Exclusion criteria: pregnancy or lactation; asthma; other respiratory diseases such as alpha‐1 antitrypsin deficiency, active lung infection (tuberculosis), lung cancer, clinically significant bronchiectasis, pulmonary hypertension, sarcoidosis or interstitial lung disease; significant cardiovascular, neurological, psychiatric, renal, hepatic, immunological, endocrine (including uncontrolled diabetes or thyroid disease), or haematological abnormalities; history of allergy or hypersensitivity to any LAMA or LABA; hospitalisation for COPD or pneumonia within 12 weeks; lung volume reduction surgery within the past 12 months; significant abnormalities on chest X‐ray or CT scan not due to the presence of COPD; abnormal cardiac rhythms on ECG; clinical chemistry and haematological abnormalities; use of systemic corticosteroids or antibiotics 4 weeks before screening, and use of other medications with bronchodilation action such as ICS/LABA, PDE4 inhibitors, other LAMAs, theophyllines, leukotriene inhibitors, oral beta2‐agonists, inhaled sodium cromoglycate, or nedocromil sodium |
| Interventions | Intervention: inhaled UME 62.5 μg once daily via novel DPI |
| Outcomes | Primary outcome: Weighted mean clinic visit FEV1 over 0 to 6 hours post dose at day 1 |
| Starting date | 9 May 2016 |
| Contact information | US GSK Clinical Trials Call Centre 877‐379‐3718 |
| Notes | Estimated study completion date: 14 October 2017 Source of support: GlaxoSmithKline |
ATS: American Thoracic Society: COPD: chronic obstructive pulmonary disease: CT: computed tomography; DPI: dry powder inhaler; ECG: electrocardiogram; ERS: Eurpoean Respiratory Society; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICSs: inhaled corticosteroids; LABAs: long‐acting beta2‐agonists; LAMAs: long‐acting muscarinic antagonists; PDE4: phosphodiesterase 4 inhibitor; TDI: Transitional Dyspnoea Index; UME: umeclidinium bromide.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with exacerbations requiring steroids, antibiotics, or both Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| Analysis 1.1 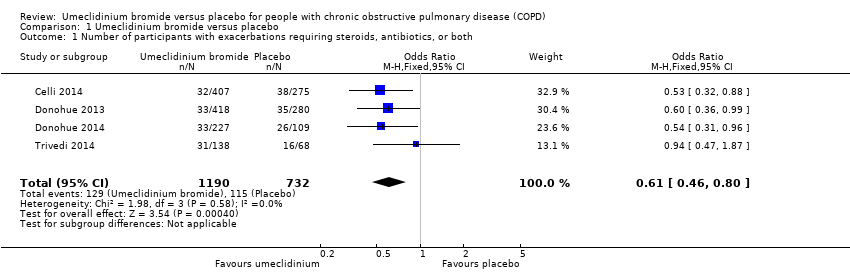 Comparison 1 Umeclidinium bromide versus placebo, Outcome 1 Number of participants with exacerbations requiring steroids, antibiotics, or both. | ||||
| 2 Quality of life: change from baseline in SGRQ total score Show forest plot | 3 | 1119 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.84, ‐0.75] |
| Analysis 1.2  Comparison 1 Umeclidinium bromide versus placebo, Outcome 2 Quality of life: change from baseline in SGRQ total score. | ||||
| 2.1 Umeclidinium 62.5 μg | 2 | 584 | Mean Difference (IV, Random, 95% CI) | ‐4.53 [‐6.97, ‐2.10] |
| 2.2 Umeclidinium 125 μg | 2 | 535 | Mean Difference (IV, Random, 95% CI) | ‐5.04 [‐15.05, 4.97] |
| 3 Quality of life: number of participants with ≥ 4 units improvement in SGRQ total score Show forest plot | 3 | 1397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.16, 1.82] |
| Analysis 1.3  Comparison 1 Umeclidinium bromide versus placebo, Outcome 3 Quality of life: number of participants with ≥ 4 units improvement in SGRQ total score. | ||||
| 3.1 Umeclidinium 62.5 μg | 2 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.19, 2.21] |
| 3.2 Umeclidinium 125 μg | 2 | 665 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 4 Non‐fatal serious adverse events Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.89, 2.00] |
| Analysis 1.4  Comparison 1 Umeclidinium bromide versus placebo, Outcome 4 Non‐fatal serious adverse events. | ||||
| 5 Total number of deaths Show forest plot | 4 | 1922 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.68 [0.52, 5.48] |
| Analysis 1.5 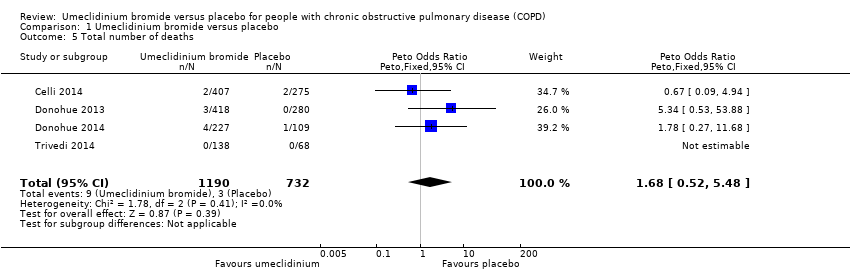 Comparison 1 Umeclidinium bromide versus placebo, Outcome 5 Total number of deaths. | ||||
| 6 Number of participants with hospital admissions due to COPD exacerbation Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.25, 2.92] |
| Analysis 1.6  Comparison 1 Umeclidinium bromide versus placebo, Outcome 6 Number of participants with hospital admissions due to COPD exacerbation. | ||||
| 6.1 Umeclidinium 62.5 μg | 2 | 801 | Odds Ratio (M‐H, Random, 95% CI) | 3.20 [0.91, 11.24] |
| 6.2 Umeclidinium 125 μg | 3 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.18, 1.03] |
| 7 Improvement in symptoms: TDI focal score Show forest plot | 3 | 1193 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.43, 1.09] |
| Analysis 1.7  Comparison 1 Umeclidinium bromide versus placebo, Outcome 7 Improvement in symptoms: TDI focal score. | ||||
| 8 Number of participants with ≥ 1 unit improvement in TDI focal score Show forest plot | 3 | 1441 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.37, 2.15] |
| Analysis 1.8  Comparison 1 Umeclidinium bromide versus placebo, Outcome 8 Number of participants with ≥ 1 unit improvement in TDI focal score. | ||||
| 9 Lung function: change from baseline in trough FEV1 (L) Show forest plot | 4 | 1381 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.12, 0.17] |
| Analysis 1.9  Comparison 1 Umeclidinium bromide versus placebo, Outcome 9 Lung function: change from baseline in trough FEV1 (L). | ||||
| 10 Lung function: change from baseline in trough FVC (L) Show forest plot | 4 | 1381 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [0.17, 0.26] |
| Analysis 1.10  Comparison 1 Umeclidinium bromide versus placebo, Outcome 10 Lung function: change from baseline in trough FVC (L). | ||||
| 11 Lung function: change from baseline in peak FEV1 (L) Show forest plot | 2 | 1035 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.14, 0.19] |
| Analysis 1.11 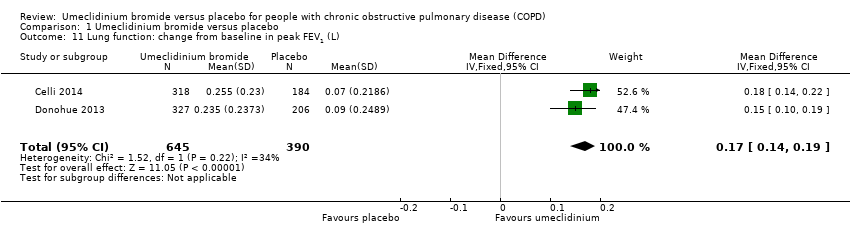 Comparison 1 Umeclidinium bromide versus placebo, Outcome 11 Lung function: change from baseline in peak FEV1 (L). | ||||
| 12 Adverse events (not including serious adverse events) Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.31] |
| Analysis 1.12  Comparison 1 Umeclidinium bromide versus placebo, Outcome 12 Adverse events (not including serious adverse events). | ||||
| 13 Use of rescue medications (change from baseline in number of puffs per day) Show forest plot | 4 | 1531 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.76, ‐0.14] |
| Analysis 1.13  Comparison 1 Umeclidinium bromide versus placebo, Outcome 13 Use of rescue medications (change from baseline in number of puffs per day). | ||||
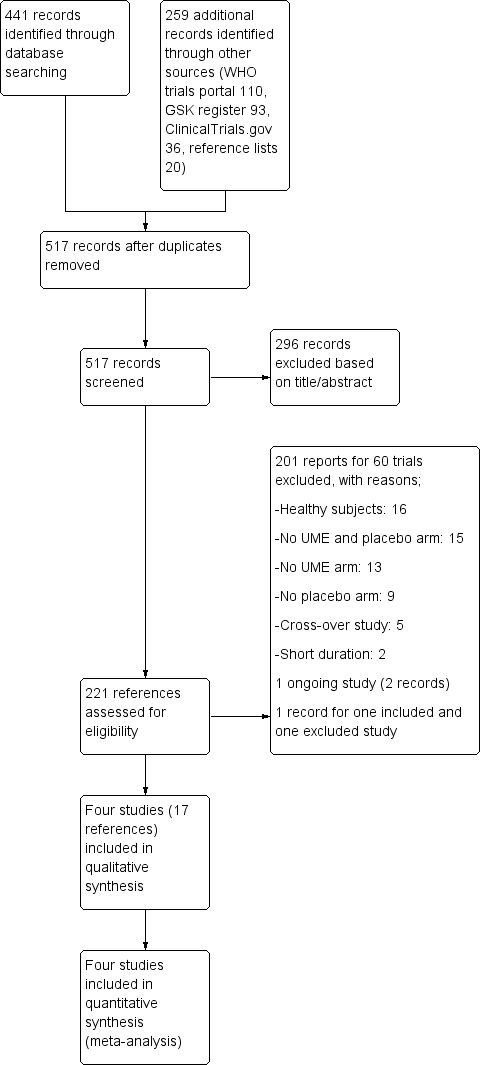
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Umeclidinium bromide versus placebo, outcome: 1.1 Number of participants with exacerbations requiring steroids, antibiotics, or both.
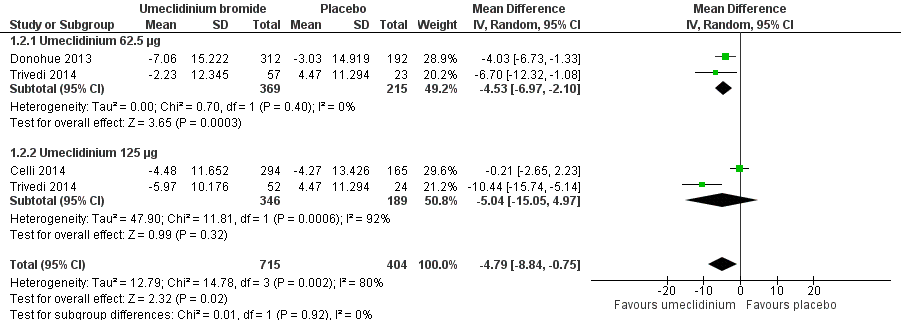
Forest plot of comparison: 1 Umeclidinium bromide versus placebo, outcome: 1.2 Quality of life: change from baseline in SGRQ total score.

Forest plot of comparison: 1 Umeclidinium bromide versus placebo, outcome: 1.8 Number of participants with ≥ 1 unit improvement in TDI focal score.

Forest plot of comparison: 1 Umeclidinium bromide versus placebo, outcome: 1.13 Use of rescue medications (change from baseline in number of puffs per day).
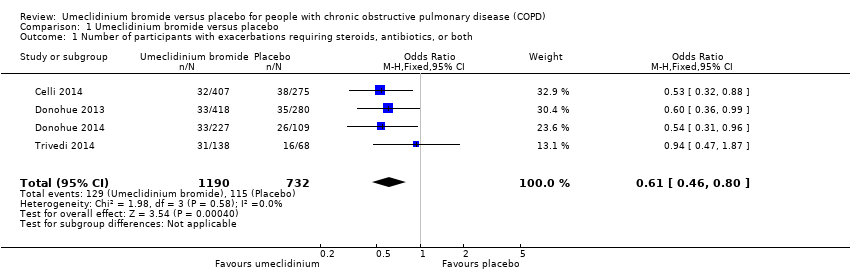
Comparison 1 Umeclidinium bromide versus placebo, Outcome 1 Number of participants with exacerbations requiring steroids, antibiotics, or both.
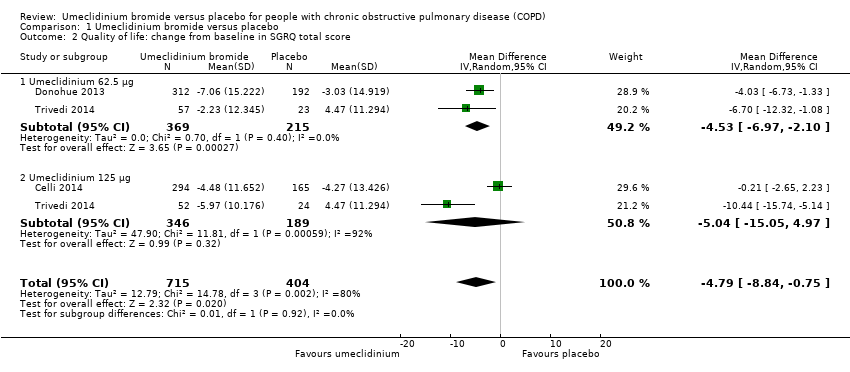
Comparison 1 Umeclidinium bromide versus placebo, Outcome 2 Quality of life: change from baseline in SGRQ total score.
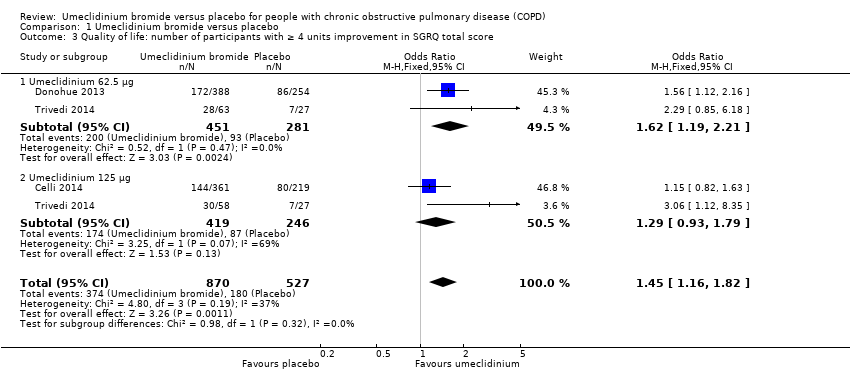
Comparison 1 Umeclidinium bromide versus placebo, Outcome 3 Quality of life: number of participants with ≥ 4 units improvement in SGRQ total score.
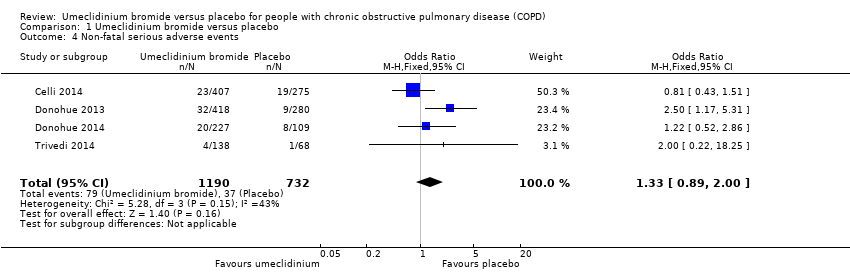
Comparison 1 Umeclidinium bromide versus placebo, Outcome 4 Non‐fatal serious adverse events.

Comparison 1 Umeclidinium bromide versus placebo, Outcome 5 Total number of deaths.

Comparison 1 Umeclidinium bromide versus placebo, Outcome 6 Number of participants with hospital admissions due to COPD exacerbation.

Comparison 1 Umeclidinium bromide versus placebo, Outcome 7 Improvement in symptoms: TDI focal score.

Comparison 1 Umeclidinium bromide versus placebo, Outcome 8 Number of participants with ≥ 1 unit improvement in TDI focal score.

Comparison 1 Umeclidinium bromide versus placebo, Outcome 9 Lung function: change from baseline in trough FEV1 (L).
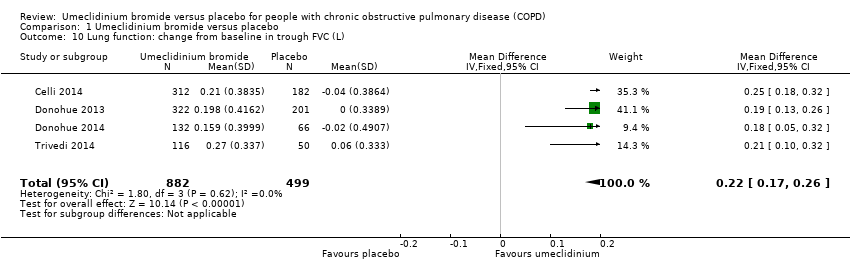
Comparison 1 Umeclidinium bromide versus placebo, Outcome 10 Lung function: change from baseline in trough FVC (L).

Comparison 1 Umeclidinium bromide versus placebo, Outcome 11 Lung function: change from baseline in peak FEV1 (L).

Comparison 1 Umeclidinium bromide versus placebo, Outcome 12 Adverse events (not including serious adverse events).

Comparison 1 Umeclidinium bromide versus placebo, Outcome 13 Use of rescue medications (change from baseline in number of puffs per day).
| Umeclidinium bromide vs placebo for stable chronic obstructive pulmonary disease | ||||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with umeclidinium bromide | |||||
| Number of participants with exacerbations requiring steroids, antibiotics, or both | 157 per 1000 | 102 per 1000 | OR 0.61 | 1922 | ⊕⊕⊕⊕ | |
| Quality of life: number of participants with ≥ 4 units improvement in SGRQ total score | 342 per 1000 | 429 per 1000 | OR 1.45 | 1397 | ⊕⊕⊕⊝ | Mean quality of life: change from baseline in SGRQ total score was 4.79 lower (8.84 lower to 0.75 lower) in umeclidinium group (1119 participants, 3 RCTs) |
| Non‐fatal serious adverse events | 51 per 1000 | 66 per 1000 | OR 1.33 | 1922 | ⊕⊕⊕⊝ | Larger studies may help refine this estimate |
| Number of participants with hospital admissions due to COPD exacerbation | 20 per 1000 | 18 per 1000 | OR 0.86 | 1922 | ⊕⊕⊝⊝ | Few events, so larger studies may help refine this estimate |
| Number of participants with ≥ 1 unit improvement in TDI focal score | 336 per 1000 | 464 per 1000 | OR 1.71 | 1441 | ⊕⊕⊕⊕ | Mean improvement in TDI focal score change from baseline was 0.76 higher (0.43 higher to 1.09 higher) in umeclidinium group (1193 participants, 3 RCTs) |
| Change from baseline in trough FEV1 (L) | Mean change from baseline in trough FEV1 (L) across control groups ranged from 0.123 to 0.139 | Mean change from baseline in trough FEV1 (L) in the intervention group was 0.14 higher (0.12 higher to 0.17 higher) | ‐ | 1381 | ⊕⊕⊕⊕ | |
| Adverse events (not including serious adverse events) | 239 per 1000 | 250 per 1000 | OR 1.06 | 1922 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a‐1 for inconsistency: unexplained significant heterogeneity b‐1 for imprecision: the CI includes non‐appreciable benefit and potential harm c‐2 for imprecision: the CI includes both appreciable benefit and harm | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with exacerbations requiring steroids, antibiotics, or both Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 2 Quality of life: change from baseline in SGRQ total score Show forest plot | 3 | 1119 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.84, ‐0.75] |
| 2.1 Umeclidinium 62.5 μg | 2 | 584 | Mean Difference (IV, Random, 95% CI) | ‐4.53 [‐6.97, ‐2.10] |
| 2.2 Umeclidinium 125 μg | 2 | 535 | Mean Difference (IV, Random, 95% CI) | ‐5.04 [‐15.05, 4.97] |
| 3 Quality of life: number of participants with ≥ 4 units improvement in SGRQ total score Show forest plot | 3 | 1397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.16, 1.82] |
| 3.1 Umeclidinium 62.5 μg | 2 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.19, 2.21] |
| 3.2 Umeclidinium 125 μg | 2 | 665 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 4 Non‐fatal serious adverse events Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.89, 2.00] |
| 5 Total number of deaths Show forest plot | 4 | 1922 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.68 [0.52, 5.48] |
| 6 Number of participants with hospital admissions due to COPD exacerbation Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.25, 2.92] |
| 6.1 Umeclidinium 62.5 μg | 2 | 801 | Odds Ratio (M‐H, Random, 95% CI) | 3.20 [0.91, 11.24] |
| 6.2 Umeclidinium 125 μg | 3 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.18, 1.03] |
| 7 Improvement in symptoms: TDI focal score Show forest plot | 3 | 1193 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.43, 1.09] |
| 8 Number of participants with ≥ 1 unit improvement in TDI focal score Show forest plot | 3 | 1441 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.37, 2.15] |
| 9 Lung function: change from baseline in trough FEV1 (L) Show forest plot | 4 | 1381 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.12, 0.17] |
| 10 Lung function: change from baseline in trough FVC (L) Show forest plot | 4 | 1381 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [0.17, 0.26] |
| 11 Lung function: change from baseline in peak FEV1 (L) Show forest plot | 2 | 1035 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.14, 0.19] |
| 12 Adverse events (not including serious adverse events) Show forest plot | 4 | 1922 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.31] |
| 13 Use of rescue medications (change from baseline in number of puffs per day) Show forest plot | 4 | 1531 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.76, ‐0.14] |

