Amitriptilina para la fibromialgia en adultos
Resumen
Antecedentes
Ésta es una versión actualizada de la revisión Cochrane original publicada en el número 12, 2012. Dicha revisión consideró la fibromialgia y el dolor neuropático, pero la eficacia de la amitriptilina para el dolor neuropático se trata actualmente en una revisión separada.
La amitriptilina es un antidepresivo tricíclico que se utiliza ampliamente para tratar la fibromialgia y se recomienda en muchas guías. Por lo general, se utiliza en dosis inferiores a las dosis del fármaco como antidepresivo.
Objetivos
Evaluar la eficacia analgésica de la amitriptilina para aliviar la fibromialgia y los eventos adversos asociados con su uso en ensayos clínicos.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL, MEDLINE y EMBASE hasta marzo de 2015, así como en las listas de referencias de los artículos recuperados, las revisiones sistemáticas anteriores y en otras revisiones, y en dos registros de ensayos clínicos. También se realizaron búsquedas manuales de los estudios más antiguos en las base de datos de los autores.
Criterios de selección
Se incluyeron estudios aleatorios doble ciego de al menos cuatro semanas de duración, que compararon amitriptilina con placebo u otro tratamiento activo para la fibromialgia.
Obtención y análisis de los datos
Se extrajeron los datos de eficacia y de los eventos adversos, y dos revisores examinaron de forma independiente aspectos de la calidad del estudio. Se realizó el análisis con tres niveles de pruebas. El primer nivel de evidencia se obtuvo a partir de los datos que cumplieron con los mejores estándares actuales y que tuvieron un riesgo de sesgo mínimo (un resultado equivalente a una reducción significativa en la intensidad del dolor, un análisis de intención de tratar sin imputación de los abandonos, al menos 200 participantes en la comparación, duración de ocho a 12 semanas, diseño paralelo); el segundo nivel a partir de los datos que no cumplieron con uno o más de estos criterios y que se consideró que tuvieron cierto riesgo de sesgo, pero con números adecuados en la comparación; y el tercer nivel a partir de los datos que incluyeron números pequeños de participantes y que se consideró que era muy probable que estuvieran sesgados o que utilizaran resultados de escasa utilidad clínica, o ambos.
Para la eficacia, se calculó el número necesario a tratar para lograr un beneficio (NNTB), y para el daño se calculó el número necesario a tratar para causar daño (NNTD) para los eventos adversos y los abandonos. Para el metanálisis se utilizó un modelo de efectos fijos.
Resultados principales
Se incluyeron siete estudios de la revisión anterior y dos estudios nuevos (nueve estudios, 649 participantes) de seis a 24 semanas de duración, que incorporaron entre 22 y 208 participantes. Ninguno tuvo 50 o más participantes en cada brazo de tratamiento. Dos estudios utilizaron un diseño cruzado (cross‐over). La dosis diaria de amitriptilina fue de 25 mg a 50 mg, y algunos estudios tuvieron un período inicial de disminución gradual de la dosis.
No hubo evidencia de primer o segundo nivel para la amitriptilina en el tratamiento de la fibromialgia. Al utilizar evidencia de tercer nivel, el cociente de riesgos (CR) para al menos el 50% de alivio del dolor, o un equivalente, con amitriptilina en comparación con placebo fue 3,0 (intervalo de confianza [IC] del 95%: 1,7 a 4,9), con un NNTB de 4,1 (2,9 a 6,7) (evidencia de calidad muy baja). No hubo diferencias consistentes entre la amitriptilina y placebo u otros comparadores activos para el alivio de los síntomas como fatiga, sueño deficiente, calidad de vida o puntos hipersensibles.
Más participantes presentaron al menos un evento adverso con amitriptilina (78%) en comparación con placebo (47%). El CR fue 1,5 (1,3 a 1,8) y el NNTD fue 3,3 (2,5 a 4,9). Los abandonos por eventos adversos y por todas las causas no fueron diferentes, pero los abandonos por falta de eficacia fueron más frecuentes con placebo (12% versus 5%; CR 0,42 [0,19 a 0,95]) (evidencia de calidad muy baja).
Conclusiones de los autores
La amitriptilina ha sido un tratamiento de primera línea para la fibromialgia durante muchos años. El hecho de que no exista evidencia no sesgada para apoyar un efecto beneficioso es decepcionante, aunque se debe considerar en relación con los años de tratamiento exitoso en muchos pacientes con fibromialgia. No existe evidencia convincente de una falta de efecto; más bien la preocupación debe ser la sobrestimación del efecto del tratamiento. La amitriptilina será una opción en el tratamiento de la fibromialgia, aunque se reconoce que solo una minoría de los pacientes logrará un alivio satisfactorio del dolor.
Es poco probable que se realicen ensayos aleatorios grandes de la amitriptilina en la fibromialgia para establecer su eficacia desde el punto de vista estadístico, o para medir el tamaño del efecto.
PICOs
Resumen en términos sencillos
Amitriptilina para la fibromialgia en adultos
El conocimiento sobre la fibromialgia (un trastorno caracterizado por dolor persistente y diseminado, así como hipersensibilidad, trastornos del sueño y fatiga) es escaso. Los analgésicos habituales, como el paracetamol y el ibuprofeno, en general no se consideran efectivos para la fibromialgia. Los fármacos que se utilizan en algunas ocasiones para tratar la epilepsia o la depresión pueden ser efectivos en algunos pacientes con fibromialgia y en otras formas de dolor crónico en que puede haber daño nervioso (dolor neuropático).
La amitriptilina es un antidepresivo, y los antidepresivos se recomiendan ampliamente para tratar la fibromialgia. Aunque la amitriptilina se utiliza con frecuencia para tratar la fibromialgia, una revisión realizada en 2012 no encontró evidencia de buena calidad que apoye su uso. La mayoría de los estudios fueron pequeños, antiguos y utilizaron métodos o informaron de resultados que, según el conocimiento actual, hacen que los beneficios parezcan mejores de lo que son.
Esta revisión es una actualización de la revisión de 2012, que consideró la fibromialgia y las afecciones de dolor neuropático. El dolor neuropático se analiza ahora en una revisión separada. Aquí se examina la efectividad de la amitriptilina en el tratamiento de la fibromialgia, y la definición utilizada para determinar si fue útil incluyó un nivel de alivio del dolor alto y la capacidad de tomar las tabletas durante más tiempo sin que los efectos secundarios fueran intolerables.
En marzo de 2015, se realizaron búsquedas de nuevos estudios y solo se encontraron dos estudios pequeños adicionales a incluir. Ninguno proporcionó evidencia de buena calidad para el beneficio ni el daño. Todavía no había estudios que pudieran brindar una respuesta fiable, ya que en su mayoría fueron relativamente antiguos y utilizaron métodos o informaron de resultados que, según el conocimiento actual, hacen que los beneficios parezcan mejores de lo que son. Esta situación es decepcionante, aunque aun así es posible formular observaciones útiles acerca del fármaco.
La amitriptilina probablemente proporciona un nivel de alivio del dolor adecuado para algunos pacientes con fibromialgia, aunque no es posible asegurarlo. La mejor suposición es que la amitriptilina brinda un buen alivio del dolor en cerca de uno de cada cuatro pacientes (25%) más en comparación con placebo. Cerca de uno de cada tres pacientes (31%) más en comparación con placebo informan que tienen uno o más eventos adversos que generalmente no son graves, pero que pueden ser problemáticos e interferir con la toma del tratamiento. En base a la información disponible, no es posible confiar en ninguna de las cifras.
El mensaje más importante es que es probable que la amitriptilina realmente proporcione un buen alivio del dolor en algunos pacientes con fibromialgia, pero solo en una minoría de ellos; la amitriptilina no será útil en la mayoría de los pacientes.
Authors' conclusions
Summary of findings
| Amitriptyline compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: amitriptyline 25 to 50 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | NNT or NNH and/or relative effect (95% CI) | No of Participants | Quality of the evidence | Comments |
| At least 50% reduction in pain or equivalent (substantial) | 360 in 1000 | 110 in 1000 | RR 2.9 (1.7 to 4.9) NNT 4.1 (2.9 to 6.7) | 4 studies, 275 participants | Very low | Small number of studies and participants |
| At least 30% reduction in pain or equivalent (moderate) | no data | |||||
| Adverse event withdrawals | 80 in 1000 | 90 in 1000 | RR 1.03 (0.49 to 2.2) NNTp not calculated | 4 studies, 298 participants | Very low | Small number of studies and participants |
| Serious adverse events | none reported | |||||
| Death | none reported | |||||
| GRADE Working Group grades of evidence | ||||||
Background
This is an update of an earlier review of amitriptyline for neuropathic pain and fibromyalgia originally published in the Cochrane Library in 2012 (Moore 2012a). The efficacy of amitriptyline for neuropathic pain conditions is now dealt with in a separate review (Moore 2015).
In the update we have used a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on current criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
Description of the condition
Fibromyalgia has been defined as widespread pain that lasts for longer than three months, with pain on palpation at 11 or more of 18 specified tender points (Wolfe 1990). It is frequently associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014). More recently, a definition of fibromyalgia has been proposed based on symptom severity and the presence of widespread pain, and which does not require palpation of tender points for diagnosis (Wolfe 2010). While some rheumatologists have thought of fibromyalgia as a specific pain disorder, other investigators have characterised it as a bodily distress syndrome or a physical symptom disorder, or somatoform disorder (Wolfe 2014). It is a heterogeneous condition in which there is abnormal processing of the sensation of pain. The cause, or causes, are not well understood, but it has features in common with neuropathic pain, including changes in the central nervous system (CNS). Moreover, people with neuropathic pain and people with fibromyalgia experience similar sensory phenomena (Koroschetz 2011).
Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years. Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. Numerous studies have investigated prevalence in different settings and countries. The Queiroz 2013 review gives a global mean prevalence of 2.7% (range 0.4% to 9.3%), and a mean in the Americas of 3.1%, in Europe of 2.5%, and in Asia of 1.7%. Fibromyalgia is more common in women, with a female to male ratio of 3:1 (4.2%:1.4%). The change in diagnostic criteria does not appear to have significantly affected estimates of prevalence (Wolfe 2013). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually not effective. Treatment is often by so‐called unconventional analgesics, such as antidepressants like duloxetine and amitriptyline (Lunn 2014; Moore 2012a; Sultan 2008), or antiepileptics like gabapentin or pregabalin (Moore 2009; Moore 2014b; Wiffen 2013). The proportion of people who achieve worthwhile pain relief (typically at least a 50% reduction in pain intensity; Moore 2013a) is small, generally only 5% to 15% more than with placebo, with numbers needed to treat to benefit (NNT) usually between 6 and 20 (Moore 2013b; Wiffen 2013). This is confirmed by individual patient level analyses for duloxetine and pregabalin (Moore 2014c; Straube 2010); and is somewhat less effective than with the same drugs in neuropathic pain (Kalso 2013).
Those who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms such as fatigue, function, sleep, depression, anxiety, and ability to work, with significant improvement in quality of life (Moore 2010b; Moore 2014a; Straube 2011). Fibromyalgia is not particularly different from other chronic pain in that only a small proportion of trial participants have a good response to treatment (Moore 2013b).
Description of the intervention
Amitriptyline is a tricyclic antidepressant. It is not licensed in the UK for treating fibromyalgia, but is commonly used for this indication, and it is commonly used for treating fibromyalgia around the world, irrespective of licensed indications. It is available as tablets (10, 25, 50 mg) and oral solutions, and is usually taken at night time to reduce any sedative effects during the day. There were over 11 million prescriptions for amitriptyline in England in 2013, mainly for 10 mg and 25 mg tablets (PCA 2014); some of these prescriptions would be for relief of depression or neuropathic pain. The main side effects are due to its anticholinergic activity, and include dry mouth, weight gain, and drowsiness.
How the intervention might work
The mechanism of action of amitriptyline in the treatment of fibromyalgia remains uncertain, although it is known to inhibit both serotonin and noradrenalin reuptake. The mechanism is likely to differ from that in depression since analgesia with antidepressants is often achieved at lower dosage than the onset of any antidepressant effect; adverse events associated with amitriptyline often wane after two or three weeks, when the benefits of the drug become apparent. In addition, there is little correlation between the effect of antidepressants on mood and pain, and antidepressants produce analgesia in patients with and without depression (Onghena 1992).
Why it is important to do this review
Amitriptyline is an established pharmacological intervention for fibromyalgia. The earlier review found some evidence of pain relief with amitriptyline compared with placebo for fibromyalgia, at the expense of increased adverse events, but this was based on small numbers of participants in studies that were susceptible to bias.
It was decided to split reviews combining neuropathic pain conditions with fibromyalgia into separate reviews, so an update was performed at the same time, to capture any new studies.
Like the earlier Cochrane review, this update assessed evidence in ways that make both statistical and clinical sense, and used developing criteria for what constitutes reliable evidence in chronic pain (Appendix 1; Moore 2010a). It followed standards set out in the PaPaS Author and Referee Guidance for pain studies of the Cochrane Pain, Palliative and Supportive Care Group (PaPaS 2012).
Objectives
To assess the analgesic efficacy of amitriptyline for relief of fibromyalgia, and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with double‐blind assessment of treatment, and outcomes reported ideally after eight weeks of treatment or longer for the highest level of evidence, but accepted studies lasting four to eight weeks as a lower level. We required full journal publication, with the exception of extended abstracts of otherwise unpublished clinical trials. We did not include short abstracts (usually meeting reports), studies that were non‐randomised, studies of experimental pain, case reports, or clinical observations. We accepted cross‐over studies only if there was clear reporting of the first phase only. We did not include studies with fewer than 10 participants in any treatment arm, or studies of topical administration.
Types of participants
We included adult participants with fibromyalgia diagnosed using the 1990 or 2010 criteria (Wolfe 1990; Wolfe 2010), aged 18 years and above, and with initial pain of at least moderate intensity
Types of interventions
Amitriptyline in any dose, by any route other than topical, administered for the relief of fibromyalgia pain, and compared to placebo or any active comparator.
Types of outcome measures
Studies needed to report pain assessment as either the primary or secondary outcome.
We anticipated that studies would use a variety of outcome measures, with most using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
-
at least 30% pain relief over baseline (moderate);
-
at least 50% pain relief over baseline (substantial);
-
much or very much improved on Patient Global Impression of Change (PGIC) (moderate);
-
very much improved on PGIC (substantial).
These outcomes were used in the earlier version of this review, but are different from many other earlier reviews, concentrating on dichotomous outcomes where pain responses are not normally distributed.
We have included a 'Summary of findings' table as set out in the author guide (PaPaS 2012), including outcomes of at least 30% and at least 50% pain intensity reduction, withdrawals due to adverse events, serious adverse events, and death, although there were no data for some of these outcomes. We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in Types of outcome measures (Schünemann 2011), as appropriate.
Primary outcomes
-
Participant‐reported pain relief of 30% or greater.
-
Participant‐reported pain relief of 50% or greater.
-
PGIC much or very much improved.
-
PGIC very much improved.
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy, adverse events, and for any cause
-
Participants experiencing any adverse event.
-
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
-
Specific adverse events, particularly somnolence, dizziness, and dry mouth.
-
Any disability‐related or mental health‐related outcome.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library 2012, Issue 9 for the earlier review and via CRSO from 2012 to 26 March 2015 for this update);
-
MEDLINE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 26 March 2015 for this update);
-
EMBASE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 26 March 2015 for this update);
-
Oxford Pain Relief database (Jadad 1996a) for the earlier review. This database is no longer being updated.
See Appendix 2 for the MEDLINE search strategy, Appendix 3 for the EMBASE search strategy, and Appendix 4 for the CENTRAL search strategy.
There was no language restriction.
Searching other resources
We reviewed the bibliographies of all identified RCTs and review articles, and searched clinical trial databases (ClinicalTrials.gov (ClinicalTrials.gov) and WHO ICTRP (apps.who.int/trialsearch/) to identify additional published or unpublished data. We did not contact investigators or study sponsors.
Data collection and analysis
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. Studies that clearly did not satisfy inclusion criteria were eliminated, and we obtained full copies of the remaining studies. Two review authors read these studies independently and reached agreement by discussion. We did not anonymise the studies in any way before assessment.
Data extraction and management
Two review authors independently extracted data using a standard form and checked for agreement before entry into RevMan (RevMan 2014) or any other analysis method. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process such as random number table or computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (for example, open list).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or used ‘baseline observation carried forward’ analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
-
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Dechartres 2013; Kjaergard 2001; Nuesch 2010). Studies were considered to be at low risk of bias if they had 200 participants or more, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We calculated numbers needed to treat to benefit (NNTs) as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat to harm (NNH) and is calculated in the same manner. We used dichotomous data to calculate risk ratio (RR) with 95% confidence intervals (CI) using a fixed‐effect model unless significant statistical heterogeneity was found (see below). Continuous data were not used in analyses.
Unit of analysis issues
The unit of analysis was the individual participant. For cross‐over studies we planned to use the first period data only, or any useable results if first period data were not available. The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement wherever possible.
Assessment of heterogeneity
We assessed statistical heterogeneity visually (L'Abbé 1987) and with the use of the I² statistic (Higgins 2003). When I² was greater than 50%, we considered the reasons.
Assessment of reporting biases
The aim of this review is to use dichotomous data of known utility and of value to patients (Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what authors of the original studies chose to report or not, though clearly difficulties arose with studies failing to report any dichotomous results. We extracted and used continuous data, which probably poorly reflect efficacy and utility, if useful for illustrative purposes only.
We undertook no assessment of publication bias due to the quality of the data identified, although we had planned to use a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008).
Data synthesis
We undertook meta‐analysis using a fixed‐effect model. A random‐effects model for meta‐analysis would have been used if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We determined that we would analyse data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
-
The first tier used data meeting current best standards, where studies reported the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of last observation carried forward analysis (LOCF) or other imputation method other than baseline observation carried forward (BOCF) for dropouts, reported an ITT analysis, lasted eight or more weeks, had a parallel‐group design, and had at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). We planned to report these top‐tier results first.
-
The second tier used data from at least 200 participants, but where one or more of the above conditions was not met (for example, reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
-
The third tier of evidence used data from fewer than 200 participants, or where there were expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible.
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We planned no sensitivity analysis because the evidence base was known to be too small to allow reliable analysis. We did examine details of dose escalation schedules in the unlikely situation that this could provide some basis for a sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Results of the search
New searches from January 2012 to 26 March 2015 identified 37 potentially relevant studies in CENTRAL, 107 in MEDLINE, and 259 in EMBASE. Of these, two were obtained and read in full to determine inclusion status.
Three studies still await classification because of translation requirements. Ataoğlu 1997 is a Turkish study comparing amitriptyline with paroxetine in 68 participants for six weeks. Jang 2010 is a Chinese study comparing amitriptyline with acupuncture plus cupping and with the combined treatments over four weeks, involving 186 participants in three treatment arms. The details of NCT00381199 are not clear, but it involved a comparison of amitriptyline 10 mg to 25 mg daily with nabilone 0.5 mg to 1 mg daily over a period of about three weeks in 32 participants.
Included studies
In this update we included two new studies (101 participants; Braz 2013; de Zanette 2014) and seven studies (548 participants; Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996; Goldenberg 1986; Goldenberg 1996; Hannonen 1998) from the previous review that fulfilled the inclusion criteria; altogether there were nine included studies with 649 participants (Figure 1).

Flow diagram.
Two studies used a cross‐over design (Carette 1995; Goldenberg 1996) and the remainder used a parallel group design. All the studies except de Zanette 2014 included a placebo control, six included an active comparator (Braz 2013; Carette 1994; de Zanette 2014; Goldenberg 1986; Goldenberg 1996; Hannonen 1998), and three additionally included a treatment arm using a combination of amitriptyline and the active comparator being tested (de Zanette 2014; Goldenberg 1986; Goldenberg 1996). Two hundred and seventy participants took amitriptyline, 209 took placebo, 195 took various active comparators, and 55 took combinations. The active comparators were:
-
Panax ginseng extract (100 mg daily, 27% of ginsenosides);
-
cyclobenzaprine 30 mg daily;
-
melatonin 10 mg daily;
-
naproxen 2 x 500 mg daily;
-
fluoxetine 20 mg daily;
-
moclobemide 450 mg daily.
The included studies individually involved between 22 and 208 participants, and only two involved over 100 participants (Carette 1994; Hannonen 1998). The vast majority of participants were female (626 women and 33 men) with three studies enrolling only women (Braz 2013; de Zanette 2014; Hannonen 1998). Study duration ranged from 6 to 24 weeks.
Excluded studies
We excluded 15 studies (Ҫapaci 2002; Fors 2002; Hampf 1989; Heymann 2001; Isomeri 1993; Jaeschke 1991; Kempenaers 1994; McQuay 1992; McQuay 1993; Özerbil 2006; Pilowsky 1982; Pilowsky 1990; Scudds 1989; Zitman 1990; Zitman 1991). Reasons for exclusion of studies were: not being convincingly double‐blind, not demonstrating that participants had initial pain of at least moderate intensity, having fewer than 10 participants in a treatment arm, or not having a clear diagnosis of the painful condition. Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias is shown in Figure 2 as a summary and in Figure 3 for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Quality scores were good using the Oxford Quality Score; one study scored 3/5 points, three scored 4/5, and five scored 5/5.
Allocation
All studies were randomised, but only four adequately described the method used to generate the random sequence, and only one adequately described the method used to conceal the allocation of the random sequence.
Blinding
Seven studies adequately described the methods used to maintain blinding.
Incomplete outcome data
Two studies had a cross‐over design, and data on all randomised participants were not available for all outcomes (Carette 1986; Goldenberg 1996), while another study reported results only for participants who completed the study (Braz 2013). Only two studies convincingly reported on all participants (Carette 1995; Ginsberg 1996).
Selective reporting
The outcomes specified in the methods of most of these studies were not those sought for the review, so selective reporting bias was not an issue.
Other potential sources of bias
None of the studies included over 50 participants per treatment arm, and all were judged at high risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison
Results from individual studies are in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Efficacy
No study met the criteria for first‐ or second‐tier evidence.
Participants with substantial pain relief
There was some third‐tier evidence that amitriptyline at 25 mg or 50 mg daily was better than placebo from four studies (Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996).
-
The proportion of participants with substantial pain relief with amitriptyline was 36% (56/157, range 22% to 58%)
-
The proportion of participants with substantial pain relief with placebo was 11% (13/118, range 0% to 19%)
-
The RR for amitriptyline compared with placebo was 2.9 (1.7 to 4.9) (Figure 4), and the NNT was 4.1 (2.9 to 6.7).

Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.
These statistics should be interpreted with caution, in particular because of the small number of participants and events. The L'Abbe plot and I2 statistic estimate show no greater variation than would be expected in this number of small studies (Figure 5). Three of the four placebo‐controlled studies that did not report dichotomous outcomes also provided some support of greater analgesic effects from amitriptyline than placebo (Goldenberg 1986; Goldenberg 1996; Hannonen 1998).
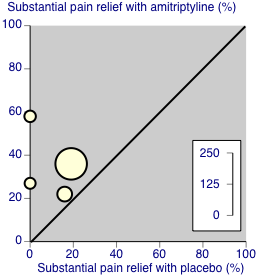
Third‐tier evidence: substantial pain relief
Amitriptyline was probably no better than cyclobenzaprine (Carette 1994), fluoxetine (Goldenberg 1996), moclobemide (Hannonen 1998) or Panax ginseng (Braz 2013) in individual small studies of limited ability to discriminate. One study claimed a benefit of melatonin over amitriptyline (de Zanette 2014).
Any disability‐related or mental health‐related outcome
All the included studies provided some information about the effects of amitriptyline on symptoms such as fatigue, sleep, tender points and quality of life (Appendix 5). These were reported as group means, but it was not always clear whether all participants were included in the analysis or what imputation method was used for missing data. Most studies reported no significant difference between treatment groups at the end of treatment for most measures, although occasionally a single measure was significantly different. There was no discernable pattern to this, and we would expect occasional significant results by chance in such a data set.
Adverse events
Participants experiencing at least one adverse event
This outcome was reported by four studies with placebo treatment arms, with 318 participants in the comparison (Carette 1986; Carette 1994; Ginsberg 1996; Hannonen 1998). At least one adverse event was experienced by 137/177 (77%) of participants taking amitriptyline, and 66/141 (47%) taking placebo. The risk ratio was 1.5 (1.3 to 1.8) (Analysis 1.2), and the number needed to treat to harm was 3.3 (2.5 to 4.9).
Serious adverse events
No studies reported any serious adverse events.
Individual adverse events
There were insufficient data for analysis of individual events. The most common events reported were dry mouth and drowsiness, somnolence or fatigue. Other events included dizziness, headache, nightmares, behavioural change, neuropsychiatric symptoms, weight gain, dyspepsia, and diarrhoea.
Withdrawals
All‐cause withdrawals were reported by seven placebo‐controlled studies (418 participants, Braz 2013; Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996; Goldenberg 1986; Hannonen 1998). Overall, 38/228 (17%) withdrew for any cause with amitriptyline and 41/190 (22%) with placebo. The risk ratio was 0.77 (0.53 to 1.1) (Analysis 1.3); the number needed to treat to harm (NNH) was not calculated.
Adverse event withdrawals were reported by four studies (298 participants, Braz 2013; Carette 1986; Carette 1994; Hannonen 1998). Overall, 14/166 (8%) withdrew because of adverse events with amitriptyline and 12/132 (9%) with placebo. The risk ratio was 1.03 (0.49 to 2.2) (Analysis 1.4); the NNH was not calculated.
Lack of efficacy withdrawals were reported by three studies (272 participants, Carette 1986; Carette 1994; Hannonen 1998). Overall, 8/153 (5%) withdrew because of lack of efficacy with amitriptyline and 14/119 (12%) with placebo. The risk ratio was 0.42 (0.19 to 0.95) (Analysis 1.5); the number needed to treat to prevent (NNTp) was 14 (7.2 to 980).
Discussion
Because amitriptyline is a crucially important drug in treating various forms of chronic pain, including fibromyalgia, and because experience from previous reviews was that most studies would be older, be small, and have methodological deficiencies according to present standards of evidence, we felt it appropriate to accept lower standards than those currently demanded for part of our analyses. It is important to recognise that the lower‐level evidence is likely to be subject to various positive biases, and that these lower levels of evidence cannot be used to make cross‐drug comparisons of efficacy with other drugs.
The most important finding of this review was that there were no studies that met current standards of evidence for chronic pain that minimise all known biases (Moore 2010a; Moore 2012b). All the studies accepted for third‐tier evidence contained features of design, conduct, or reporting that are known to be associated with bias in favour of the active treatment. Particular problems were reporting of outcomes of less than 50% pain intensity reduction or undefined 'improvement', having relatively short duration (although two thirds of the studies had treatment periods of eight weeks or more), and studies being small, in circumstances where small studies in chronic pain are known to be associated with over‐estimation of treatment effect (Dechartres 2013; Kjaergard 2001; Nüesch 2010), beyond the large random variation that occurs with small pain studies (Moore 1998). That means that the third‐tier efficacy results reported here offer only the best judgement possible on evidence that is not wholly trustworthy.
While it is possible that amitriptyline is effective in some patients with fibromyalgia, the evidence we have cannot rule out the possibility that amitriptyline is no better than placebo for this condition. This rather bleak conclusion should be tempered by many years of clinical experience indicating that amitriptyline can give really good pain relief to some patients with fibromyalgia, but only a minority of them; amitriptyline will not work for most people.
Summary of main results
There is limited evidence based on small numbers of small studies that amitriptyline may provide good pain relief in fibromyalgia. Our best estimate is that for every four people treated, one will experience a good level of pain relief (equivalent to at least 50% pain reduction) who would not have done with placebo (very low quality evidence; summary of findings Table for the main comparison). Given the caveats above, this is probably an overestimation of treatment effect, but the consistency of effect within these four studies does provide some confidence that amitriptyline benefits are real, at least for some patients.
The effect of amitriptyline on other fibromyalgia symptoms, such as fatigue, quality of sleep, and tender points is less clear. Mean data indicate that all treatment groups show an overall improvement during the study, but there were no consistent findings of significant differences between amitriptyline and placebo or other comparators. This differs from results of individual patient level analyses linking improvements in pain with changes in other outcomes; these demonstrate that pain reduction is closely linked with improvements in sleep, depression, quality of life, and ability to work (Moore 2010c; Straube 2011). The small number of studies and participants and the use of mean data for these outcomes may have limited the ability to demonstrate a significant difference.
Overall completeness and applicability of evidence
It is likely that all of the completed clinical trials have been found, but those we found and included had deficiencies because the design or reporting included features known to be associated with potential bias towards the active treatment over placebo. For example, two had a cross‐over design, all were small, and fewer than half reported efficacy outcomes based on individual participants obtaining a high degree of pain relief.
This limits considerably the applicability of the evidence. Although amitriptyline is widely used as the mainstay of treatment of fibromyalgia, there is no unbiased evidence on which to base clinical practice beyond extensive clinical experience, and no evidence for comparison with other potential treatments of fibromyalgia.
There are also significant limits in what the review can say about appropriate doses of amitriptyline. Studies used daily doses of 25 mg to 50 mg, with titration in some.
Quality of the evidence
All studies had to be randomised and double‐blind to be included, and all had to have participants with at least moderate pain relief to ensure that studies were sensitive. No single study fulfilled all the qualities of reliability now used in chronic pain. It is disappointing that the more recent studies were not of higher reporting quality.
Potential biases in the review process
We used an extensive search strategy, which was based on previous Cochrane reviews and on other reviews with different strategies, and included a comprehensive manual journal search (Jadad 1996a). It is unlikely that relevant high‐quality large studies of amitriptyline in fibromyalgia have been overlooked, especially because amitriptyline is the mainstay of treatment.
Agreements and disagreements with other studies or reviews
Our earlier review looked at both neuropathic pain and fibromyalgia, although different conditions were analysed separately for efficacy. The new studies did not contribute to efficacy data and did not change the conclusions for the analyses of withdrawals to which they did contribute.
A review of amitriptyline, duloxetine, and milnacipran in fibromyalgia included seven of the nine studies in this review, and three studies that we excluded because they had no baseline pain requirement or baseline pain data (Heymann 2001; Scudds 1989), or had fewer than 10 participants in each treatment arm (Kempenaers 1994). The authors acknowledged the poor quality of the studies, reporting an NNT of 3.5 (2.7 to 5.0) for at least 30% pain relief compared with placebo, and small or moderate effects on fatigue, quality of life, and sleep, using mean data. These results are consistent with those found in this review. Corresponding NNTs for duloxetine and milnacipran were 8 and 11, with similar small to moderate effects on other symptoms. The higher (worse) NNTs may in part be due to inclusion of larger studies of better quality for these two drugs. There were no significant differences in overall withdrawal rates between each drug and placebo, or between the three drugs, but adverse event withdrawals were not specifically reported (Hausser 2011).
An earlier review of amitriptyline for fibromyalgia again included seven of the nine studies in this review, and three studies that we excluded because they had no baseline pain requirement or baseline pain data (Fors 2002; Heymann 2001; Scudds 1989). The authors chose not to pool data, but reported a therapeutic response for amitriptyline 25 mg, but not 50 mg, compared with placebo for pain, sleep, fatigue, and global impression. Neither dose had an effect on tender points. No clear statement was made about adverse events because of inconsistent reporting, although there was no difference between amitriptyline and placebo for adverse event withdrawals (Nishishinya 2008).
Amitriptyline had similar effects on the intensity of care in a large cohort of US patients treated with a variety of antidepressant and antiepileptic drugs (Kim 2015).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.

Third‐tier evidence: substantial pain relief

Comparison 1 Amitriptyline versus placebo, Outcome 1 Third‐tier efficacy.

Comparison 1 Amitriptyline versus placebo, Outcome 2 At least 1 adverse event.

Comparison 1 Amitriptyline versus placebo, Outcome 3 All‐cause withdrawal.

Comparison 1 Amitriptyline versus placebo, Outcome 4 Adverse event withdrawal.

Comparison 1 Amitriptyline versus placebo, Outcome 5 Lack of efficacy withdrawal.
| Amitriptyline compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: amitriptyline 25 to 50 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | NNT or NNH and/or relative effect (95% CI) | No of Participants | Quality of the evidence | Comments |
| At least 50% reduction in pain or equivalent (substantial) | 360 in 1000 | 110 in 1000 | RR 2.9 (1.7 to 4.9) NNT 4.1 (2.9 to 6.7) | 4 studies, 275 participants | Very low | Small number of studies and participants |
| At least 30% reduction in pain or equivalent (moderate) | no data | |||||
| Adverse event withdrawals | 80 in 1000 | 90 in 1000 | RR 1.03 (0.49 to 2.2) NNTp not calculated | 4 studies, 298 participants | Very low | Small number of studies and participants |
| Serious adverse events | none reported | |||||
| Death | none reported | |||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Third‐tier efficacy Show forest plot | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 1.1 Fibromyalgia | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 2 At least 1 adverse event Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.29, 1.84] |
| 3 All‐cause withdrawal Show forest plot | 7 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 4 Adverse event withdrawal Show forest plot | 4 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
| 5 Lack of efficacy withdrawal Show forest plot | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.95] |

