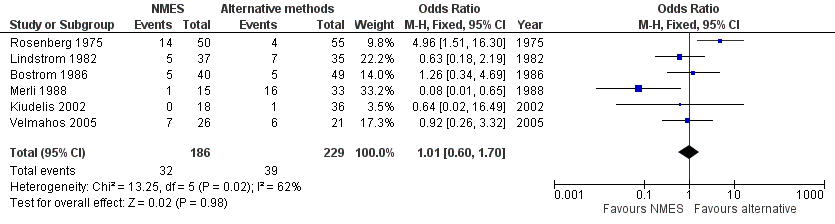
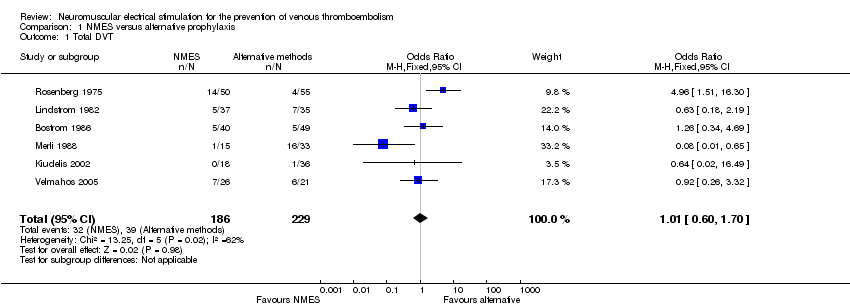
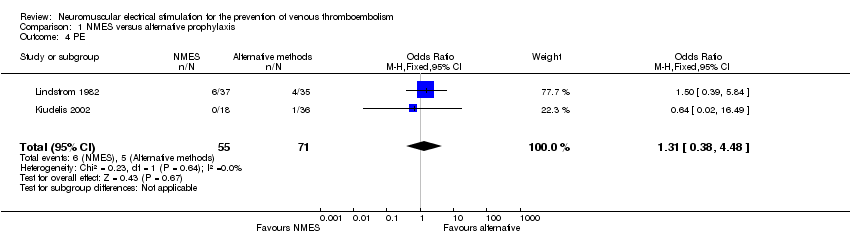
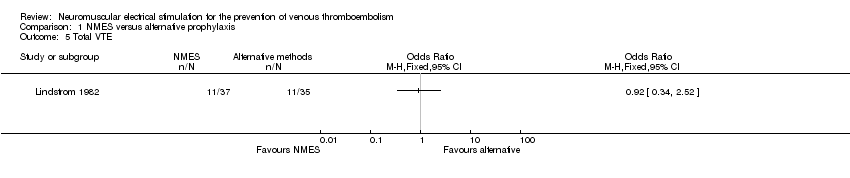
Contenido relacionado
Revisiones y protocolos relacionados
Raza Alikhan, Rachel Forster, Alexander T Cohen | 7 mayo 2014
Stavros Kakkos, George Kirkilesis, Joseph A Caprini, George Geroulakos, Andrew Nicolaides, Gerard Stansby, Daniel J Reddy | 28 enero 2022
Kezhou Dong, Yanzhi Song, Xiaodong Li, Jie Ding, Zhiyong Gao, Daopei Lu, Yimin Zhu | 31 octubre 2016
Aniek AG Zee, Kelly van Lieshout, Maaike van der Heide, Loes Janssen, Heinrich MJ Janzing | 6 agosto 2017
Jin Min Zhao, Mao Lin He, Zeng Ming Xiao, Ting Song Li, Hao Wu, Hua Jiang | 22 diciembre 2014
Alina Andras, Adriano Sala Tenna, Marlene Stewart | 24 julio 2017
Susan R Kahn, David R Morrison, Gisèle Diendéré, Alexandre Piché, Kristian B Filion, Adi J Klil‐Drori, James D Douketis, Jessica Emed, André Roussin, Vicky Tagalakis, Martin Morris, William Geerts | 24 abril 2018
Fabio CF Amaral, Jose CC Baptista-Silva, Luis CU Nakano, Ronald LG Flumignan | 22 noviembre 2022
Anne WS Rutjes, Ettore Porreca, Matteo Candeloro, Emanuele Valeriani, Marcello Di Nisio | 18 diciembre 2020
David K Cundiff, Juliet Manyemba, John C Pezzullo | 25 enero 2006