Diferentes tipos de insulina y regímenes para pacientes embarazadas con diabetes preexistente
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011880.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All six review authors were involved in the development of this review. Sinéad O'Neill co‐ordinated and drafted the review with the help of Helen West, and is the guarantor of the review. Louise Kenny, Rebecca Smyth, and Patricia Kearney assisted in the conception of the review, and offered a clinical perspective. Ali Khashan provided statistical advice, as well as general advice on the review. Paul Beirne assisted with securing fellowship funding, and provided detailed comments on the protocol, but was not involved in the full review.
Sources of support
Internal sources
-
The Irish Centre for Fetal and Neonatal Translational Research (INFANT), University College Cork (UCC), Ireland.
LK is a Science Foundation Ireland (SFI) Principal Investigator (08/IN/.1/B2083) and the Director of the SFI funded centre INFANT (12/RC/2272)
-
University College Cork (UCC), Ireland.
PB, SON, AK, and LK are employees of UCC and receive support from the University in the form of a salary.
-
Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
-
Health Research Board (HRB), Ireland.
SON is the recipient of a full Cochrane Fellowship (Grant no: CTF‐2014‐884) from the HRB, Ireland
-
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
SON: received support from a Health Research Board Cochrane Fellowship in order to prepare this review.
AK: none known.
LK: is As Director of the Irish Centre for Fetal and Neonatal Translational Research, and as such, has numerous grant applications under review at any given time. She has been paid by Alere to give invited symposia on a proprietary screening test for preeclampsia. She is the editor of Te Teachers and received royalties from the publishers. LK is also a limited share holder in Metabolomic Diagnostics, an SME who have licensed technology she has developed pertaining to the screening of preeclampsia.
HW: Helen West is paid to work on Cochrane reviews by a grant to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors, and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
RS: none known.
PK: none known.
Acknowledgements
We would like to acknowledge the assistance of the Pregnancy and Childbirth Group Editors, Denise Atherton for administrative assistance and Lynn Hampson for the literature search.
Sinéad O'Neill's contribution to this review was supported by the Health Research Board (HRB), Ireland, in the form of a full Cochrane Fellowship (Grant no: CTF‐2014‐884).
Helen West's contribution to this project was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding and Cochrane Programme Grant funding (13/89/05) to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 03 | Different insulin types and regimens for pregnant women with pre‐existing diabetes | Review | Sinéad M O'Neill, Louise C Kenny, Ali S Khashan, Helen M West, Rebecca MD Smyth, Patricia M Kearney | |
| 2015 Sep 27 | Different insulin types and regimens for pregnant women with pre‐existing diabetes | Protocol | Sinéad M O'Neill, Louise C Kenny, Ali S Khashan, Paul V Beirne, Rebecca MD Smyth, Patricia M Kearney | |
Differences between protocol and review
-
HW has been added as an author.
-
We added additional outcomes and labelled these as 'not prespecified outcomes'. For infants, these included: birthweight (g); infant fasting C‐peptide level at three months (pmol/mL); infant C‐peptide level one hour after glucose‐amino acid challenge at three months (pmol/mL)); infant fasting glucose level at three months (pmol/mL); infant glucose level one hour after glucose‐amino acid challenge at three months (pmol/mL), and gestational age at delivery. Additional maternal outcomes included: ventouse delivery; maternal ketonuria, and a maternal compliance with treatment score (1 = best, 5 = worst compliance).
-
We have reworded other outcomes to be in line with the list of core outcomes for diabetes in pregnancy (use of healthcare resources now includes maternal hospital days, pre‐eclampsia includes pregnancy‐induced hypertension, neonatal adiposity includes body weight percentile).
-
SON and HW performed the screening for eligibility, data extraction, and risk of bias for the included studies
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Diabetes Mellitus, Type 1 [*drug therapy];
- Diabetes Mellitus, Type 2 [*drug therapy];
- Hypoglycemic Agents [administration & dosage, *therapeutic use];
- Insulin [administration & dosage, *therapeutic use];
- Insulin Aspart [therapeutic use];
- Insulin Detemir [therapeutic use];
- Insulin Lispro [therapeutic use];
- Pregnancy Complications [*drug therapy];
- Pregnancy in Diabetics [*drug therapy];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.
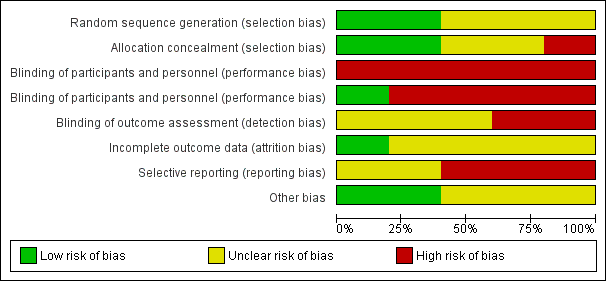
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
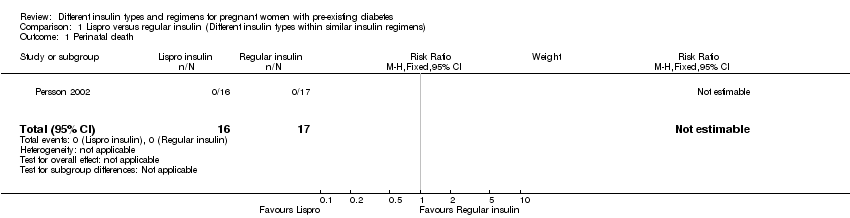
Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 1 Perinatal death.
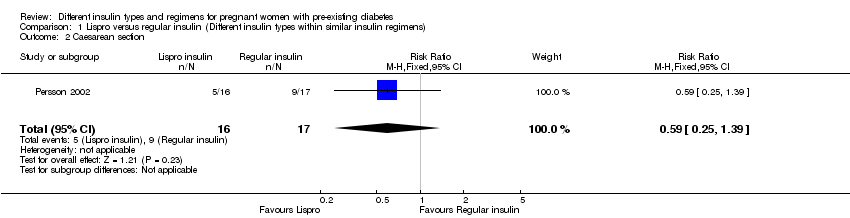
Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 2 Caesarean section.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 3 Pregnancy‐induced hypertension and pre‐eclampsia.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 4 Fetal anomaly.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture.
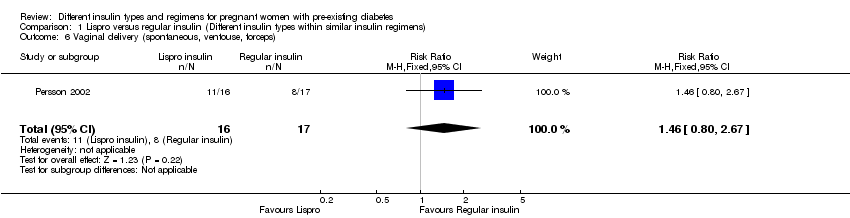
Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 6 Vaginal delivery (spontaneous, ventouse, forceps).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 7 Blood glucose (mmol/L) week 14 (after lunch).
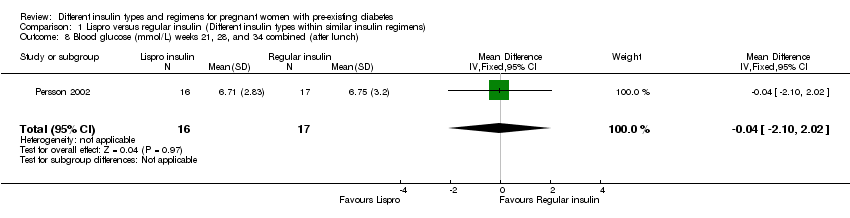
Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch).
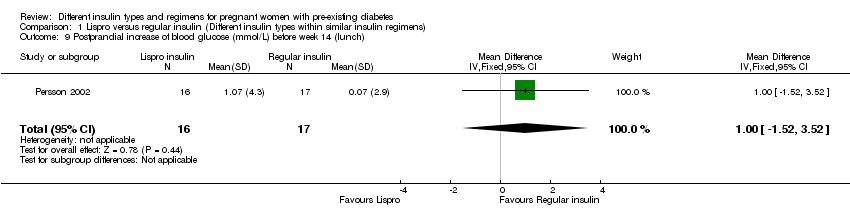
Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 12 Retinopathy.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 13 Ventouse delivery.
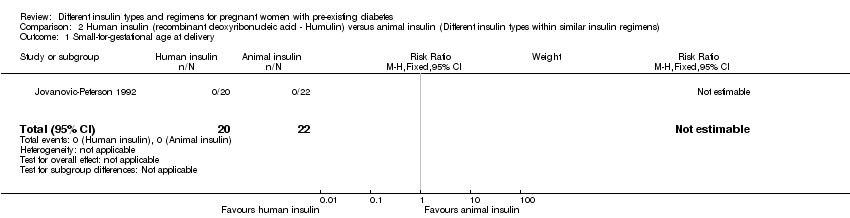
Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 1 Small‐for‐gestational age at delivery.
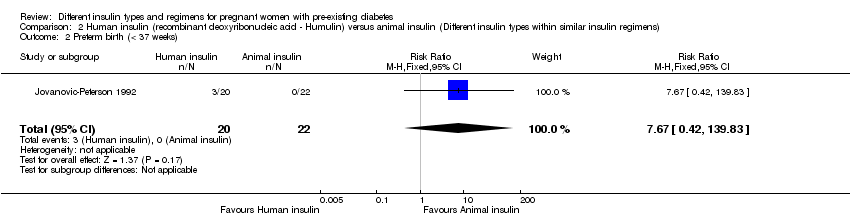
Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 2 Preterm birth (< 37 weeks).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 3 Birthweight centile (%).
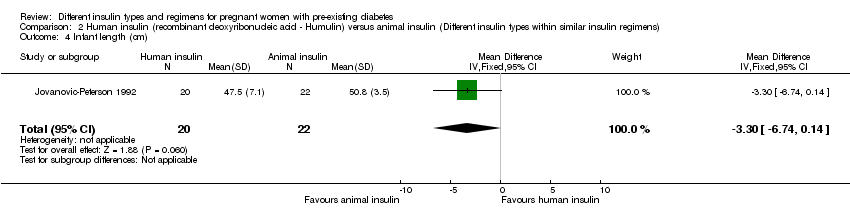
Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 4 Infant length (cm).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 5 Skinfold thickness (mm).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 6 Body weight percentile (%).
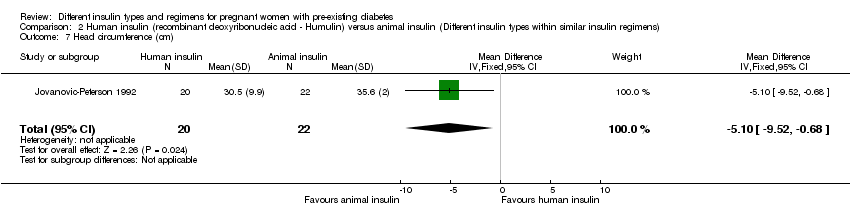
Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 7 Head circumference (cm).
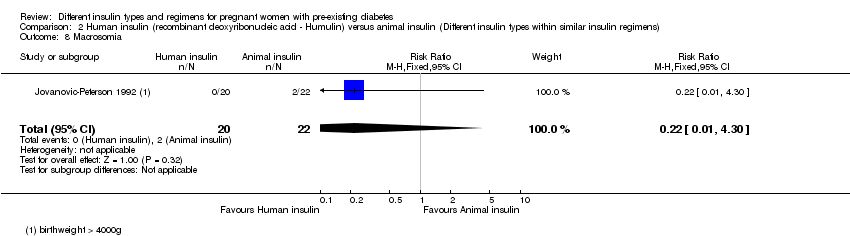
Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 8 Macrosomia.

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 9 Insulin requirement during pregnancy (U/kg/24 hour).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 10 Birthweight (g).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 11 Infant fasting C‐peptide level at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 13 Infant glucose fasting level at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 15 Gestational age at delivery (weeks).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 16 Maternal ketonuria.
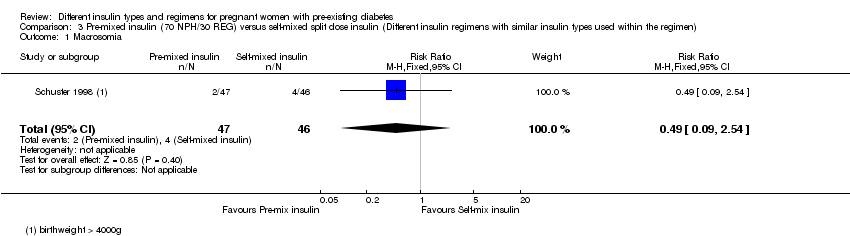
Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch).
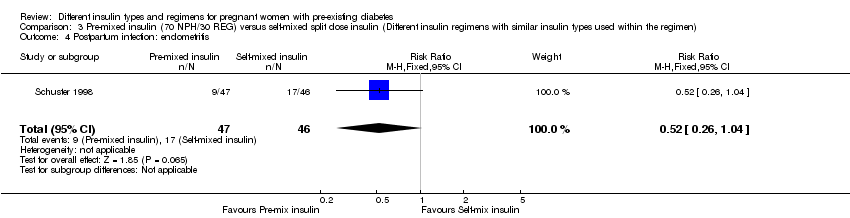
Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days).

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g).

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia.
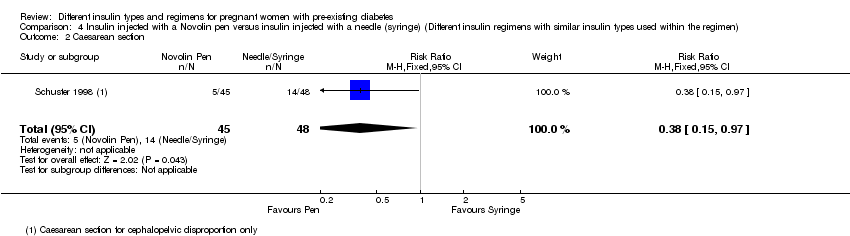
Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section.
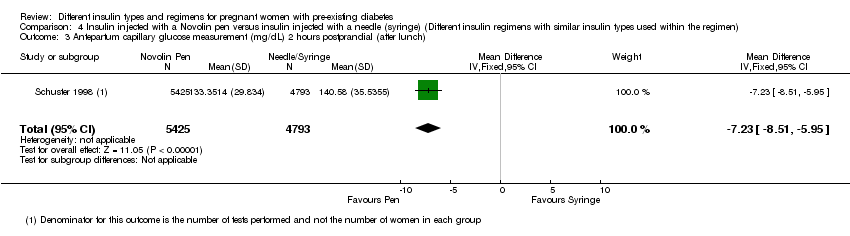
Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch).

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days).
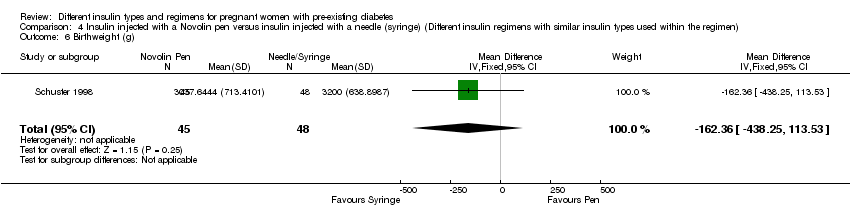
Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g).

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score.
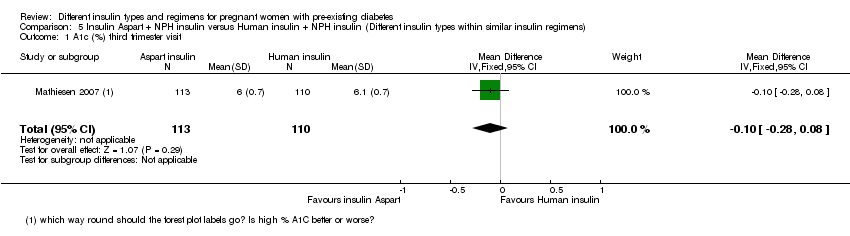
Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 1 A1c (%) third trimester visit.
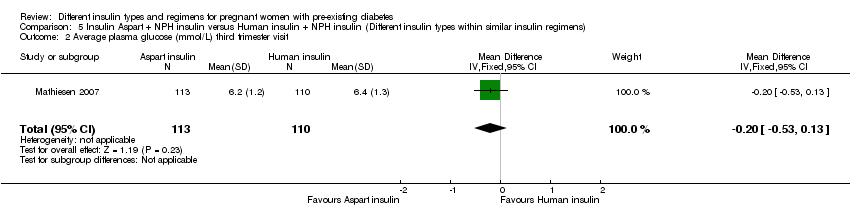
Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 2 Average plasma glucose (mmol/L) third trimester visit.

Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 3 Maternal hypoglycaemic episodes.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 1 Major congenital malformation.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 2 Major congenital malformation.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 3 Minor congenital malformation.
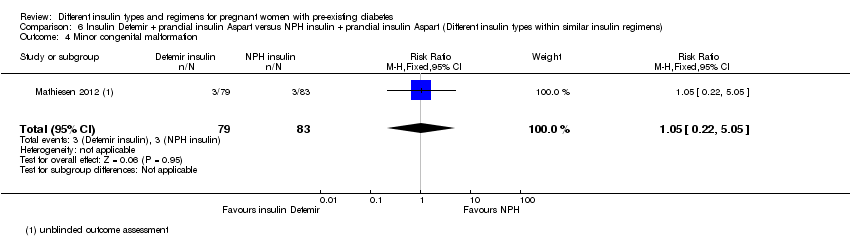
Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 4 Minor congenital malformation.
| Lispro versus regular insulin (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with regular insulin | Risk with Lispro | |||||
| Macrosomia | (0 studies) | Not reported | ||||
| Perinatal death | Study population | not estimable | 33 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Pre‐eclampsia | Study population | RR 0.68 | 33 | ⊕⊝⊝⊝ | ||
| 647 per 1000 | 440 per 1000 | |||||
| Caesarean section | Study population | RR 0.59 | 33 | ⊕⊝⊝⊝ | ||
| 529 per 1000 | 312 per 1000 | |||||
| Fetal anomaly | Study population | RR 0.35 | 33 | ⊕⊝⊝⊝ | ||
| 59 per 1000 | 21 per 1000 | |||||
| Birth trauma, including shoulder dystocia, nerve palsy, and fracture | Study population | Not estimable | 33 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High or unclear risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases 2 Small sample size and no events 3 One study with design limitations 4 Very wide 95% confidence intervals crossing the line of no effect 5 Small sample size with few events | ||||||
| Human insulin versus animal insulin (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with animal insulin | Risk with human insulin (Humulin) | |||||
| Macrosomia | Study population | RR 0.22 | 42 | ⊕⊝⊝⊝ | ||
| 91 per 1000 | 20 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section | (0 studies) | Not reported | ||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, and fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias was high or unclear for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting 2 One study with serious design limitations including lack of blinding for allocation concealment 3 Very wide 95% confidence intervals crossing the line of no effect 4 Small sample size and few events | ||||||
| Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with self‐mixed split dose insulin | Risk with pre‐mixed insulin (70 NPH/30 REG) | |||||
| Macrosomia | Study population | RR 0.49 | 93 | ⊕⊝⊝⊝ | ||
| 87 per 1000 | 43 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section 4 | Study population | RR 0.57 | 93 | ⊕⊝⊝⊝ | ||
| 261 per 1000 | 149 per 1000 | |||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Very wide 95% confidence intervals crossing the line of no effect 2 Small sample size and few events 3 One study with serious design limitations 4 Caesarean section for cephalo‐pelvic disproportion | ||||||
| Insulin injected with a Novolin pen versus insulin injected with a needle or syringe (Different insulin regimens with similar insulin types used within the regimen) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with insulin injected with a needle or syringe | Risk with insulin injected with a Novolin pen | |||||
| Macrosomia | Study population | RR 0.21 | 93 | ⊕⊝⊝⊝ | ||
| 104 per 1000 | 22 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section 4 | Study population | RR 0.38 | 93 | ⊕⊝⊝⊝ | ||
| 292 per 1000 | 111 per 1000 | |||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Very wide 95% confidence intervals crossing the line of no effect 2 Small sample size with few events 3 One study with serious design limitations 4 Caesarean section for cephalo‐pelvic disproportion | ||||||
| Insulin Aspart (+ NPH) compared to human insulin (+ NPH) for pregnant women with pre‐existing diabetes (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with human insulin (+ NPH) | Risk with insulin Aspart (+ NPH) | |||||
| Macrosomia | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Pre‐eclampsia | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Fetal anomaly | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Birth trauma including shoulder dystocia, nerve palsy and fracture | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Composite outcome measure of neonatal morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with NPH (+ Aspart) | Risk with insulin Detemir (+ Aspart) | |||||
| Macrosomia | (0 studies) | Not reported | ||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section | (0 studies) | Not reported | ||||
| Fetal anomaly (major) 1 | Study population | RR 3.15 | 162 | ⊕⊝⊝⊝ | ||
| 12 per 1000 | 38 per 1000 | |||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assessed by an expert who was blinded to the outcome 2 One study with design limitations 3 Very wide 95% confidence intervals crossing the line of no effect 4 Large effect estimate 5 Small sample size with few events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.39] |
| 3 Pregnancy‐induced hypertension and pre‐eclampsia Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.30] |
| 4 Fetal anomaly Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.08] |
| 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Vaginal delivery (spontaneous, ventouse, forceps) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.80, 2.67] |
| 7 Blood glucose (mmol/L) week 14 (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐3.60, 1.42] |
| 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐2.10, 2.02] |
| 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.52, 3.52] |
| 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.12, 2.32] |
| 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 12 Retinopathy Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.17, 6.67] |
| 13 Ventouse delivery Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.37, 27.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Small‐for‐gestational age at delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Preterm birth (< 37 weeks) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [0.42, 139.83] |
| 3 Birthweight centile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.64, 10.24] |
| 4 Infant length (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.74, 0.14] |
| 5 Skinfold thickness (mm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.10 [‐13.28, 5.08] |
| 6 Body weight percentile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.74, 10.34] |
| 7 Head circumference (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.52, ‐0.68] |
| 8 Macrosomia Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 9 Insulin requirement during pregnancy (U/kg/24 hour) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.45, ‐0.21] |
| 10 Birthweight (g) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐591.0 [‐1066.27, ‐115.73] |
| 11 Infant fasting C‐peptide level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.03] |
| 13 Infant glucose fasting level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.62, 0.22] |
| 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.04, 1.04] |
| 15 Gestational age at delivery (weeks) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐3.70, 4.70] |
| 16 Maternal ketonuria Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.08, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.54] |
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.32] |
| 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐11.25 [‐12.55, ‐9.95] |
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.40, 0.41] |
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐116.56 [‐391.81, 158.69] |
| 7 Compliance score Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.87, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.76] |
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.97] |
| 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐7.23 [‐8.51, ‐5.95] |
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.28, 1.14] |
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.45, 0.33] |
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐162.36 [‐438.25, 113.53] |
| 7 Compliance score Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A1c (%) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 2 Average plasma glucose (mmol/L) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.53, 0.13] |
| 3 Maternal hypoglycaemic episodes Show forest plot | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.33, 29.67] |
| 2 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| 3 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 4 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 5.05] |

