Aumento de la dosis del antipsicótico versus cambio de antipsicótico para la falta de respuesta en la esquizofrenia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011884.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Myrto Samara: protocol development, study selection, data extraction, writing the report.
Elisabeth Klupp: study selection, data extraction.
Bartosz Helfer: protocol development.
Philipp Rothe: protocol development.
Johannes Schneider‐Thoma: writing of a summary report for the review.
Stefan Leucht: protocol development, study selection, data extraction, writing the report.
Sources of support
Internal sources
-
Klinik und Poliklinik für Psychiatrie und Psychotherapie, Technische Universität München Klinikum rechts der Isar, München, Germany.
Employs review authors Myrto T Samara, Bartosz Helfer, Philipp H Rothe, Johannes Schneider‐Thoma and Stefan Leucht.
External sources
-
Bundesministerium für Bildung und Forschung (BMBF), Germany.
Grant number: FKZ 01KG1407
Declarations of interest
Myrto Samara: none known.
Elisabeth Klupp: none known.
Bartosz Helfer: none known.
Philipp Rothe: none known.
Johannes Schneider‐Thoma: none known.
Stefan Leucht ‐ has received honoraria for consulting from LB Pharma, Lundbeck, Otsuka, TEVA, Geodon Richter, Recordati, LTS Lohmann, and Boehringer Ingelheim; and for lectures from Janssen, Lilly, Lundbeck, Otsuka, SanofiAventis, and Servier.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
The search term was developed by the Trial Search Co‐ordinator of the Cochrane Schizophrenia Group and the contact author of this review.
We would like to thank Dr Georgios Mikellides for peer reviewing the protocol and Joey Kwong for copy editing. We also thank Wing Chung Chang, Yuen‐Shan Ho and Brittany Dutton for peer reviewing the full review version.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 11 | Increasing antipsychotic dose versus switching antipsychotic for non response in schizophrenia | Review | Myrto T Samara, Elisabeth Klupp, Bartosz Helfer, Philipp H Rothe, Johannes Schneider‐Thoma, Stefan Leucht | |
| 2015 Oct 21 | Increasing antipsychotic dose versus switching antipsychotic for non response in schizophrenia | Protocol | Myrto T Samara, Bartosz Helfer, Philipp H Rothe, Stefan Leucht | |
Differences between protocol and review
We have removed the second sentence from Objectives ("We will include any antipsychotic drug studies in any people with schizophrenia, however diagnosed") as this is also described in Types of participants.
We have renamed outcomes from 'Clinically significant response' to 'Clinically important change'.
We have now specified 'Summary of findings' table outcomes should be 'Clinically important change' but if data were not available for these pre‐specified outcomes but were available for ones that are similar, we presented the closest outcome to the pre‐specified one in the table but took this into account when grading the finding.
We have updated the methods template to the latest version provided by Cochrane Schizophrenia. This does not involve a change to the methods but updating of references and rewording of some sections.
We have reworded some of the background to harmonise this review with its 'sibling review' Helfer 2015
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram for trial selection up to March 2017

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 1 Global state: clinically relevant response – as defined by trial.
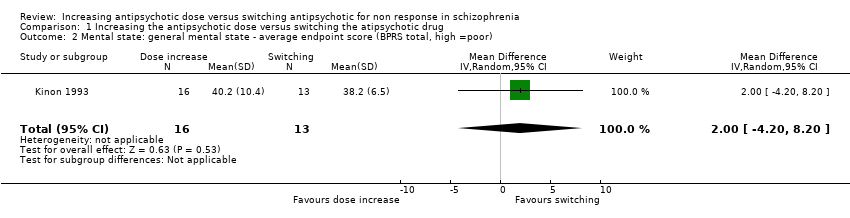
Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor).

Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor).
| Methods | Randomisation: random |
| Participants | Diagnosis: people with schizophrenia, schizoaffective disorder or schizophreniform disorder N > 450 |
| Interventions | All participants firstly receive treatment with one antipsychotic drug for at least 2 weeks. Those participants who do not at least minimally improve after 2 weeks, are considered non‐responders and are randomised to: 1. increasing the dose of the initial antipsychotic drug above the officially recommended dose range; or 2. switching the initial antipsychotic drug to another one with a different receptor profile; or 3. continuing treatment with the initial antipsychotic drug and at the same, initial dose (within the officially recommended dose range). |
| Outcomes | Response (defined as PANSS or BPRS decrease ≥ 50%)* Relapse Leaving the study early due to any reason Leaving the study early due to side effects General mental state: average change in general mental state scores Adverse effects: at least one adverse effect; clinically important general adverse effects; sudden and unexpected death Service use: time in hospital Quality of life All outcomes by time ‒ short term (up to 12 weeks), medium term (13 to 26 weeks) and long term (over 26 weeks) |
| Notes | *Primary outcome of interest |
| Increasing the antipsychotic dose compared to switching the antipsychotic drug for non responsein schizophrenia | ||||||
| Patient or population: patients with non response in schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Switching the atipsychotic drug | Increasing the antipsychotic dose | |||||
| Global state: Clinically relevant response – as defined by trial | 77 per 1000 | 125 per 1000 | RR 1.63 | 29 | ⊕⊝⊝⊝ | |
| Leaving the study early: Tolerability ‒leaving the study early due to side effects | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Leaving the study early: Acceptability ‒leaving the study early due to any reason | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome, |
| General mental state ‒BPRS total score at endpoint* | The mean general mental state ‒ BPRS total score at endpoint in the control groups was | The mean general mental state ‐ BPRS total score at endpoint in the intervention groups was | 29 | ⊕⊝⊝⊝ | Data for prespecified outcome: Clinically important change were not reported. | |
| Adverse effects ‒at least one adverse effect | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Service use ‒time in hospital | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Quality of life ‒average change in quality of life | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: total (cumulative) sample size was just 29 participants and 95% confidence interval around the estimate of effect included no effect and appreciable benefit and appreciable harm; thus, very serious imprecision was present. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically relevant response – as defined by trial Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.17, 15.99] |
| 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐4.20, 8.20] |
| 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐12.56, 19.36] |

