Amitriptilina para la fibromialgia en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011824Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 julio 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
PW, RAM, and SD wrote the protocol.
For the original review, and again for this update, RAM and SD carried out searches, assessed studies for inclusion, and extracted data. RAM acted as arbitrator. All authors were involved in writing the review.
RAM will be responsible for updating the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
DA has no conflicts relating to this review or any similar product.
PC has received research support from industry sources at various times but none related to this review.
For transparency SD, PW and RAM have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Acknowledgements
Support for this review came from the Oxford Pain Relief Trust.
The protocol for this review was written with funding support from the NHS Cochrane Collaboration Programme Grant Scheme (UK) and European Union Biomed 2 Grant no. BMH4 CT95 0172 (UK). We are grateful to the peer reviewers for some very useful comments relating to that protocol, and the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jul 31 | Amitriptyline for fibromyalgia in adults | Review | R Andrew Moore, Sheena Derry, Dominic Aldington, Peter Cole, Philip J Wiffen | |
Differences between protocol and review
This update considers fibromyalgia only. Neuropathic pain conditions are the subject of a separate review. It is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
We have used three‐tiers of evidence, not two, to better distinguish the strength of evidence and in line with other reviews of interventions for neuropathic pain. We assessed the data according to different neuropathic pain conditions, and planned no further subgroup analysis because the amount of data was expected to be small.
PICO
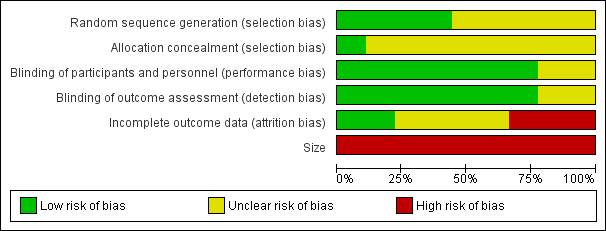
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.
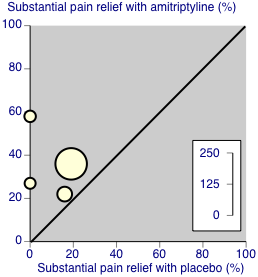
Third‐tier evidence: substantial pain relief
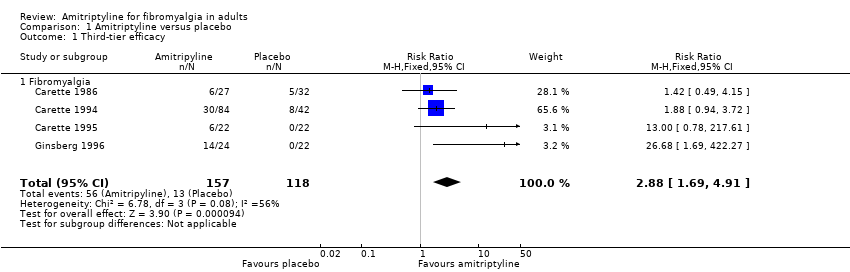
Comparison 1 Amitriptyline versus placebo, Outcome 1 Third‐tier efficacy.

Comparison 1 Amitriptyline versus placebo, Outcome 2 At least 1 adverse event.
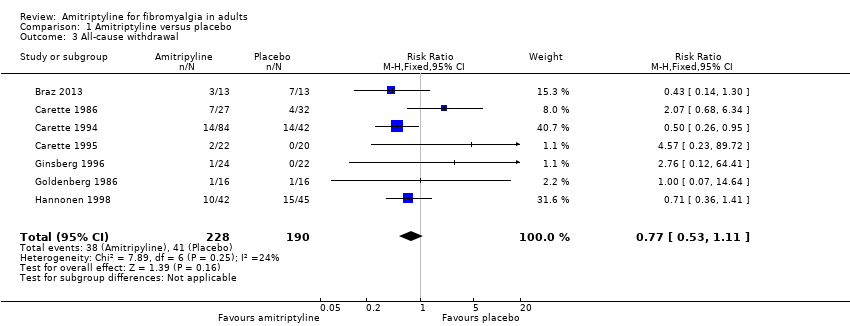
Comparison 1 Amitriptyline versus placebo, Outcome 3 All‐cause withdrawal.
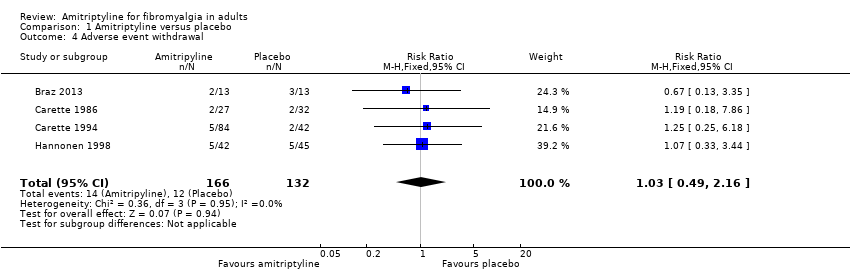
Comparison 1 Amitriptyline versus placebo, Outcome 4 Adverse event withdrawal.

Comparison 1 Amitriptyline versus placebo, Outcome 5 Lack of efficacy withdrawal.
| Amitriptyline compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: amitriptyline 25 to 50 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | NNT or NNH and/or relative effect (95% CI) | No of Participants | Quality of the evidence | Comments |
| At least 50% reduction in pain or equivalent (substantial) | 360 in 1000 | 110 in 1000 | RR 2.9 (1.7 to 4.9) NNT 4.1 (2.9 to 6.7) | 4 studies, 275 participants | Very low | Small number of studies and participants |
| At least 30% reduction in pain or equivalent (moderate) | no data | |||||
| Adverse event withdrawals | 80 in 1000 | 90 in 1000 | RR 1.03 (0.49 to 2.2) NNTp not calculated | 4 studies, 298 participants | Very low | Small number of studies and participants |
| Serious adverse events | none reported | |||||
| Death | none reported | |||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Third‐tier efficacy Show forest plot | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 1.1 Fibromyalgia | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 2 At least 1 adverse event Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.29, 1.84] |
| 3 All‐cause withdrawal Show forest plot | 7 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 4 Adverse event withdrawal Show forest plot | 4 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
| 5 Lack of efficacy withdrawal Show forest plot | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.95] |


