Različiti režimi oralnih kortikosteroida za liječenje akutne astme
Abstract
Background
Asthma is a common long‐term breathing condition that affects approximately 300 million people worldwide. People with asthma may experience short‐term worsening of their asthma symptoms; these episodes are often known as ‘exacerbations’, ‘flare‐ups’, ‘attacks’ or 'acute asthma'. Oral steroids, which have a potent anti‐inflammatory effect, are recommended for all but the most mild asthma exacerbations; they should be initiated promptly. The most often prescribed oral steroids are prednisolone and dexamethasone, but current guidelines on dosing vary between countries, and often among different guideline producers within the same country. Despite their proven efficacy, use of steroids needs to be balanced against their potential to cause important adverse events. Evidence is somewhat limited regarding optimal dosing of oral steroids for asthma exacerbations to maximise recovery while minimising potential side effects, which is the topic of this review.
Objectives
To assess the efficacy and safety of any dose or duration of oral steroids versus any other dose or duration of oral steroids for adults and children with an asthma exacerbation.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) and reference lists of all primary studies and review articles. This search was up to date as of April 2016.
Selection criteria
We included parallel randomised controlled trials (RCTs), irrespective of blinding or duration, that evaluated one dose or duration of oral steroid versus any other dose or duration, for management of asthma exacerbations. We included studies involving both adults and children with asthma of any severity, in which investigators analysed adults and children separately. We allowed any other co‐intervention in the management of an asthma exacerbation, provided it was not part of the randomised treatment. We included studies reported as full text, those published as abstract only and unpublished data.
Data collection and analysis
Two review authors independently screened the search results for included trials, extracted numerical data and assessed risk of bias; all data were cross‐checked for accuracy. We resolved disagreements by discussion with the third review author or with an external advisor.
We analysed dichotomous data as odds ratios (ORs) or risk differences (RDs) using study participants as the unit of analysis; we analysed continuous data as mean differences (MDs). We used a random‐effects model, and we carried out a fixed‐effect analysis if we detected statistical heterogeneity. We rated all outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system and presented results in 'Summary of findings' tables.
Main results
We included 18 studies that randomised a total of 2438 participants ‐ both adults and children ‐ and performed comparisons of interest. Included studies assessed higher versus lower doses of prednisolone (n = 4); longer versus shorter courses of prednisolone (n = 3) or dexamethasone (n = 1); tapered versus non‐tapered courses of prednisolone (n = 4); and prednisolone versus dexamethasone (n = 6). Follow‐up duration ranged from seven days to six months. The smallest study randomised just 15 participants, and the largest 638 (median 93). The varied interventions and outcomes reported limited the number of meaningful meta‐analyses that we could perform.
For two of our primary outcomes ‐ hospital admission and serious adverse events ‐ events were too infrequent to permit conclusions about the superiority of one treatment over the other, or their equivalence. Researchers in the included studies reported asthma symptoms in different ways and rarely used validated scales, again limiting our conclusions. Secondary outcome meta‐analysis was similarly hampered by heterogeneity among interventions and outcome measures used. Overall, we found no convincing evidence of differences in outcomes between a higher dose or longer course and a lower dose or shorter course of prednisolone or dexamethasone, or between prednisolone and dexamethasone.
Included studies were generally of reasonable methodological quality. Review authors assessed most outcomes in the review as having low or very low quality, meaning we are not confident in the effect estimates. The predominant reason for downgrading was imprecision, but indirectness and risk of bias also reduced our confidence in some estimates.
Authors' conclusions
Evidence is not strong enough to reveal whether shorter or lower‐dose regimens are generally less effective than longer or higher‐dose regimens, or indeed that the latter are associated with more adverse events. Any changes recommended for current practice should be supported by data from larger, well‐designed trials. Varied study design and outcome measures limited the number of meta‐analyses that we could perform. Greater emphasis on palatability and on whether some regimens might be easier to adhere to than others could better inform clinical decisions for individual patients.
PICO
Laički sažetak
Različite doze i različite duljine trajanja liječenja astmatskih napada oralnim steroidima
Dosadašnje spoznaje: Osobe s astmom ponekad pate od astmatskih napada, u kojima simptomi poput kašlja, pritiska u prsima i zaduhe postaju izraženiji. Mnogi se pacijenti s astmatskim napadima liječe steroidima, i to najčešće kratkoročnim režimima u obliku tableta ili tekuće formulacije. Steroidi smanjuju upalni odgovor u dišnim putevima u plućima, no mogu imati nuspojave poput zastoja u rastu kod djece, hiperaktivnosti, te mučnine.
Pitanje sustavnog pregleda: U ovom Cochrane sustavnom pregledu literature uspoređene su različite doze ili trajanja terapija oralnim steroidima (steroidi koji se uzimaju na usta) u osoba s astmatskim napadom. To je važno zbog toga što se u različitim državama koriste različiti režimi terapije oralnim steroidima, no nije poznato koji je od njih najučinkovitiji u ublažavanju simptoma, a da istovremeno izazivaju što manje nuspojava.
Obilježja istraživanja: Uključeno je 18 studija u kojima je sudjelovalo 2438 ispitanika (odraslih i djece). U njima su uspoređene dvije vrste steroida ‐ prednizolon i deksametazon ‐ ili dvije različite doze ili trajanja terapije jednim od tih lijekova. Najmanja je studija uključivala samo 15 osoba, dok je u najvećoj sudjelovalo 638 ispitanika. Ispitanici su praćeni od sedam dana do šest mjeseci kako bi se uočili rezultati terapijskog režima. Obuhvaćena su istraživanja objavljena do travnja 2016.
Ključni rezultati: Uspoređivanje rezultata uključenih studija bilo je otežano zbog toga što su u njima korištene različite doze i trajanja terapijskih režima, te su autori svoje rezultate mjerili na različite načine. Isto tako, vrlo su rijetko zabilježeni događaji poput prijema u bolnicu i ozbiljnih nuspojava, zbog čega je teško procijeniti jesu li bolji i sigurniji kraći ili dulji režimi, odnosno režimi sa višim ili nižim dozama, te je li prednizolon općenito više ili manje učinkovit od deksametazona. Neke od uključenih studija bile su stare i u njima se nisu koristile doze ili trajanja terapije koje se koriste u današnje vrijeme.
Da bi se promijenio sadašnji način liječenja astmatskih napada oralnim steroidima nužno je dobiti rezultate novih istraživanja koja će biti veća od onih koja su nam trenutno na raspolaganju.
Kvaliteta dokaza: Dokazi izneseni u ovom Cochrane sustavnom pregledu općenito se smatraju dokazima niske ili vrlo niske kvalitete, što znači da autori nisu potpuno sigurni jesu li rezultati točni, poglavito iz razloga što nisu bili u mogućnosti kombinirati mnogo studija. Neke studije nisu jasno opisale način na koji su istraživači odlučili koji će sudionici primati određene doze steroida, a postojale su i studije u kojima su i sudionici i istraživači znali koja se doza lijeka primjenjuje. Moguće je da je to utjecalo na rezultate istraživanja.
Authors' conclusions
Summary of findings
| Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration range: 3 to 26 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission in follow‐up period | Longer vs shorter course prednisolone | OR 1.35 | 142 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 97 per 1000 | |||||
| Asthma symptoms Asthma severity score | Longer vs shorter course prednisolone | ‐ | 44 | ⊕⊕⊝⊝ | Higher score = Worse symptoms | |
| Mean asthma severity score was 2.6 | Mean asthma severity score in the longer course group was 0.7 lower (1.28 lower to 0.12 lower) | |||||
| Asthma symptoms Complete resolution by day 28 | Longer vs shorter course prednisolone | OR 0.55 | 35 | ⊕⊕⊝⊝ | ||
| 412 per 1000 | 278 per 1000 | |||||
| New exacerbation in follow‐up period Requiring visit to healthcare provider | Longer vs shorter course prednisolone | OR 0.98 | 55 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 109 per 1000 | |||||
| Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | OR 3.56 | 41 | ⊕⊝⊝⊝ | No events were reported in the tapered arm and only 2 events in the stable arm, so we were unable to calculate a baseline risk | ||
| No events | Risk difference in the stable (higher total dose) group was 9% (0 to 26%) | |||||
| New exacerbation in follow‐up period Oral corticosteroids prescribed | Longer vs shorter course prednisolone | OR 0.62 | 122 | ⊕⊕⊝⊝ | Lederle 1987 dominates this analysis, as the event rate was much higher than in the other 2 studies, possibly reflecting co‐morbid COPD in the study population. Result should be interpreted with caution | |
| 241 per 1000 | 165 per 1000 (68 to 348) | |||||
| Lung function tests FEV1% predicted | Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | ‐ | 41 | ⊕⊝⊝⊝ | Higher percentage = Better lung function | |
| Mean FEV1% predicted was 70.6 | Mean FEV1% predicted in the stable dose (higher total dose) was 1.02 lower (4.62 lower to 2.58 higher) | |||||
| All adverse events | Longer vs shorter course prednisolone | OR 4.15 | 43 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 409 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLederle 1987 carried a large proportion of the analysis weight for this outcome because event rates were higher in both groups. This may reflect co‐morbid COPD (participants were older and most had an extensive smoking history). Downgraded once for indirectness bConfidence intervals include no difference and an important benefit of a longer or shorter course. Downgraded once for imprecision cConfidence intervals excluded possible benefit of a shorter course, but the effect was based on only 1 study of 44 people. Downgraded once for imprecision dA 1‐7 scale of symptom severity averaged over days 6‐21 was used, making clinical benefit difficult to interpret. Downgraded once for indirectness eNeither treatment regimen used in the one study in this analysis is consistent with current international guidance. Downgraded once for indirectness fThe study contributing most of the analysis weight was unblinded and uncertainties surrounded the selection procedure. Downgraded once for risk of bias gBoth trials contributing to the analysis used a treatment regimen that was inconsistent with current international guidance. Downgraded once for indirectness hThe effect was derived from 2 very similar studies including 41 people in total. Studies had smaller standard deviations than would be expected given the sample sizes. Downgraded once for imprecision iThe result is based on 1 small study and has wide confidence intervals, which do not exclude the possibility of no difference or an important increase in adverse events in the longer course arm, Downgraded twice for imprecision | ||||||
| Adults: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration: 2 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Re‐admission during follow‐up period | 29 per 1000 | 10 per 1000 | OR 0.35 | 200 | ⊕⊝⊝⊝ | |
| Asthma symptoms Returned to normal activities within 3 days | 901 per 1000 | 800 per 1000 | OR 0.44 | 191 | ⊕⊕⊝⊝ | |
| New exacerbation during follow‐up period Any ED visit after discharge | 48 per 1000 | 63 per 1000 | OR 1.32 | 200 | ⊕⊝⊝⊝ | |
| New exacerbation during follow‐up period Unscheduled visit to primary healthcare provider | 29 per 1000 | 52 per 1000 | OR 1.85 | 200 | ⊕⊝⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed to this outcome with very few events reported in total, resulting in an imprecise estimate with confidence intervals including both important harms and benefits of either regimen. Downgraded twice for imprecision bOnly contributing study judged to be at high risk of attrition bias because of post‐randomisation exclusions and large numbers lost to follow‐up. Downgraded once for risk of bias cOnly 1 study contributed to this outcome with imprecise estimate and confidence intervals not completely excluding the possibility of no differences. Downgraded once for imprecision | ||||||
| Children: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: children with an acute exacerbation of asthma Duration range: 1 to 4 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission during follow‐up period | Higher‐ vs lower‐dose prednisolone | Not estimable | 98 | ⊕⊝⊝⊝ | Only one 3‐arm study (Langton Hewer 1998) contributed events to this analysis. Two lower‐dose arms pooled for this outcome. OR 1.55 (0.24 to 9.78) favouring lower dose | |
| Not pooled | Not pooled | |||||
| Longer vs shorter course prednisolone | OR 0.33 | 201 | ⊕⊕⊝⊝ | |||
| 10 per 1000 | 3 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 2.22 | 100 | ⊕⊝⊝⊝ | |||
| 19 per 1000 | 42 per 1000 | |||||
| Asthma symptoms Symptom free by 7 days | Longer vs shorter course prednisolone | OR 1.22 | 201 | ⊕⊕⊕⊝ | One other study (Langton Hewer 1998) randomising 98 children to high‐ vs medium‐ vs low‐dose prednisolone reported clinical asthma score at discharge. Small differences in scores were reported with uncertain clinical importance and no consistent dose‐response effect | |
| 307 per 1000 | 351 per 1000 | |||||
| Serious adverse events | Longer vs shorter course prednisolone | Not estimable | 201 | No events occurred in either trial arm | ||
| 0 per 1000 | 0 per 1000 | |||||
| New exacerbation during follow‐up period Oral corticosteroids prescribed | Higher‐ vs lower‐dose prednisolone | OR 1.38 | 231 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 24 per 1000 | |||||
| Longer vs shorter course prednisolone | OR 0.61 | 201 | ⊕⊕⊕⊝ | |||
| 79 per 1000 | 50 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 0.24 | 100 | ⊕⊕⊝⊝ | |||
| 154 per 1000 | 42 per 1000 | |||||
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | Longer vs shorter course dexamethasone | OR 2.17 | 100 | ⊕⊝⊝⊝ | ||
| 96 per 1000 | 188 per 1000 | |||||
| Lung function tests FEV1% predicted at discharge | High vs medium vs low dose | ‐ | 34 | This outcome includes only 1 small study (Langton Hewer 1998) in which a subset of participants were able to perform PFTs. Reported between‐group differences were small and of uncertain clinical importance with no consistent dose‐response effect. | ||
| ‐ | ‐ | |||||
| All adverse events | Longer vs short course prednisolone | OR 0.67 | 201 | ⊕⊕⊕⊝ | ||
| 30 per 1000 | 20 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed events to this outcome and was assessed to be at high risk of attrition bias because of unbalanced drop‐out from intervention arms. Downgraded once for risk of bias bThe study contributing events had 3 different dose arms, 1 of which is outside the current dosing guidelines. Two other studies reported no events, but intervention involved much higher doses of prednisolone. Downgraded once for indirectness cOnly 1 study contributed to this analysis. Imprecise estimate with confidence intervals including possibility of important harms or benefits. Downgraded twice for imprecision dOnly contributing study considered at high risk of bias in multiple domains. Downgraded once for risk of bias eOnly 1 study contributed to this outcome, resulting in imprecise estimate and confidence intervals including the possibility of important harms or benefits. Downgraded once for imprecision fOnly 2 studies contributed to this outcome with few events, resulting in imprecise estimate and wide confidence intervals including the possibility of important harms or benefits. Downgraded twice for imprecision gOnly 1 study contributed to this outcome, resulting in imprecise estimate, which does not exclude the possibility of no difference. Downgraded once for imprecision | ||||||
| Children: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: children with acute exacerbation of asthma Duration range: 1.5 to 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Admission at initial presentation | 116 per 1000 | 124 per 1000 | OR 1.08 (0.74 to 1.58) | 1007 | ⊕⊕⊝⊝ | |
| Re‐admission during follow‐up period | 22 per 1000 | 10 per 1000 | OR 0.44 (0.15 to 1.33) | 985 | ⊕⊕⊝⊝ | |
| Asthma symptoms scores Pulmonary Index Score (PIS); Patient Self Assessment Score (PSAS); Paediatric Respiratory Assessment Measure (PRAM) | Not pooled | Not pooled | ‐ | 328 (2 RCTs) | ⊕⊝⊝⊝ | Altamimi 2006 reported PIS and PSAS Cronin 2015 reported PRAM (we extracted the result, which excluded re‐enrolments) No between‐group differences were detected |
| Asthma symptoms Persistent cough, wheeze, chest tightness, night‐time wakening and difficulty maintaining normal activities | Not pooled | Not pooled | ‐ | 533 | The number of people experiencing these symptoms at day 10 was not found to be significantly different between the 2 intervention arms | |
| Serious adverse events | Not pooled | Not pooled | Not estimable | 255 | No events were reported in either study | |
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | 97 per 1000 | 83 per 1000 | OR 0.85 (0.54 to 1.34) | 981 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe 2 studies contributing most events to this outcome were considered to be at high or unclear risk of selection (Qureshi 2001) and performance and detection bias (Cronin 2015; Qureshi 2001). In addition, Cronin 2015 allowed 19 participants to enrol more than once in the study. Downgraded once for risk of bias bConfidence intervals include possible harms or benefits of either intervention. Downgraded once for imprecision cThe pulmonary index score may lack rigorous evaluation, so clinical interpretation of this score is limited. Downgraded once for indirectness dConfidence intervals for PIS and PSAS include no difference, but we are unsure whether either end of the confidence intervals includes a clinically important effect. Downgraded once for imprecision eThe PSAS score has been adapted from National Institute of Health guidelines and may lack rigorous evaluation, so clinical interpretation is limited. Downgraded once for indirectness fWe were unable to combine the results of these different scales. Downgraded once for inconsistency | ||||||
Background
Description of the condition
Asthma is a common long‐term breathing condition that affects approximately 300 million people worldwide and causes an estimated 250,000 deaths every year (WHO 2007). Between 1% and 18% of people in different countries are affected by asthma (GINA 2015), which is characterised by chronic airway inflammation and airway hyperresponsiveness, leading to shortness of breath, wheeze, chest tightness and cough. Symptoms are typically worse at night and in the early morning and may vary over time (CDC 2012; GINA 2015). Treatments are largely aimed at reducing airway smooth muscle constriction through the use of inhaled bronchodilators (e.g. short‐ and long‐acting beta2‐agonists) and reducing airway inflammation through the use of corticosteroids, which usually are also inhaled (BTS/SIGN 2014).
People with asthma may experience short‐term worsening of their asthma symptoms; these episodes are known as ‘exacerbations’, ‘flare‐ups’, ‘attacks’ or 'acute asthma'. Exacerbations are characterised by episodes of “progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms” (NAEPP 2007). International consensus on the definition of an attack or exacerbation has not been reached, but a working group in the USA recently suggested the definition as “a worsening of asthma requiring the use of systemic corticosteroids to prevent a serious outcome” (Fuhlbrigge 2012).
In the USA in 2008, more than half of adults and children with asthma had at least one asthma exacerbation (CDC 2011). Asthma exacerbation triggers vary from person to person but commonly include tobacco smoke, respiratory tract infection, house dust mites, air pollution, pets and mould (CDC 2006). Depending on severity, asthma exacerbations usually require a temporary change in the medication regimen for a person with asthma, for example, increased use of short‐acting bronchodilators such as salbutamol and a course of systemic steroids. More severe exacerbations may require treatment in an emergency department or admission to the hospital (BTS/SIGN 2014).
Description of the intervention
Oral steroids are recommended for all but the most mild asthma exacerbations (BTS/SIGN 2014); they should be initiated promptly (Rowe 2001). It is thought that the intravenous or intramuscular route offers no advantage over the oral route unless compliance with treatment or intestinal absorption is a matter of concern (Krishnan 2009; Lahn 2004). It is advised that oral steroids be taken as a single dose after breakfast (BNF).
Current guidelines on dosing vary slightly between countries, and often among different guideline producers within the same country. In the UK, the most recent (BTS/SIGN 2014) guidelines recommend for adults 40 to 50 mg daily oral prednisolone for at least five days, or until recovery. The same guidelines recommend a dose of 20 mg of prednisolone for children two to five years old, and 30 to 40 mg for children older than five years. GINA 2015 recommendations are similar and suggest a dose of 1 mg/kg for adult patients, up to a maximum daily dose of 50 mg, and 1 to 2 mg/kg for children aged six to 11 years, up to a maximum daily dose of 40 mg. GINA 2015 guidance advises that a five‐ to seven‐day course in adults and three to five days in children is usually adequate.
Currently evidence is insufficient to suggest that alternative steroids, such as dexamethasone, offer any advantage over prednisolone (BTS/SIGN 2014). Prednisolone is widely used internationally and is relatively inexpensive; a packet 28 × 5 mg tablets costs just £1.29 in the UK (BNF). It is not necessary to taper the dose when stopping, provided the patient is already using inhaled corticosteroids, is not taking long‐term oral steroids or has required an acute course of over three weeks’ duration (BTS/SIGN 2014; GINA 2015).
How the intervention might work
Glucocorticoids, including prednisolone, are potent inhibitors of inflammation and are used to treat a wide variety of inflammatory and autoimmune conditions, including asthma (Barnes 2003; van der Velden 1998). Glucocorticoids are thought to work by binding to a cellular glucocorticoid receptor, leading to down‐regulation of the expression of various genes involved in maintaining the inflammatory process. This in turn leads to decreased inflammatory cell recruitment and activation, up‐regulation of beta2‐receptors, decreased microvascular permeability and decreased mucus production (Barnes 1992). Research findings suggest more rapid resolution of symptoms and reduced relapse rates among patients treated with oral steroids (Alangari 2014; Krishnan 2009; Rowe 2007).
Why it is important to do this review
Despite their proven efficacy, use of steroids needs to be balanced against their potential to cause important adverse events. The problems associated with longer‐term steroid therapy are well established and include diabetes, osteoporosis, muscle wasting, Cushing’s syndrome and linear growth restriction in children (BNF). Indeed, regular use of even low to moderate daily doses of inhaled corticosteroids is associated with a mean reduction in linear growth velocity of 0.48 cm/y among children (Zhang 2014). However, many important adverse events are associated with shorter‐term use, which is commonly recommended for asthma exacerbations. These side effects include insomnia, nausea, abdominal distension, dyspepsia, malaise, vertigo, headache and (especially in children) behavioural changes (BNF; Kayani 2002).
Current evidence regarding optimal dosing of oral steroids for asthma exacerbations is somewhat limited. Bowler 1992 randomised 76 participants to receive low‐, medium‐ or high‐dose intravenous hydrocortisone in an inpatient setting for 48 hours, followed by low, medium or high doses of oral steroids give over 12 days. Study authors concluded that low‐dose hydrocortisone (50 mg, four times a day for 48 hours), followed by low‐dose prednisolone (20 mg daily, reduced to 5 mg over 12 days), was as effective as higher doses. In a similar study of 20 participants in the year 2000, researchers concluded that a one‐week course of oral steroids after a three‐day course of intravenous steroids was as effective as a two‐week course (Hasegawa 2000). A study of 86 children aged two to 16 years concluded that an oral prednisolone dose of 1 mg/kg was equally effective as 2 mg/kg but was associated with fewer behavioural adverse events (Kayani 2002). Similarly, Hewer 1998 identified no advantage of a 1 or 2 mg/kg dose over a 0.5 mg/kg dose in a study of 98 children admitted to hospital with acute asthma.
An overview or 'umbrella review' of corticosteroid use in acute asthma also addressed this question, suggesting that no evidence shows that doses above 50 to 100 mg daily are beneficial, and that a course duration of five to 10 days is sufficient for most discharged patients (Krishnan 2009). Similar findings were reported in Manser 2001. However, the conclusions presented in both of these reviews are based on studies of hospitalised patients wherein participants in at least one of the trial arms were receiving parenteral steroids.
Objectives
To assess the efficacy and safety of any dose or duration of oral steroids versus any other dose or duration of oral steroids for adults and children with an asthma exacerbation.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs), both blinded and unblinded, that evaluated any dose or duration of oral steroids versus any other dose or duration of oral steroids for management of an asthma exacerbation. We excluded cross‐over trials because of the long‐term effects of treatment with oral steroids and the unpredictable timing of a second exacerbation. We included studies reported as full text, those published as abstract only and unpublished data.
Types of participants
We included studies of both adults and children with asthma, diagnosed by clinician or according to national or international guidelines, who were experiencing an exacerbation. We recorded the severity of the exacerbation and the criteria used to define this. We excluded studies that recruited participants with other respiratory co‐morbidities and those taking long‐term oral steroids.
Types of interventions
We included studies comparing any dose or duration of oral steroids with any other dose or duration of oral steroids. We included studies that allowed any other co‐interventions for management of an asthma exacerbation, such as inhaled or nebulised short‐acting beta2‐agonists, provided they were not part of the randomised treatment.
We included participants who had presented to a primary care‐based healthcare facility or emergency department and those who had been admitted to hospital. We included participants who had received intravenous or intramuscular steroid therapy before commencing oral steroids, provided this was not part of the randomised treatment and this route of administration had ceased before randomisation to different oral dose or duration arms.
Eligable study comparisons included, but were not limited to, the following examples.
-
Short versus long duration of the same dose, e.g. 40 mg oral prednisolone daily for five days versus 40 mg oral prednisolone daily for 10 days.
-
High versus low dose of the same duration, e.g. 20 mg oral prednisolone daily for five days versus 40 mg oral prednisolone daily for five days.
-
Short duration and high dose versus long duration and low dose, e.g. 50 mg oral prednisolone for three days versus 20 mg oral prednisolone daily for 10 days.
Types of outcome measures
Primary outcomes
-
Admission/re‐admission to hospital.
-
Asthma symptoms at end of steroid course.
-
Serious adverse events.
Secondary outcomes
-
New exacerbation during post‐treatment follow‐up period.
-
Lung function tests at end of treatment/follow‐up period (trough forced expiratory volume in one second (FEV1) preferred if available).
-
All adverse events/side effects.
Reporting by investigators of one or more of the outcomes listed here was not an inclusion criterion for the review. Outcomes were chosen as those most important to patients after consultation with a patient representative.
If more than one scale measuring the same construct was reported within a study, or if different scales were used across studies, we analysed them together using standardised mean differences, provided clinical heterogeneity was sufficiently low to make a pooled analysis meaningful (e.g. we avoided combining different un‐validated symptom scales).
When possible, we extracted the types of adverse events experienced; our user group research suggests that psychological/emotional/behavioural side effects can be particularly troublesome during short‐term steroid courses. This has been reported narratively when meta‐analysis was not possible.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for details). We searched all records in the CAGR using the search strategy presented in Appendix 2. We performed the search in April 2016.
We conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/), also in April 2016.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. In a change to our protocol, we did not search manufacturers' websites, as the intervention medication is made generically by a large number of manufacturers worldwide.
We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) in April 2016 and identified no errata or retractions.
Data collection and analysis
Selection of studies
Two review authors (RN and KMK or GM) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications; two review authors (RN and KMK or GM) independently screened full‐text reports and identified studies for inclusion, while identifying and recording reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted the third review author. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least two studies in the review to record study characteristics and outcome data. In a change from the protocol, one review author (RN) extracted study characteristics from included studies and another review author (KMK) independently spot‐checked the extracted information for accuracy. We extracted the following information.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected and time points reported.
-
Notes: funding for trial and notable conflicts of interest of all trial authors.
Two review authors (RN and KMK or GM) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving the third person (RN, KMK or GM). One review author (RN or KMK) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review with data provided in the study reports. We ensured that KMK was not involved in both transferring data into RevMan and spot‐checking for accuracy.
Assessment of risk of bias in included studies
Two review authors (RN and KMK or GM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussing them or by involving another review author (RN, KMK or GM). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios or (for very rare events) as risk differences, which takes into account the zero cells in an analysis. We analysed continuous data as mean differences or standardised mean differences. We entered data presented as a scale with a consistent direction of effect. We extracted change from baseline scores in preference to endpoint scores, if both were reported.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we planned to include only the relevant arms. However, no included study reported a treatment arm irrelevant to this review. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) are combined in the same meta‐analysis, we will halve the control group to avoid double‐counting.
We dealt with children (i.e. average age of participants younger than 16) and adults separately in the review.
For our analyses, we attempted to group data into 'high‐dose' courses (e.g. > 50 mg daily dose in adults or > 2 mg/kg in children, i.e. higher than current recommendations) versus 'low‐dose' courses (i.e. within current recommendations), and 'longer duration' courses (e.g. > 7 days, again longer than most recommendations) versus 'short duration' courses.
Further grouping, determined by comparisons made within the studies, will be described later in the review.
Unit of analysis issues
The unit of analysis was the patient (i.e. number of participants admitted to hospital at least once rather than number of admissions per participant).
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, we reported this. We were not able to carry out any of our pre‐specified subgroup analyses because combinable data were lacking.
Assessment of reporting biases
We were unable to pool more than 10 trials, and so we could not create a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model.
Summary of findings table
We created a 'Summary of findings' table using the following outcomes.
-
Admission/re‐admission to hospital.
-
Asthma symptoms at end of steroid course.
-
Serious adverse events.
-
New exacerbation in post‐treatment follow‐up period.
-
All adverse events/side effects.
-
Lung function tests at end of treatment/follow‐up period.
We used the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), while using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses if we found significant heterogeneity. However, we anticipated correctly that we would identify few studies contributing data to each outcome within the possible comparisons outlined under Types of interventions. Therefore, we did not attempt to perform these subgroup analyses and instead presented information on these potential effect modifiers in Table 1.
| Study ID | Total n | Country | Age range, years | Duration of follow‐up | Comparison | Total dose comparison (converted to prednisolone equivalent) |
| 58 | USA | Not reported | 4 weeks | Prednisone 40 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 200 mg vs 213 mg | |
| 134 | Canada | 2 to16 | 3 weeks (maximum) | Predisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 1 day | 200 mg vs 80 mg (based on 20 kg child) | |
| 201 | Australia | 2 to15 | 4 weeks | Prednisolone 1 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 3 days | 100 mg vs 60 mg (based on 20 kg child) | |
| 226 | Ireland | 2 to 16 | 2 weeks | Prednisolone 1 mg/kg daily for 3 days vs 0.3 mg/kg dexamethasone once daily for 1 day | 60 mg vs 40 mg (based on 20 kg child) | |
| 15 | USA | 19 to 50 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 125 | USA | 2 to 17 | 1 week | Dexamethasone 0.6 mg/kg once daily for 2 doses (days 1 and 3) versus dexamethasone 0.6 mg/kg once daily for 1 day | 160 mg vs 80 mg (based on 20 kg child) | |
| 167 | USA | 2 to 18 | 1.5 weeks | Prednisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 200 mg vs 160 mg (based on 20 kg child) | |
| 20 | Japan | Not reported | 26 weeks | Prednisolone 0.5 mg/kg daily for 14 days vs prednisolone 0.5 mg/kg once daily for 7 days | 490 mg vs 245 mg (based on 70 kg adult) | |
| 47 | UK | 16 to 60 | 4‐6 weeks | Prednisolone 40 mg once daily for 10 days vs prednisolone 40 mg once daily for 5 days | 400 mg vs 200 mg | |
| 26 | India | 17 to 70 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 88 | USA | 2 to 18 | 4 weeks (maximum) | Prednisolone 2 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 5 days | 200 mg vs 100 mg (based on 20 kg child) | |
| 285 | USA | 18 to 45 | 2 weeks | Prednisolone 50 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 250 mg vs 213 mg | |
| 98 | UK | 1 to 15 | 2 weeks | Prednisolone 2 mg/kg once daily vs prednisolone 1 mg/kg once daily vs prednisolone 0.5 mg/kg once daily while inpatient and for up to 3 days post discharge | 200 mg vs 100 mg vs 50 mg (based on 20 kg child receiving a 5‐day course) | |
| 43 | USA | 30 to 78 | 12 weeks | Prednisolone 45 mg daily reducing to 0 mg daily over 7 weeks vs prednisolone 45 mg daily reducing to 0 mg daily over 7 days | 1575 mg vs 225 mg | |
| 152 | USA | 2 to 18 | 2 weeks | Prednisolone 4 mg/kg daily for 2 days, then 2 mg/kg daily for duration of admission vs prednisolone 2 mg/kg daily for duration of admission | 400 mg vs 200 mg (based on 20 kg child receiving a 5‐day course) | |
| 39 | UK | 16 to 55 | 4‐6 weeks | Prednisolone 40 mg daily for 10 days followed by 7‐day taper vs prednisolone 40 mg daily for 10 days | 540 mg vs 400 mg | |
| 628 | USA | 2 to 18 | 2 weeks | Prednisolone 2 mg/kg initial dose, then 1 mg/kg daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 120 mg vs 160 mg (based on 20 kg child) | |
| 86 | Indonesia | "Adults" | 6 weeks | Prednisolone 36 mg daily for 2 weeks vs prednisolone 12 mg daily for 2 weeks | 504 mg vs 168 mg |
-
Severity of asthma exacerbation according to mean baseline characteristics (e.g. mild vs moderate vs severe).
-
Hospitalised participants versus non‐hospitalised participants.
-
Treatment with intramuscular or intravenous steroids before randomisation versus no treatment with intramuscular or intravenous steroids before randomisation.
-
Asthma severity according to reported background characteristics (e.g. Global Initiative for Asthma (GINA) 1 and 2 vs GINA 3 and 4).
We planned to use the following outcomes in subgroup analyses.
-
Admission/re‐admission to hospital.
-
Asthma symptoms at end of treatment course.
-
Serious adverse events.
-
All adverse events.
We planned to use the formal test for subgroup interactions in Review Manager 5 (RevMan 2014), had subgroup analysis been possible.
We included all adverse events as an outcome in the subgroup analysis, as user group feedback suggests that many of the adverse events experienced would not be classified as 'serious' according to standard definitions in research, but can nonetheless have a substantial impact on daily functioning.
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
-
Studies at high risk of selection bias.
-
Unpublished data (i.e. no peer‐reviewed full paper available).
Results
Description of studies
Full details of the conduct and characteristics of each included study can be found in the Characteristics of included studies tables and reasons for exclusion when full texts had to be viewed are given in the Characteristics of excluded studies table.
Results of the search
We identified 1297 references through electronic database searches and an additional 109 records through searches of clinicaltrials.gov and the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/). We excluded most (n = 1335) of these references on the basis of title and abstract. We retrieved 71 full texts for more detailed assessment and at this stage excluded 47 additional references (related to 39 individual studies). Reasons for exclusion included wrong comparator, wrong intervention and not a randomised controlled trial. We also excluded three studies that were ongoing, and one study (reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria. We present trial flow in Figure 1.
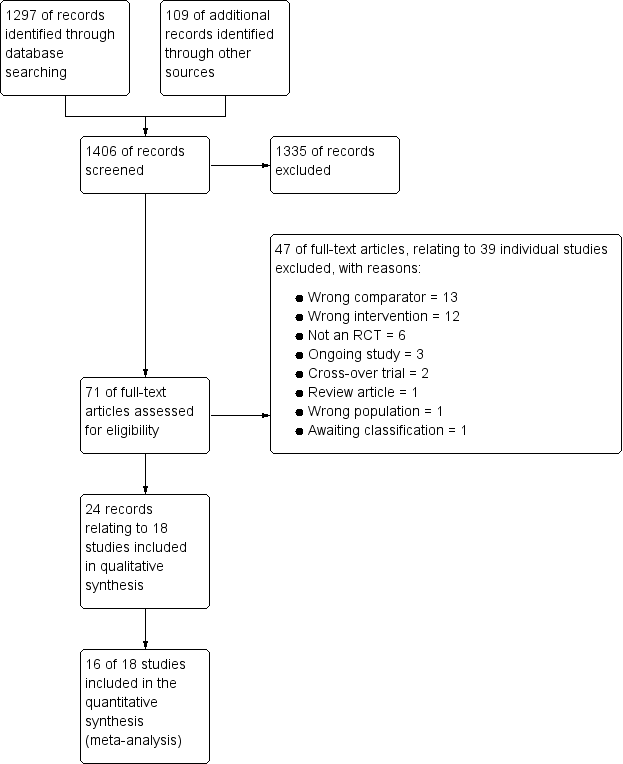
Study flow diagram.
Included studies
Eighteen studies met our inclusion criteria, 16 of which contributed data to at least one meta‐analysis. These studies included a total of 2438 participants who were randomly assigned to comparisons of interest in this review. The largest study included 628 participants, and the smallest just 15. The mean total number of participants was 135, and the median 93. Investigators reported 14 trials as full peer‐reviewed articles, three as abstracts only (Aboeed 2014; Ghafouri 2010; Viska 2008) and one on the clinicaltrials.gov website (NCT00257933), for which we obtained additional unpublished data directly from the trial contact person. We present a summary of the characteristics of included studies in Table 1.
Methods
As per our protocol, all included trials were RCTs with parallel design that compared one dose or duration of oral steroids versus another dose or duration. One study included three relevant arms: high‐, medium‐ and low‐dose oral prednisolone. Trial duration varied, with oral steroid treatment courses ranging from just a single dose to seven weeks of treatment. All studies included a post‐treatment follow‐up period, which ranged in duration from seven days to six months. No studies reported a run‐in period, as recruitment was triggered by an unscheduled presentation with an acute exacerbation of asthma. Outcomes data were extracted at the end of steroid treatment or at the last time point reported, or at both times if available. Trials were conducted in a variety of countries worldwide, but most were carried out in the USA (Aboeed 2014; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Kayani 2002; Kravitz 2011; Lederle 1987; NCT00257933; Qureshi 2001) and the UK (Jones 2002; Langton Hewer 1998; O’Driscoll 1993). The remainder were carried out in Australia (Chang 2008), Canada (Altamimi 2006), Japan (Hasegawa 2000), Indonesia (Viska 2008), India (Karan 2002) and Ireland (Cronin 2015).
Participants
We included studies involving both children and adults. Nine studies (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001) recruited only children (age range one to 18 years depending on the individual study), and seven studies (Cydulka 1998; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993; Viska 2008) recruited only adults (age range 16 to 78 years depending on the individual study). Two studies (Aboeed 2014; Hasegawa 2000) did not report the age range of participants, but the steroid doses administered in Aboeed 2014 would be consistent with adult participants. Most studies did not specify the ethnicity of participants.
All studies included participants with acute exacerbations of asthma. Although reported as having asthma, most of the participants in Lederle 1987 were older men who were current smokers or ex‐smokers, and many may in fact have had chronic obstructive pulmonary disease (COPD) with a degree of reversibility. In most cases, researchers did not report baseline asthma severity and severity of the asthma attack. However, in the majority of studies (Aboeed 2014; Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Karan 2002; Kayani 2002; Kravitz 2011; Qureshi 2001), researchers recruited participants in the emergency department (ED) or at an outpatient clinic, and the inclusion criteria in most of these studies required that they must be well enough to be discharged home. Four studies (Jones 2002; Langton Hewer 1998; Lederle 1987; O’Driscoll 1993) recruited participants and commenced randomised treatment on an inpatient basis but completed treatment at home. In one study (NCT00257933), randomised steroid treatment was continued for 48 hours or until discharge, whichever came sooner, followed by five to 10 days of standard oral steroid treatment at the discretion of the treating physician. One study did not report the specific setting in which treatment was commenced (Viska 2008), and in Hasegawa 2000, treatment was initiated in hospital, but it is not clear whether participants remained as inpatients for the duration of their steroid treatment.
Interventions
Studies included a variety of comparisons: longer versus shorter course of prednisolone (Chang 2008; Hasegawa 2000; Jones 2002); higher versus lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933; Viska 2008); longer course of prednisolone versus shorter course of dexamethasone (Aboeed 2014; Altamimi 2006; Cronin 2015; Greenberg 2008; Kravitz 2011; Qureshi 2001); tapering versus non‐tapering course of prednisolone (Cydulka 1998; Karan 2002; O’Driscoll 1993); long‐tapering versus short‐tapering course of prednisolone (Lederle 1987); and finally long versus short course of dexamethasone (Ghafouri 2010). Dosing also varied across studies; we have extracted this information and presented it in the Characteristics of included studies tables, along with the 'prednisolone‐equivalent' total dose received. All participants in Hasegawa 2000 received three days of intravenous methylprednisolone before commencing randomised oral steroid treatment.
Although we did not set out to compare different types of oral steroids, we included the dexamethasone versus prednisolone comparison because these agents were given over different durations, and this was part of our scope. We meta‐analysed these trials separately because, unlike studies that compared a different dose or duration of the same drug, most of these studies gave almost equivalent total doses of steroid in each intervention arm, so any between‐group differences may be related to drug‐specific factors including adherence or palatability. We recognise that in a clinical setting, drug‐specific factors, such as convenience for the patient, may affect an individual practitioner’s choice of drug or regimen.
Most studies stated that participants were allowed to continue use of specified rescue and preventer medication for asthma throughout the study, and in some trials, frequency of use of rescue medication, such as a short‐acting beta2‐agonist, was an efficacy outcome.
Outcomes
Outcomes reported were not consistent across reviews, and validated scales were not always used. Most studies (n = 13) reported some measure of asthma symptoms, at the end of treatment or follow‐up, or time taken for resolution of symptoms. Most (n = 13) also reported relapse rates, defined usually as an unscheduled visit to the ED or another healthcare provider during the follow‐up period. Three studies specifically reported hospitalisation during the follow‐up period, and seven studies reported new exacerbations or another course of oral steroids prescribed during the follow‐up period. Various measures of lung function were also frequently reported (n = 10), as was compliance with prescribed steroid therapy (n = 6). Adverse events were explicitly stated as an outcome measure in only six studies. Four studies recorded rescue medication use, four reported vital signs and three reported asthma severity scores. Two studies assessed adrenal suppression. One study reported Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), two reported school days or workdays missed and another used the asthma control test.
Excluded studies
We excluded 46 references (related to 38 individual studies) after assessment of full‐text articles. We excluded 13 studies, as they used a comparator not of interest in this review, for example, intravenous or inhaled steroids were compared with oral steroids. We excluded 12 studies because the intervention was not of interest in this review, for example, studies comparing different doses of intravenous steroids in the acute setting, or interventions including additional randomised treatments not of interest in this review. We excluded six studies as they were not randomised controlled trials and another two because they used a cross‐over trial design. One study was in fact a review article, and another study recruited a mixed population of patients with COPD and asthma. We excluded two studies that were ongoing (NCT01241006; NCT02192827), and one study (Tanifuji 2001; reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria.
Risk of bias in included studies
For details of the risk of bias rating for each study and the supporting evidence for each rating, see the Characteristics of included studies table. A summary of risk of bias judgements by study and domain (sequence generation, allocation concealment, blinding, incomplete data and selective reporting) can be found in Figure 2.
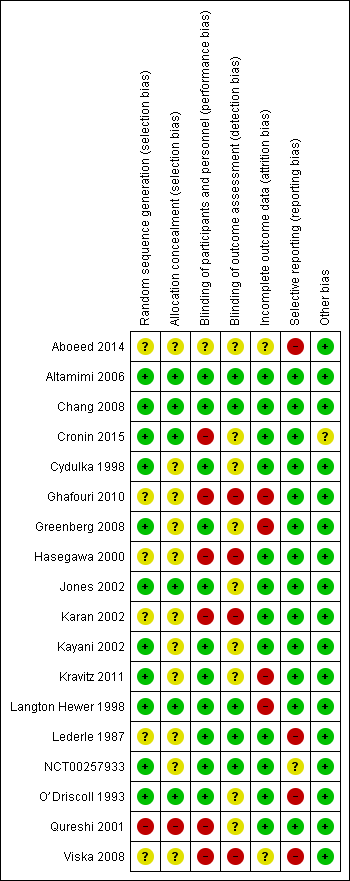
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six studies (Altamimi 2006; Chang 2008; Cronin 2015; Jones 2002; Langton Hewer 1998; O’Driscoll 1993) described the generation of a random sequence and concealment of allocation of participants in sufficient detail for review authors to assess them as having low risk of selection bias. We considered five other studies (Cydulka 1998; Greenberg 2008; Kayani 2002; Kravitz 2011; NCT00257933) to be at low risk of bias for random sequence generation but at unclear risk of bias for allocation concealment, which was not described in sufficient detail to allow a judgement.
Six studies (Aboeed 2014; Ghafouri 2010; Hasegawa 2000; Karan 2002; Lederle 1987; Viska 2008) did not provide sufficient details of random sequence generation or allocation concealment for review authors to make a judgement, and so we considered these studies to be at unclear risk of bias in both domains. We assessed Qureshi 2001 as having high risk of bias for random sequence generation and allocation concealment, as participants were allocated to the two intervention arms on the basis of the day of the month they presented to the ED.
Blinding
We judged most studies (n = 11; Altamimi 2006; Chang 2008; Cydulka 1998; Greenberg 2008; Jones 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Lederle 1987; NCT00257933; O’Driscoll 1993) to be at low risk of performance bias, as participants and trial personnel were adequately blinded. Five studies (Altamimi 2006; Chang 2008; Langton Hewer 1998; Lederle 1987; NCT00257933) clearly described blinding of outcome assessors, and we judged these studies to be at low risk of detection bias. We assessed the remaining six studies as having unclear risk of detection bias, as blinding of outcome assessors was not clearly described.
We considered Aboeed 2014 to be at unclear risk of bias for both performance and detection bias, as the abstract did not contain enough detail to allow a judgement. Four studies (Ghafouri 2010; Hasegawa 2000; Karan 2002; Viska 2008) were open‐label and were considered to be at high risk of performance and detection bias. In Cronin 2015, also an open‐label study, outcome assessors for the primary outcome (paediatric respiratory assessment measure (PRAM)) at day 4 were unaware of group allocation, but other participant‐reported or influenced outcomes (e.g. decision to re‐present to a healthcare practitioner) may have been affected by knowledge of group allocation, so we rated this study as having unclear risk of detection bias and high risk of performance bias. We considered one study (Qureshi 2001) to be at high risk of performance bias, as the trial was unblinded, but the primary outcome ‐ decision to seek medical care for deteriorating symptoms ‐ was assessed independently of study investigators, and so we rated the risk of detection bias as unclear.
Incomplete outcome data
We assessed 12 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Lederle 1987; NCT00257933; O’Driscoll 1993; Qureshi 2001) to be at low risk of attrition bias, as they had low and balanced withdrawal, and all participants who withdrew were clearly accounted for in the trial flow. We assessed Aboeed 2014 and Viska 2008, both conference abstracts, as having unclear risk, as they did not describe the number randomised to, or withdrawn from, each treatment arm.
We assessed Langton Hewer 1998 to be at high risk; attrition in the intervention groups was unbalanced (< 10% in the medium‐ and low‐dose groups and 20% in the higher‐dose group), and although all withdrawals were accounted for in the text of the report, this imbalance may have affected the findings. We assessed Ghafouri 2010, a conference abstract, to be at high risk of attrition bias because of unbalanced attrition in intervention groups, and because the reasons for withdrawal were not stated. We assessed Greenberg 2008 also to be at high risk, as approximately half of the participants randomised to each treatment did not complete the trial, and although baseline details are given for those who completed and those who did not, how this high level of attrition may have affected the findings is unclear. Finally, we assessed Kravitz 2011 as having high risk of attrition bias, as 30% (85 out of 285) of all randomised participants did not complete the trial as the result of admission to hospital after they were randomised or loss to follow‐up, and their outcomes remain unknown.
Selective reporting
We assessed 13 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Qureshi 2001) to be at low risk of reporting bias, although we were able to find prospectively registered protocols only for Chang 2008, Cronin 2015 and Ghafouri 2010.
We assessed Aboeed 2014 and Viska 2008, both conference abstracts, to be at high risk, as they provided minimal details and could not be included in the quantitative synthesis. We assessed NCT00257933 to be at unclear risk, as the trial has not yet been published. Some results are posted on clinicaltrials.gov, and the study authors kindly provided us with an unpublished manuscript, but some listed outcomes are as yet not fully reported (peak flow, clinical asthma score).
We considered Lederle 1987 to be at high risk, as not all outcomes were reported in a way that allowed meta‐analysis, including FEV1 (reported as percentage of baseline value without variance) and diary outcomes (reported narratively in the text with minimal supporting numerical data). Similarly, we assessed O’Driscoll 1993 to be at high risk, as many of the diary outcomes were not reported numerically, and data were displayed graphically with no variance.
Other potential sources of bias
Most studies did not report their funding source, and for those that did, this was not considered to be a likely source of bias. We assessed Cronin 2015 as being at unclear risk of other bias, as investigators allowed participants to enrol more than once in the trial. This may have led to the same participant contributing to outcomes twice; how the trial authors adjusted the analyses to take this into account is not clear, as they simply state that a "descriptive analysis of the patients enrolled multiple times was performed".
Effects of interventions
See: Summary of findings for the main comparison Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma; Summary of findings 2 Adults: prednisolone compared with dexamethasone for acute asthma; Summary of findings 3 Children: higher dose/longer course compared with lower dose/shorter course for acute asthma; Summary of findings 4 Children: prednisolone compared with dexamethasone for acute asthma
Structure of the analysis
We chose to analyse trials in adults and trials in children completely separately in this review.
Structure of the meta‐analysis
We created four main comparison headings within the analysis tree. For each comparison, we chose to meta‐analyse results only when the interventions and outcomes measured were sufficiently similar for pooling to make sense.
Adults: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in adults that compared a higher dose or a longer course with a lower dose or a shorter course of the same oral steroid (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), for example, 40 mg of prednisolone once daily for 10 versus five days, or 36 mg versus 12 mg of prednisolone daily for two weeks.
Adults: prednisolone versus dexamethasone
This comparison included all studies in adults that compared prednisolone with dexamethasone (Aboeed 2014; Kravitz 2011), for example, 40 mg prednisolone daily for five days versus 16 mg of dexamethasone daily for two days.
Children: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in children that compared a higher dose or a longer course with a lower dose of a shorter course of the same oral steroid (Chang 2008; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933), for example, 1 mg/kg daily prednisolone for five versus three days, or 2 mg/kg daily versus 1 mg/kg daily prednisolone for five days.
Children: prednisolone versus dexamethasone
This comparison included all studies in children that compared prednisolone with dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001), for example, 1 mg/kg prednisolone twice daily for five days versus dexamethasone 0.6 mg/kg once daily for one day.
Structure of the narrative synthesis
Below, we present the results narratively according to our pre‐specified outcomes. We begin with the primary outcomes: admission/re‐admission to hospital; asthma symptoms; and serious adverse events. Within each outcome, we describe effects of the interventions in adults, followed by effects in children, clearly specifying which of the above comparisons yielded the extracted data. We then describe the secondary outcomes: new exacerbation in the follow‐up period; lung function tests; and all adverse events/side effects, according to the same pattern.
Primary outcomes
Admission/re‐admission to hospital
Overall, our results demonstrated no difference in admission or re‐admission to hospital between participants prescribed a longer course or a higher dose of oral steroids and those prescribed a shorter course or a lower dose, or between those prescribed prednisolone and those prescribed dexamethasone. The requirement for admission at initial presentation was an exclusion criterion for many of the included studies. In those reporting admissions or re‐admissions, events were generally rare, and differences between interventions and populations in the included studies precluded meaningful meta‐analysis, resulting in imprecise estimates and low confidence in the result.
Admission at initial presentation: children
Four studies in children (Altamimi 2006; Cronin 2015; Ghafouri 2010; Qureshi 2001) reported admission at initial presentation. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ studies comparing prednisolone and dexamethasone ‐ did not detect a difference in admission rates between intervention groups (Analysis 4.1; odds ratio (OR) 1.08, 95% confidence interval (CI) 0.74 to 1.58; participants = 1007; I2 = 0%), but the confidence intervals include an important reduction and increase in admissions. In addition, one of the studies contributing to this analysis (Qureshi 2001) was considered to be at high risk of selection bias, and another study (Cronin 2015) was open‐label and therefore was at high risk of performance and detection bias for this outcome. We therefore have low confidence in the finding. Ghafouri 2010, a study comparing a longer course versus a shorter course of the same dose of dexamethasone, also reported no difference in admissions at initial presentation (Analysis 3.1; OR 1.66, 95% CI 0.60 to 4.61; participants = 125) but again with wide confidence intervals. It is important to note that admission at initial presentation would have been measured before the differing durations of treatment would have had an impact, and so this result is of limited value.
Re‐admission during follow‐up period: adults
Re‐admission to hospital during the follow‐up period was reported by five studies of adult participants (Hasegawa 2000; Jones 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993).
In four studies that compared a longer course versus a shorter course of prednisolone (Hasegawa 2000; Jones 2002; Lederle 1987; O’Driscoll 1993), no difference in re‐admissions was found between intervention groups, but events were rare and confidence intervals include the possibility of harm and the possibility of benefit from a longer or a shorter course (Analysis 1.1; OR 1.35, 95% CI 0.38 to 4.79; participants = 142; studies = 4; I2 = 0%). Of note, the study carrying the greatest weight in this analysis (Lederle 1987) likely recruited participants with co‐morbid COPD, so this outcome was additionally downgraded for indirectness of the study population. Similarly, the study comparing prednisolone versus dexamethasone in adults (Kravitz 2011) reported infrequent re‐admissions to hospital and consequently an imprecise result, and was considered to be at high risk of attrition bias (Analysis 2.1; OR 0.35, 95% CI 0.04 to 3.47; participants = 200).
Re‐admission during follow‐up period: children
Re‐admission to hospital during the follow‐up period was reported by eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001).
Three studies in children compared a higher dose versus a lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933), one compared a longer course versus a shorter course of prednisolone (Chang 2008) and one compared a longer course versus a shorter course of dexamethasone (Ghafouri 2010). Again, events were rare, with only nine participants requiring re‐admission across all five studies (with two studies reporting no events), resulting in wide confidence intervals in each of the three studies reporting events (Analysis 3.2). As the interventions were not sufficiently similar, we did not perform a meta‐analysis and our confidence in these estimates is low or very low.
Altamimi 2006, Cronin 2015 and Qureshi 2001 compared prednisolone versus dexamethasone, and although all three studies reported re‐admissions, they were infrequent, resulting in wide confidence intervals (Analysis 4.2; OR 0.44, 95% CI 0.15 to 1.33; participants = 985; I2 = 0%), and our confidence in the finding was further reduced by the risk of selection bias identified in Qureshi 2001 and by lack of blinding in Cronin 2015.
Asthma symptoms
Asthma symptoms were reported by several included studies, but investigators used a variety of measures and time points, limiting meaningful meta‐analysis. In general, individual studies did not detect an important difference between intervention arms but with a high level of imprecision.
Adults
In adults, asthma severity score was reported by Jones 2002 (mean of individuals' mean overall severity 1 to 7; 1 = no symptoms, 7 = worst symptoms) on days six to 21; Analysis 1.2). The result showed modest benefit with a longer course of prednisolone over a shorter course, but the clinical importance of this is not clear (mean difference (MD) ‐0.70, 95% CI ‐1.28 to ‐0.12; participants = 44), and our confidence in this estimate is low. O’Driscoll 1993, a small study comparing a tapered (longer) course of prednisolone versus a non‐tapered (shorter) course, reported the number of participants with complete resolution of asthma symptoms by day 28 but provided insufficient data to allow conclusions (Analysis 1.3; OR 0.55, 95% CI 0.13 to 2.26; participants = 35), and again we have low confidence in this estimate. Kravitz 2011, a trial that compared prednisolone versus dexamethasone, reported the number of participants who had resumed normal activities within three days. Results suggest a modest benefit of dexamethasone over prednisolone (Analysis 2.2; OR 0.44, 95% CI 0.19 to 1.01; participants = 191), but the confidence intervals do not fully exclude no differences, and the one study contributing to this outcome was assessed to be at high risk of attrition bias.
Children
In children, clinical asthma score at discharge was reported by Langton Hewer 1998, a study that compared high‐, medium‐ and low‐dose prednisolone. These findings are inconsistent, have uncertain clinical importance and show no clear benefit of a higher or a lower dose (Analysis 3.3). Chang 2008, a trial of a five‐ versus three‐day course of prednisolone, reported the number of children symptom free at seven days and did not detect a difference between intervention groups (Analysis 3.4; OR 1.22, 95% CI 0.67 to 2.19; participants = 201). We downgraded this outcome once for imprecision, but we are otherwise moderately confident in this estimate. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ reported asthma symptoms using different scales. Altamimi 2006 reported both the pulmonary index score (PIS) at day five and the mean number of days for the patient self assessment sheet (PSAS) score to return to normal. Researchers detected no between‐group differences (Analysis 4.3; MD ‐0.10, 95% CI ‐0.45 to 0.25; participants = 110; Analysis 4.4; MD 0.01, 95% CI ‐0.67 to 0.69; participants = 110), but we have low confidence in both estimates as the result of imprecision and lack of clarity about the rigorous validation of the scoring systems used. Cronin 2015 reported the paediatric respiratory assessment measure (PRAM) score at day four as the primary outcome for which the study was powered and detected no between‐group differences (Analysis 4.5; MD 0.00, 95% CI ‐0.36 to 0.36). Qureshi 2001, again a trial of prednisolone versus dexamethasone, reported separately persistent cough, wheeze, chest tightness, night wakening and difficulty maintaining normal activities (Analysis 4.6). This study detected no between‐group differences, but we assessed this trial as having high risk of selection and performance bias.
Serious adverse events
Included studies infrequently reported serious adverse events, and none of the studies in adults specifically reported this outcome. Five studies in children (Altamimi 2006; Chang 2008; Langton Hewer 1998; NCT00257933; Qureshi 2001), including a total of 695 participants, reported that there were no serious adverse events.
Secondary outcomes
New exacerbations during the follow‐up period
New exacerbations during the follow‐up period were reported by seven studies in adults (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993) and eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; NCT00257933; Qureshi 2001). New exacerbations were classified in two main ways: those requiring an unscheduled visit to a healthcare provider, and those requiring the prescription of additional oral corticosteroids. Overall, no included study reported a clear, unbiased benefit of one regimen over another, and varied interventions and definitions of an exacerbation prevented a unifying meta‐analysis.
Exacerbation requiring a visit to a healthcare provider: adults
Four small studies in adults (Cydulka 1998; Hasegawa 2000; Karan 2002; O’Driscoll 1993; total n = 96; Analysis 1.4) that compared longer versus shorter courses of prednisolone or stable versus tapered prednisolone reported exacerbations requiring a visit to a healthcare professional during the follow‐up period. Only eight events were reported across the four studies, resulting in insufficient data to ascertain possible differences between interventions for this outcome. Our confidence in these estimates was further reduced by concerns about selection, performance and detection bias in two of the contributing studies (Hasegawa 2000; Karan 2002) and by indirectness of the treatment regimens used, which deviated widely from current standard practice.
Kravitz 2011, a study involving adults that compared prednisolone versus dexamethasone, separately reported exacerbations requiring an emergency department visit and those requiring a visit to a primary healthcare provider. Investigators detected no differences between the two interventions for this outcome, but confidence intervals did not exclude the possibility of risk or harm for either intervention (Analysis 2.3; Analysis 2.4); we assessed this study to be at high risk of attrition bias, further limiting our confidence in this estimate.
Exacerbation requiring a visit to a healthcare provider: children
Five studies in children ‐ one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010; Analysis 3.7) and four comparing prednisolone versus dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001; Analysis 4.8) reported exacerbations requiring an unscheduled visit to a healthcare provider during the follow‐up period. The results reported by Ghafouri 2010 favoured a shorter over a longer course of dexamethasone for this outcome but with wide confidence intervals, which do not exclude the possibility that the longer course may be more beneficial (OR 2.17, 95% CI 0.67 to 7.01; participants = 100). In addition to our serious concerns about imprecision, we considered this study to be at high risk of bias in several domains.
The four studies investigating prednisolone versus dexamethasone favoured prednisolone, but again the confidence intervals did not exclude potential risk or benefit of either steroid for this outcome (Analysis 4.8; OR 0.85, 95% CI 0.54 to 1.34; participants = 981; I2 = 0%). Of note, Qureshi 2001 carried out an intention‐to‐treat analysis for this outcome, assuming that all children excluded because of vomiting or lost to follow‐up had a relapse; this analysis favoured dexamethasone, but confidence intervals did not exclude the possibility of no differences (OR 0.61, 95% CI 0.35 to 1.05), and we assessed this study as having high risk of selection and performance bias. We also rated Cronin 2015 as having high risk of performance and detection bias for this outcome, and we are uncertain about the effect that repeated enrolment of the same participants may have had on this outcome.
Exacerbation requiring additional oral corticosteroids: adults
Three studies in adults (Jones 2002; Lederle 1987; O’Driscoll 1993) that compared longer courses versus shorter courses of prednisolone reported exacerbations requiring an additional course of oral steroids during the follow‐up period. Results favoured a longer course of steroids, but the confidence intervals did not exclude the possibility of no differences or benefit derived from a shorter course (Analysis 1.5; OR 0.62, 95% CI 0.23 to 1.68; participants = 122; I2 = 0%). In addition, as already described, our confidence in the applicability of this finding to a population with asthma is reduced by the likelihood that many of the participants in Lederle 1987 had co‐morbid COPD, and that the higher event rate in this study dominated the analysis.
Viska 2008, a conference abstract, also reported 'relapse'. We did not include this study in the quantitative synthesis, as the total 'n' for each intervention group (higher‐ vs lower‐dose prednisolone) was not given. However, the abstract reported no differences between the two treatment arms for this outcome.
Exacerbation requiring additional oral corticosteroids: children
Finally, five studies in children ‐ two comparing higher versus lower doses of prednisolone (Kayani 2002; NCT00257933), one comparing a longer versus a shorter course of prednisolone (Chang 2008), one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010) and one comparing prednisolone and dexamethasone (Cronin 2015) ‐ reported exacerbations requiring an additional course of oral steroids. As for previous outcomes, events in Chang 2008, Ghafouri 2010, Kayani 2002 and NCT00257933 were rare, and none of these analyses demonstrated a conclusive benefit of one regimen over the other (Analysis 3.6). Our confidence in these estimates is moderate (Chang 2008) or low (Ghafouri 2010; Kayani 2002; NCT00257933) because of concerns about imprecision and risk of bias. Cronin 2015 detected benefit in favour of prednisolone (Analysis 4.9; OR 0.29, 95% CI 0.10 to 0.81; participants = 242). However, as the study authors discuss, this finding may be related to unblinded clinicians who tended to favour prednisolone over dexamethasone and were more inclined to prescribe additional steroids for those in the dexamethasone intervention group, reducing our confidence in this result.
Lung function tests
Some included studies reported lung function test results, predominantly peak expiratory flow rates (PEFRs) and forced expiratory volume in one second (FEV1), but overall these studies did not identify a conclusive benefit of one steroid regimen over another.
PEFR: adults
Two studies of adult participants that compared longer courses versus shorter courses of prednisolone (Jones 2002; O’Driscoll 1993) reported trough PEFR. Although a combined analysis of results of these two studies did not suggest differences between treatment regimens, the confidence intervals did not rule out a perceivable difference between trial arms (Analysis 1.6; MD ‐4.81, 95% CI ‐45.82 to 36.20; participants = 79; I2 = 0%). Viska 2008, a conference abstract, randomised adult participants to higher‐ versus lower‐dose prednisolone and reported PEFR at four weeks but did not reveal total 'n' for each group and reported no variance, so we were unable to include this study in the quantitative synthesis. Mean PEFR at four weeks (two weeks post treatment) for the higher‐dose group was 272.89 L/min, and for the lower‐dose group 296.11 L/min.
FEV1: adults
Two small studies of stable (higher total dose) versus tapered (lower total dose) prednisolone, given for the same duration (Cydulka 1998; Karan 2002), reported FEV1% predicted at 21 days (exact timing of the test not specified). Again, although investigators detected no differences between treatment regimens, we cannot conclude that the regimens are equivalent because data provided were insufficient (Analysis 1.7; MD ‐1.02, 95% CI ‐4.62 to 2.58; participants = 41; I2 = 0%); our confidence in this result is further reduced by the indirectness of treatment regimens used in these studies and by the unusually small standard deviations reported.
PEFR and FEV1: children
In children, only one study, which compared high‐, medium‐ and low‐dose prednisolone (Langton Hewer 1998), measured FEV1% predicted (Analysis 3.8) and PEFR% predicted (Analysis 3.9) at discharge in a small subgroup of participants who were able to perform these tests. Results were inconsistent (i.e. did not demonstrate a dose‐response relationship) and confidence intervals were overlapping for all three comparisons (high vs medium, high vs low and medium vs low) for both outcomes.
All adverse events/side effects
Similarly to serious adverse events, all adverse events were not frequently reported by the included studies, and when they were reported, benefit of one regimen over another was not generally shown.
Adults
Lederle 1987, a study of long tapering (seven weeks) versus short tapering (seven days) of prednisolone, was the only study including adults that reported adverse events. These were defined as 'steroid side effects', including weight gain, oedema, acne and easy bruising. Findings favoured a shorter taper but with very wide confidence intervals, which did not exclude the possibility of no differences (Analysis 1.9; OR 4.15, 95% CI 0.94 to 18.41; participants = 43). Of note, many participants likely had COPD with reversibility and may represent a distinctly different group from participants in the other included studies. Our confidence in this result is very low.
Children
In children, only one study of a five‐ versus three‐day course of prednisolone (Chang 2008) reported all adverse events. Events were too infrequent to permit conclusions about the relative safety of a longer course versus a shorter course (Analysis 3.10; OR 0.67, 95% CI 0.11 to 4.08; participants = 201). Two studies of higher‐dose versus lower‐dose prednisolone (Kayani 2002; NCT00257933) specifically reported recognised steroid side effects (facial fullness, facial erythema, change in appetite, abdominal pain, diarrhoea, anxiety, euphoria, depression, quiet and reserved manner, hyperactivity and aggressive behaviour). Langton Hewer 1998 also specifically reported 'hyperactivity related to beta‐agonist use', which we combined with findings of the two aforementioned studies in a meta‐analysis. None of the meta‐analyses showed clear benefit of one regimen over another. Of note, analyses of anxiety, hyperactivity and aggressive behaviour demonstrated high levels of heterogeneity, and many showed substantial imprecision (Analysis 3.11).
Finally, Cronin 2015, Greenberg 2008 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ specifically reported the adverse event of vomiting. Findings favoured dexamethasone, but with moderate heterogeneity, and the confidence interval did not exclude the possibility of no difference or modest benefit with use of prednisolone (Analysis 4.10; OR 3.05, 95% CI 0.88 to 10.55; participants = 867; I2 = 53%).
Discussion
Summary of main results
This review includes 18 studies that randomised a total of 2438 participants to comparisons of interest. Nine studies recruited only children, and seven only adults. Two studies did not report the age range of participants; we assumed one to be a study in adults, as the steroid doses described were consistent with treatment of adults (Aboeed 2014); the other was presented as a conference abstract, which did not contribute to the quantitative synthesis (Viska 2008). The included studies assessed higher versus lower doses of prednisolone (n = 4); longer versus shorter courses of prednisolone (n = 3) or dexamethasone (n = 1); tapered versus non‐tapered courses of prednisolone (n = 4); and prednisolone versus dexamethasone (n = 6). The varied interventions and outcomes reported limited the number of meaningful meta‐analyses that we could perform.
Overall, we did not find convincing evidence of a difference in outcomes between a higher dose or a longer course and a lower dose or a shorter course prednisolone or dexamethasone, or between prednisolone and dexamethasone. For two of our primary outcomes ‐ hospital admission and serious adverse events ‐ events were too infrequent to allow a conclusion about the superiority of one treatment over the other, or about their equivalence. Included studies reported asthma symptoms several different ways and rarely used validated scales, again limiting the conclusions that we could reach. Secondary outcome meta‐analysis was similarly hampered by heterogeneity among the interventions and outcomes measures used.
Included studies generally were of reasonable methodological quality, but generation of the randomisation sequence, allocation procedures and blinding of outcome assessors were frequently inadequately described. In six studies, participants were not blinded to their group allocation (Figure 2). Most outcomes in the review were assessed to be of low or very low quality, meaning that we are not confident in the effect estimates. The predominant reason for downgrading was imprecision, but indirectness and risk of bias also reduced our confidence in some estimates (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4).
Overall completeness and applicability of evidence
Although oral steroids are commonly used for asthma exacerbations worldwide, we identified only 18 studies of variable methodological quality that met our inclusion criteria.
Management of asthma exacerbations differs internationally, affecting the definition of a 'high‐' or 'low‐' dose regimen, or a 'short' or 'long' course. Some guidelines define a recommended range for the course of steroids (GINA 2015; NAEPP 2007); others emphasise that courses should be no less than five days but keep the length otherwise open‐ended (BTS/SIGN 2014). Regimens recommended by guidelines are likely to differ in cost and possibly in adherence, leaving practitioners in doubt about the preferred plan.
The recommendation to use a low or high dose or a short or long course might be understood differently in different countries if attention is not paid to the individual studies from which the evidence has been drawn. Also, practice has changed over time. The dates of studies included in this review range from 1987 (Lederle 1987) to 2015 (Cronin 2015), and what was considered a 'shorter regimen' in an earlier study might be considered a 'longer regimen' today. Indeed, although many of the included studies compared currently used regimens, others used uncommon doses or lengths of treatment in one or both trial arms that are not recommended by current guidelines and are not commonly used in practice today (Cydulka 1998; Hasegawa 2000; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), limiting the applicability of evidence derived from these trials.
In terms of choice of steroid, prednisolone is recommended as first‐line in all guidelines, whether for adults or for paediatric patients, and the evidence presented in this review is not strong enough to indicate whether the usual second‐line option, dexamethasone, is better or worse than prednisolone. Of note, this review did not consider other head‐to‐head steroid comparisons, as the primary objective was to assess the evidence for different doses and durations. Indeed, several included studies, which compared dexamethasone versus prednisolone, gave very similar total steroid doses within each intervention arm (e.g. Aboeed 2014; Greenberg 2008; Kravitz 2011; Table 1) and so addressed a slightly different question. This may explain why researchers detected little difference between arms. We did not combine these studies with any that assessed a different total dose or duration of the same steroid.
An important question that is addressed by some of the included studies (Aboeed 2014; Altamimi 2006; Cronin 2015; Cydulka 1998; Karan 2002; Lederle 1987; Qureshi 2001) is whether duration or complexity of the regimen affects participant adherence. Potential benefits of a longer treatment course risk may be underestimated if adherence is suboptimal compared with a shorter or less complex course. In clinical practice, this may be a factor that affects an individual clinician's choice, depending on the behaviour and needs of a particular patient. For example, a clinician might choose a shorter course or the option with the fewest daily doses for patients who have trouble adhering to medications. This may be particularly true for the head‐to‐head comparison of dexamethasone versus prednisolone described in this review, wherein factors such as palatability may have resulted in differential adherence to treatment regimens. This review did not seek to address this question, but it may be an important topic for future research.
In addition, almost all of the included studies recruited participants from an emergency department setting, which limits the applicability of our findings to people with asthma exacerbations who present to a primary care provider.
We had planned to perform subgroup analyses to explore whether background asthma severity or severity of the exacerbation was an important effect modifier. However, this was not possible, as this information was not consistently reported by the included studies, and heterogeneity of the studies limited meta‐analysis.
Quality of the evidence
We assessed the quality of the evidence presented in this review according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) criteria (Higgins 2011) using GRADEpro software (GRADEpro GDT) and presented these assessments in the 'Summary of findings' tables.
summary of findings Table for the main comparison presents a higher dose/longer course versus a lower dose/shorter course of oral steroids in adults; summary of findings Table 2 presents prednisolone versus dexamethasone in adults; summary of findings Table 3; presents a higher dose/longer course versus a lower dose/shorter course of oral steroids in children; and summary of findings Table 4 presents prednisolone versus dexamethasone in children. We assessed most outcomes to be of low or very low quality, meaning that we have limited confidence in the estimates.
We downgraded all outcomes at least once for imprecision, reflecting the small size of most of the included studies and the limited pooling that we were able to perform. Many effect estimates included a potentially important harm or benefit from either intervention, particularly for outcomes in which events were rare, such as admission to hospital or new exacerbations during the follow‐up period.
We also downgraded several outcomes because of concerns about possible performance and detection bias in the contributing studies (Cronin 2015; Ghafouri 2010; Karan 2002; Qureshi 2001), uncertainty about allocation procedures (Qureshi 2001) or attrition bias (Ghafouri 2010; Kravitz 2011; Langton Hewer 1998). Indirectness was a concern for outcomes contributed to by studies that used an intervention not currently used in common practice (Lederle 1987; Viska 2008) or that recruited a study sample likely to include many participants with co‐morbid COPD (Lederle 1987). We downgraded other outcomes for indirectness, as we had concerns about the rigorous validation of the measurement scales used (Altamimi 2006).
We did not suspect publication bias for any of the outcomes assessed. Pooled results appeared consistent, with low heterogeneity for almost all outcomes, likely reflecting our circumspect approach to combining data for which treatments, participants and underlying clinical questions were not similar enough for pooling to make sense.
Potential biases in the review process
We followed standard procedure according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to minimise bias in the review process. We performed a comprehensive search and think it unlikely that we failed to identify relevant studies. Two review authors independently screened the search, extracted study characteristics, and spot‐checked them for accuracy; and independently extracted all outcomes data, then checked them against the original report. Two or more review authors independently assessed risk of bias , showing a high level of agreement. These review authors resolved a few discrepancies by discussion, carried out GRADE assessments and achieved consensus by discussion.
However, our approach to the analysis required some flexibility, as we were unable to fully anticipate the nature of the outcome data that we would find. The precise comparisons used were inevitably performed post hoc as a result, and this introduced the risk of a data‐led analysis. We believe we mitigated for this risk by extensively discussing different approaches to the analysis and by seeking the independent opinion of the contact editor for the review.
Agreements and disagreements with other studies or reviews
Several systematic reviews have been published that address the question of the most effective dose of oral steroids for exacerbations of asthma. An 'umbrella review' (Krishnan 2009) concluded that doses of corticosteroids in excess of 50 to 100 mg per day offer no advantage over lower doses, and that a non‐tapering course given over five to 10 days is adequate for most patients. Although we did not consider the evidence presented in this review to be of sufficient quality to suggest that giving a dose over 50 to 100 mcg per day confers an advantage, we would agree that high doses have not generally proved more effective than lower doses. Furthermore, a daily dose of 50 to 100 mg exceeds the dose recommended by some current guidelines (BTS/SIGN 2014), and a five‐ to 10‐day range still leaves uncertainty for practitioners, which is especially important as many patients report that they experience unpleasant side effects while taking steroids.
An earlier Cochrane review (Manser 2001) assessed the evidence for the optimal dose of steroids, given by any route, for patients with severe asthma exacerbations requiring hospitalisation. This review also concluded that no evidence indicated that higher doses were associated with better outcomes or indeed with more adverse events. However, Manser 2001 included a cohort of patients with much more severe disease, and the doses given in the included studies far exceeded those assessed in this review, for example, high dose was considered greater than 360 mg per day methylprednisolone‐equivalent, medium dose between 80 and 360 mg and low dose 80 mg or less.
A meta‐analysis conducted to address the question of whether dexamethasone is an equivalent alternative treatment to prednisolone in children with acute asthma (Keeney 2014) included six studies, three of which are included in the current review. The additional three studies included in Keeney 2014 used dexamethasone given intramuscularly; therefore we excluded them. We also included Cronin 2015, published after Keeney 2014. However, the overall conclusions of Keeney 2014 are similar to ours; in terms of efficacy, one drug does not appear to be superior to the other. Study authors also note that dexamethasone may be associated with fewer episodes of vomiting and better adherence to prescribed therapy, but this is perhaps to be expected in a review that includes studies that used the intramuscular route for dexamethasone administration. We were unable to locate a systematic review that addressed this question in adult patients.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 1 Re‐admission during follow‐up period.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 2 Asthma symptoms: asthma severity score.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: complete resolution.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 4 New exacerbation during follow‐up period: requiring visit to healthcare provider.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 5 New exacerbation during follow‐up period: oral corticosteroids prescribed.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 6 Lung function tests: trough PEFR.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 7 Lung function tests: FEV1% predicted.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: number of participants achieving personal best at 4 weeks.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 9 All adverse events.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 1 Re‐admission during follow‐up period.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 2 Asthma symptoms: returned to normal activities within 3 days.
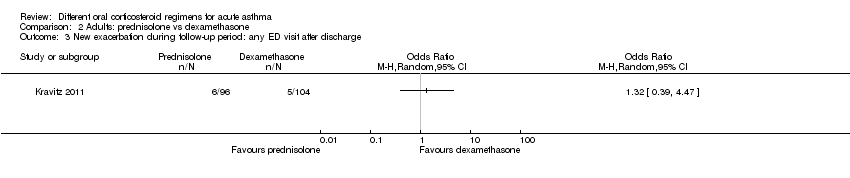
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 3 New exacerbation during follow‐up period: any ED visit after discharge.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 1 Admission at initial presentation.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 2 Re‐admission during follow‐up period.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: clinical asthma score at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 4 Asthma symptoms: symptom free by 7 days.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 5 Serious adverse events.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 6 New exacerbation during follow‐up period: oral corticosteroids prescribed.
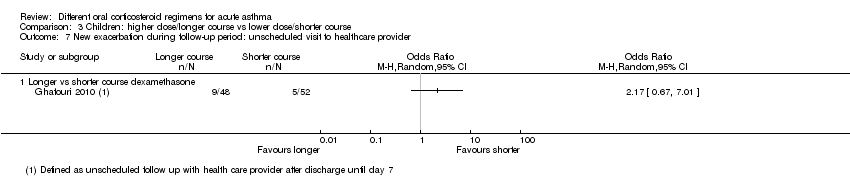
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: FEV1% predicted at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 9 Lung function tests: PEFR% predicted at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 10 All adverse events: longer vs short course prednisolone.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 11 All adverse events: higher‐dose vs lower‐dose prednisolone.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 1 Admission at initial presentation.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 2 Re‐admission during follow‐up period.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 3 Asthma symptoms: PIS.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 4 Asthma symptoms: PSAS.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 5 Asthma symptoms: PRAM.
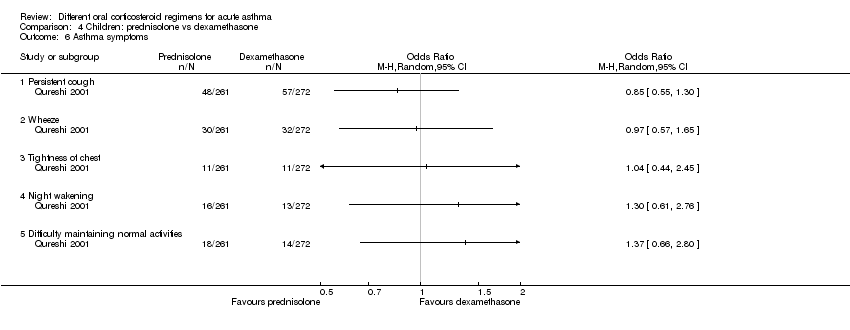
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 6 Asthma symptoms.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 7 Serious adverse events.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 9 New exacerbation during follow‐up period: oral corticosteroids prescribed.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 10 Adverse event: vomiting.
| Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration range: 3 to 26 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission in follow‐up period | Longer vs shorter course prednisolone | OR 1.35 | 142 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 97 per 1000 | |||||
| Asthma symptoms Asthma severity score | Longer vs shorter course prednisolone | ‐ | 44 | ⊕⊕⊝⊝ | Higher score = Worse symptoms | |
| Mean asthma severity score was 2.6 | Mean asthma severity score in the longer course group was 0.7 lower (1.28 lower to 0.12 lower) | |||||
| Asthma symptoms Complete resolution by day 28 | Longer vs shorter course prednisolone | OR 0.55 | 35 | ⊕⊕⊝⊝ | ||
| 412 per 1000 | 278 per 1000 | |||||
| New exacerbation in follow‐up period Requiring visit to healthcare provider | Longer vs shorter course prednisolone | OR 0.98 | 55 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 109 per 1000 | |||||
| Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | OR 3.56 | 41 | ⊕⊝⊝⊝ | No events were reported in the tapered arm and only 2 events in the stable arm, so we were unable to calculate a baseline risk | ||
| No events | Risk difference in the stable (higher total dose) group was 9% (0 to 26%) | |||||
| New exacerbation in follow‐up period Oral corticosteroids prescribed | Longer vs shorter course prednisolone | OR 0.62 | 122 | ⊕⊕⊝⊝ | Lederle 1987 dominates this analysis, as the event rate was much higher than in the other 2 studies, possibly reflecting co‐morbid COPD in the study population. Result should be interpreted with caution | |
| 241 per 1000 | 165 per 1000 (68 to 348) | |||||
| Lung function tests FEV1% predicted | Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | ‐ | 41 | ⊕⊝⊝⊝ | Higher percentage = Better lung function | |
| Mean FEV1% predicted was 70.6 | Mean FEV1% predicted in the stable dose (higher total dose) was 1.02 lower (4.62 lower to 2.58 higher) | |||||
| All adverse events | Longer vs shorter course prednisolone | OR 4.15 | 43 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 409 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLederle 1987 carried a large proportion of the analysis weight for this outcome because event rates were higher in both groups. This may reflect co‐morbid COPD (participants were older and most had an extensive smoking history). Downgraded once for indirectness bConfidence intervals include no difference and an important benefit of a longer or shorter course. Downgraded once for imprecision cConfidence intervals excluded possible benefit of a shorter course, but the effect was based on only 1 study of 44 people. Downgraded once for imprecision dA 1‐7 scale of symptom severity averaged over days 6‐21 was used, making clinical benefit difficult to interpret. Downgraded once for indirectness eNeither treatment regimen used in the one study in this analysis is consistent with current international guidance. Downgraded once for indirectness fThe study contributing most of the analysis weight was unblinded and uncertainties surrounded the selection procedure. Downgraded once for risk of bias gBoth trials contributing to the analysis used a treatment regimen that was inconsistent with current international guidance. Downgraded once for indirectness hThe effect was derived from 2 very similar studies including 41 people in total. Studies had smaller standard deviations than would be expected given the sample sizes. Downgraded once for imprecision iThe result is based on 1 small study and has wide confidence intervals, which do not exclude the possibility of no difference or an important increase in adverse events in the longer course arm, Downgraded twice for imprecision | ||||||
| Adults: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration: 2 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Re‐admission during follow‐up period | 29 per 1000 | 10 per 1000 | OR 0.35 | 200 | ⊕⊝⊝⊝ | |
| Asthma symptoms Returned to normal activities within 3 days | 901 per 1000 | 800 per 1000 | OR 0.44 | 191 | ⊕⊕⊝⊝ | |
| New exacerbation during follow‐up period Any ED visit after discharge | 48 per 1000 | 63 per 1000 | OR 1.32 | 200 | ⊕⊝⊝⊝ | |
| New exacerbation during follow‐up period Unscheduled visit to primary healthcare provider | 29 per 1000 | 52 per 1000 | OR 1.85 | 200 | ⊕⊝⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed to this outcome with very few events reported in total, resulting in an imprecise estimate with confidence intervals including both important harms and benefits of either regimen. Downgraded twice for imprecision bOnly contributing study judged to be at high risk of attrition bias because of post‐randomisation exclusions and large numbers lost to follow‐up. Downgraded once for risk of bias cOnly 1 study contributed to this outcome with imprecise estimate and confidence intervals not completely excluding the possibility of no differences. Downgraded once for imprecision | ||||||
| Children: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: children with an acute exacerbation of asthma Duration range: 1 to 4 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission during follow‐up period | Higher‐ vs lower‐dose prednisolone | Not estimable | 98 | ⊕⊝⊝⊝ | Only one 3‐arm study (Langton Hewer 1998) contributed events to this analysis. Two lower‐dose arms pooled for this outcome. OR 1.55 (0.24 to 9.78) favouring lower dose | |
| Not pooled | Not pooled | |||||
| Longer vs shorter course prednisolone | OR 0.33 | 201 | ⊕⊕⊝⊝ | |||
| 10 per 1000 | 3 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 2.22 | 100 | ⊕⊝⊝⊝ | |||
| 19 per 1000 | 42 per 1000 | |||||
| Asthma symptoms Symptom free by 7 days | Longer vs shorter course prednisolone | OR 1.22 | 201 | ⊕⊕⊕⊝ | One other study (Langton Hewer 1998) randomising 98 children to high‐ vs medium‐ vs low‐dose prednisolone reported clinical asthma score at discharge. Small differences in scores were reported with uncertain clinical importance and no consistent dose‐response effect | |
| 307 per 1000 | 351 per 1000 | |||||
| Serious adverse events | Longer vs shorter course prednisolone | Not estimable | 201 | No events occurred in either trial arm | ||
| 0 per 1000 | 0 per 1000 | |||||
| New exacerbation during follow‐up period Oral corticosteroids prescribed | Higher‐ vs lower‐dose prednisolone | OR 1.38 | 231 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 24 per 1000 | |||||
| Longer vs shorter course prednisolone | OR 0.61 | 201 | ⊕⊕⊕⊝ | |||
| 79 per 1000 | 50 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 0.24 | 100 | ⊕⊕⊝⊝ | |||
| 154 per 1000 | 42 per 1000 | |||||
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | Longer vs shorter course dexamethasone | OR 2.17 | 100 | ⊕⊝⊝⊝ | ||
| 96 per 1000 | 188 per 1000 | |||||
| Lung function tests FEV1% predicted at discharge | High vs medium vs low dose | ‐ | 34 | This outcome includes only 1 small study (Langton Hewer 1998) in which a subset of participants were able to perform PFTs. Reported between‐group differences were small and of uncertain clinical importance with no consistent dose‐response effect. | ||
| ‐ | ‐ | |||||
| All adverse events | Longer vs short course prednisolone | OR 0.67 | 201 | ⊕⊕⊕⊝ | ||
| 30 per 1000 | 20 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed events to this outcome and was assessed to be at high risk of attrition bias because of unbalanced drop‐out from intervention arms. Downgraded once for risk of bias bThe study contributing events had 3 different dose arms, 1 of which is outside the current dosing guidelines. Two other studies reported no events, but intervention involved much higher doses of prednisolone. Downgraded once for indirectness cOnly 1 study contributed to this analysis. Imprecise estimate with confidence intervals including possibility of important harms or benefits. Downgraded twice for imprecision dOnly contributing study considered at high risk of bias in multiple domains. Downgraded once for risk of bias eOnly 1 study contributed to this outcome, resulting in imprecise estimate and confidence intervals including the possibility of important harms or benefits. Downgraded once for imprecision fOnly 2 studies contributed to this outcome with few events, resulting in imprecise estimate and wide confidence intervals including the possibility of important harms or benefits. Downgraded twice for imprecision gOnly 1 study contributed to this outcome, resulting in imprecise estimate, which does not exclude the possibility of no difference. Downgraded once for imprecision | ||||||
| Children: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: children with acute exacerbation of asthma Duration range: 1.5 to 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Admission at initial presentation | 116 per 1000 | 124 per 1000 | OR 1.08 (0.74 to 1.58) | 1007 | ⊕⊕⊝⊝ | |
| Re‐admission during follow‐up period | 22 per 1000 | 10 per 1000 | OR 0.44 (0.15 to 1.33) | 985 | ⊕⊕⊝⊝ | |
| Asthma symptoms scores Pulmonary Index Score (PIS); Patient Self Assessment Score (PSAS); Paediatric Respiratory Assessment Measure (PRAM) | Not pooled | Not pooled | ‐ | 328 (2 RCTs) | ⊕⊝⊝⊝ | Altamimi 2006 reported PIS and PSAS Cronin 2015 reported PRAM (we extracted the result, which excluded re‐enrolments) No between‐group differences were detected |
| Asthma symptoms Persistent cough, wheeze, chest tightness, night‐time wakening and difficulty maintaining normal activities | Not pooled | Not pooled | ‐ | 533 | The number of people experiencing these symptoms at day 10 was not found to be significantly different between the 2 intervention arms | |
| Serious adverse events | Not pooled | Not pooled | Not estimable | 255 | No events were reported in either study | |
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | 97 per 1000 | 83 per 1000 | OR 0.85 (0.54 to 1.34) | 981 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe 2 studies contributing most events to this outcome were considered to be at high or unclear risk of selection (Qureshi 2001) and performance and detection bias (Cronin 2015; Qureshi 2001). In addition, Cronin 2015 allowed 19 participants to enrol more than once in the study. Downgraded once for risk of bias bConfidence intervals include possible harms or benefits of either intervention. Downgraded once for imprecision cThe pulmonary index score may lack rigorous evaluation, so clinical interpretation of this score is limited. Downgraded once for indirectness dConfidence intervals for PIS and PSAS include no difference, but we are unsure whether either end of the confidence intervals includes a clinically important effect. Downgraded once for imprecision eThe PSAS score has been adapted from National Institute of Health guidelines and may lack rigorous evaluation, so clinical interpretation is limited. Downgraded once for indirectness fWe were unable to combine the results of these different scales. Downgraded once for inconsistency | ||||||
| Study ID | Total n | Country | Age range, years | Duration of follow‐up | Comparison | Total dose comparison (converted to prednisolone equivalent) |
| 58 | USA | Not reported | 4 weeks | Prednisone 40 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 200 mg vs 213 mg | |
| 134 | Canada | 2 to16 | 3 weeks (maximum) | Predisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 1 day | 200 mg vs 80 mg (based on 20 kg child) | |
| 201 | Australia | 2 to15 | 4 weeks | Prednisolone 1 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 3 days | 100 mg vs 60 mg (based on 20 kg child) | |
| 226 | Ireland | 2 to 16 | 2 weeks | Prednisolone 1 mg/kg daily for 3 days vs 0.3 mg/kg dexamethasone once daily for 1 day | 60 mg vs 40 mg (based on 20 kg child) | |
| 15 | USA | 19 to 50 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 125 | USA | 2 to 17 | 1 week | Dexamethasone 0.6 mg/kg once daily for 2 doses (days 1 and 3) versus dexamethasone 0.6 mg/kg once daily for 1 day | 160 mg vs 80 mg (based on 20 kg child) | |
| 167 | USA | 2 to 18 | 1.5 weeks | Prednisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 200 mg vs 160 mg (based on 20 kg child) | |
| 20 | Japan | Not reported | 26 weeks | Prednisolone 0.5 mg/kg daily for 14 days vs prednisolone 0.5 mg/kg once daily for 7 days | 490 mg vs 245 mg (based on 70 kg adult) | |
| 47 | UK | 16 to 60 | 4‐6 weeks | Prednisolone 40 mg once daily for 10 days vs prednisolone 40 mg once daily for 5 days | 400 mg vs 200 mg | |
| 26 | India | 17 to 70 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 88 | USA | 2 to 18 | 4 weeks (maximum) | Prednisolone 2 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 5 days | 200 mg vs 100 mg (based on 20 kg child) | |
| 285 | USA | 18 to 45 | 2 weeks | Prednisolone 50 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 250 mg vs 213 mg | |
| 98 | UK | 1 to 15 | 2 weeks | Prednisolone 2 mg/kg once daily vs prednisolone 1 mg/kg once daily vs prednisolone 0.5 mg/kg once daily while inpatient and for up to 3 days post discharge | 200 mg vs 100 mg vs 50 mg (based on 20 kg child receiving a 5‐day course) | |
| 43 | USA | 30 to 78 | 12 weeks | Prednisolone 45 mg daily reducing to 0 mg daily over 7 weeks vs prednisolone 45 mg daily reducing to 0 mg daily over 7 days | 1575 mg vs 225 mg | |
| 152 | USA | 2 to 18 | 2 weeks | Prednisolone 4 mg/kg daily for 2 days, then 2 mg/kg daily for duration of admission vs prednisolone 2 mg/kg daily for duration of admission | 400 mg vs 200 mg (based on 20 kg child receiving a 5‐day course) | |
| 39 | UK | 16 to 55 | 4‐6 weeks | Prednisolone 40 mg daily for 10 days followed by 7‐day taper vs prednisolone 40 mg daily for 10 days | 540 mg vs 400 mg | |
| 628 | USA | 2 to 18 | 2 weeks | Prednisolone 2 mg/kg initial dose, then 1 mg/kg daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 120 mg vs 160 mg (based on 20 kg child) | |
| 86 | Indonesia | "Adults" | 6 weeks | Prednisolone 36 mg daily for 2 weeks vs prednisolone 12 mg daily for 2 weeks | 504 mg vs 168 mg |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐admission during follow‐up period Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Longer vs shorter course prednisolone | 4 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.38, 4.79] |
| 2 Asthma symptoms: asthma severity score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Longer vs shorter course prednisolone | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: complete resolution Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 New exacerbation during follow‐up period: requiring visit to healthcare provider Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Longer vs shorter course prednisolone | 2 | 55 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.14, 0.14] |
| 4.2 Stable vs tapered prednisolone | 2 | 41 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [‐0.07, 0.26] |
| 5 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 5.1 Longer vs shorter course prednisolone | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 6 Lung function tests: trough PEFR Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Longer vs shorter prednisolone (trough PEFR) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐4.81 [‐45.82, 36.20] |
| 7 Lung function tests: FEV1% predicted Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Stable vs tapered prednisolone (FEV1% predicted) | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐4.62, 2.58] |
| 8 Lung function tests: number of participants achieving personal best at 4 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 All adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐admission during follow‐up period Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Asthma symptoms: returned to normal activities within 3 days Show forest plot | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.01] |
| 3 New exacerbation during follow‐up period: any ED visit after discharge Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admission at initial presentation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Re‐admission during follow‐up period Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Higher‐dose vs lower‐dose prednisolone | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: clinical asthma score at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Asthma symptoms: symptom free by 7 days Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 4.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 5 Serious adverse events Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Higher‐dose vs lower‐dose prednisolone | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.25, 7.47] |
| 6.2 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.19, 1.94] |
| 6.3 Longer vs shorter course dexamethasone | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.19] |
| 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Lung function tests: FEV1% predicted at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lung function tests: PEFR% predicted at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 All adverse events: longer vs short course prednisolone Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.08] |
| 11 All adverse events: higher‐dose vs lower‐dose prednisolone Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Facial fullness | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.58, 2.80] |
| 11.2 Facial erythema | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.06] |
| 11.3 Change in appetite | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.72] |
| 11.4 Abdominal pain | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.57, 3.25] |
| 11.5 Diarrhoea | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.43, 13.84] |
| 11.6 Anxiety | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.20, 15.49] |
| 11.7 Euphoria | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.10] |
| 11.8 Depression | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.16, 1.79] |
| 11.9 Quiet and reserved | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.69, 4.36] |
| 11.10 Hyperactive | 3 | 318 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.52] |
| 11.11 Aggressive behaviour | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.02, 267.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admission at initial presentation Show forest plot | 3 | 1007 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 2 Re‐admission during follow‐up period Show forest plot | 3 | 985 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.33] |
| 3 Asthma symptoms: PIS Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.45, 0.25] |
| 4 Asthma symptoms: PSAS Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.67, 0.69] |
| 5 Asthma symptoms: PRAM Show forest plot | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 6 Asthma symptoms Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Persistent cough | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Wheeze | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Tightness of chest | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Night wakening | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Difficulty maintaining normal activities | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse events Show forest plot | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider Show forest plot | 4 | 981 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.34] |
| 9 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 1 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.81] |
| 10 Adverse event: vomiting Show forest plot | 3 | 867 | Odds Ratio (M‐H, Random, 95% CI) | 3.05 [0.88, 10.55] |

