Suturas absorbibles versus no absorbibles para el cierre de la piel después de la cirugía de descompresión del túnel carpiano
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011757.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 febrero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
RGW: identified need for review on this topic and preliminary review of available evidence.
RGW and JCRW: developed protocol.
AF: provided clinical input.
JCRW: provided methodology input and developed initial search strategy alongside AAG.
The authors completed this review within their own time, as there is no allocated time or funding for such activities within their job plans.
| TASK | WHO HAS AGREED TO UNDERTAKE THE TASK? |
| Draft the protocol | Ryckie G Wade, Justin CR Wormald |
| Develop criteria for a search strategy (in conjunction with the Trials Search Co‐ordinator) | Justin CR Wormald |
| Search identified titles and abstracts for trials (usually 2 people) | Ryckie G Wade, Justin CR Wormald |
| Obtain copies of trials | All review authors |
| Select which trials to include (2 + 1 arbiter) | All review authors |
| Extract data from trials (2 people) | Justin Wormald, Ryckie G Wade |
| Enter data into RevMan (1 + 1 to check) | Justin CR Wormald, Ryckie G Wade |
| Carry out the analysis | Justin CR Wormald |
| Develop 'Summary of findings' tables | Justin CR Wormald, Ryckie G Wade |
| Interpret the analysis | All review authors |
| Draft the final review | All review authors |
| Update the review | Ryckie G Wade, Justin CR Wormald |
Sources of support
Internal sources
-
NIHR Academic Clinical Fellowship, UK.
Ryckie G Wade is an Academic Clinical Fellow in Plastic Surgery, funded by the National Institute for Health Research. He conducted part of this review during this fellowship,
-
None, Other.
Justin Wormald and Andrea Figus complete this review within their own time, as there is no allocated time or funding for such activities within their jobs plans. Ryckie Wade began this review in his own time, but later used the above fellowship time to complete the works.
External sources
-
No sources of support supplied
Declarations of interest
RGW: none
JCRW: none
AF: none
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
We owe thanks to:
Dr Michael Lunn, Mr Richard Wormald and the late Professor M Felix Freshwater for their constructive comments;
Mrs Angela A Gunn, Information Specialist for Cochrane Neuromuscular for her expert advice and supervision of the search strategy;
Dr Ruth Brassington, Managing Editor of Cochrane Neuromuscular for her extensive support and advice throughout the review process, as well as her amendments and suggestions to the protocol and full review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Feb 01 | Absorbable versus non‐absorbable sutures for skin closure after carpal tunnel decompression surgery | Review | Ryckie G Wade, Justin CR Wormald, Andrea Figus | |
| 2015 Jun 23 | Absorbable versus non‐absorbable sutures for carpal tunnel release | Protocol | Ryckie Wade, Justin CR Wormald, Andrea Figus | |
Differences between protocol and review
-
Our protocol described using primary data from studies on "open or minimally invasive carpal tunnel decompression surgery" and accordingly, we included one trial on ECTD because this is a form of minimally invasive surgery. The incision(s) required for ECTD are slightly smaller, and sometimes two incisions are required. Because such incisions warrant closure with a suture and the participants, methods and outcomes are sufficiently similar, we chose to include the ECTD data, but presented the data separately.
-
We intended to define postoperative pain as: short term (first 48 hours postoperatively), medium term (48 hours to seven days postoperatively) and long term (more than seven days postoperatively). However, following review of the included literature, we found that the majority of studies reported pain at 10 days and at 6 weeks and so we have deviated from protocol in order to report these outcomes at time points which both reflected the original data and enabled us to perform a meta‐analysis.
-
In general, outcome reporting was not as extensive as we had expected and so we were largely limited to reporting postoperative pain and wound inflammation, with the one ECTD trial reporting hand function and—on an ordinal, probably nonvalidated scale—scar satisfaction. Our protocol described secondary outcomes including: 1) postoperative hand function, e.g. as measured by the validated Disabilities of the Arm, Shoulder and Hand questionnaire, 2) scar satisfaction, e.g. as measured by the validated Vancouver Scar Scale, and 3) postoperative adverse events. Adverse events were not reported in any of the included studies and so we are unable to investigate them.
-
We included five studies so did not produce a funnel plot to investigate publication bias.
-
We changed the title of the review in order to better describe the nature of the intervention under scrutiny.
-
We added scar inflammation as a secondary outcome because this was reported in two trials and we felt would be supplement the secondary outcomes (particularly scar satisfaction) which we could not fully assess owing to insufficient primary data.
-
We planned to carry out subgroup analyses stratified by: 1) disease severity of carpal tunnel syndrome, and 2) medical comorbidity, but these data were not reported in the primary trials so could not be undertaken.
-
We followed guidance in the Cochrane Handbook for Systematic Reviews of Interventions for rule of thumb interpretation of SMD and I2 (Deeks 2011; Higgins 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Sutures;
- *Wound Closure Techniques;
- Carpal Tunnel Syndrome [*surgery];
- Cicatrix [psychology];
- Decompression, Surgical [methods];
- Inflammation [epidemiology, etiology];
- Pain Measurement;
- Pain, Postoperative [diagnosis];
- Postoperative Complications [epidemiology, psychology];
- Randomized Controlled Trials as Topic;
- Treatment Outcome;
Medical Subject Headings Check Words
Female; Humans; Male; Middle Aged;
PICO

Flow diagram illustrating the study selection process.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.1 Postoperative pain (10 days) after CTD.
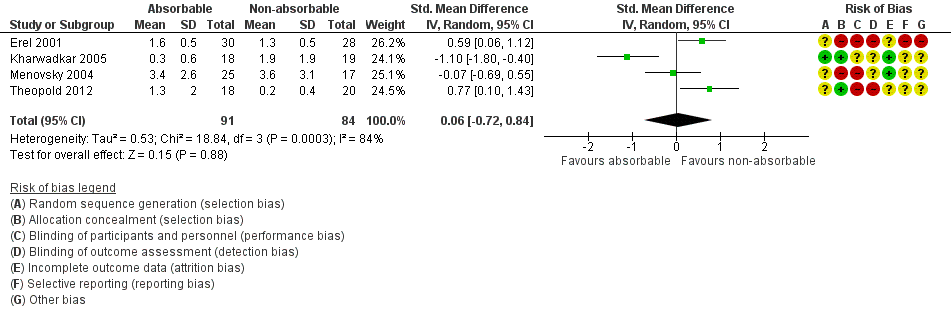
Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.2 Postoperative pain (6 weeks) after open CTD.

Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.3 Wound inflammation.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 1 Postoperative pain (10 days) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 2 Postoperative pain (6 weeks) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 3 Postoperative hand function (BCQ‐FSS) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 4 Wound inflammation.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 1 Postoperative pain (10 days) after CTD.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 2 Wound inflammation.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 3 Scar satisfaction (scar assessed as 'nice') by participant after endoscopic CTD.
| Absorbable sutures compared with non‐absorbable sutures for carpal tunnel decompression after open carpal tunnel decompression surgery | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by open carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting : secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after OCTD in the non‐absorbable suture groups was 0.03 SMD higher (0.43 lower to 0.48 higher) | 137 (3 RCTs) | ⊕⊝⊝⊝ | SMD 0.03 (95% CI ‐0.43 to 0.48) A SMD of 0.03 represents little or no difference between groups3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative pain (6 weeks) after open CTD in the non‐absorbable suture groups was | 175 (4 RCTs) | ⊕⊝⊝⊝ | SMD 0.06 (95% CI ‐0.72 to 0.84) A SMD of 0.06 represents little or no difference between groups. It is uncertain whether there is any difference in postoperative pain scores at 6 weeks because the quality of evidence is very low. | |
| Postoperative hand function (2 weeks postoperatively) Mean | The mean FSS score in the non‐absorbable suture group 2 weeks postoperatively was 1.6 | The mean FSS score 2 weeks postoperatively was 0.1 lower (0.53 lower to 0.33 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ | MD ‐0.10, 95% CI ‐0.53 to 0.33) It is uncertain whether there is any difference between groups in postoperative hand function because the quality of evidence is very low. MD at 6 and 12 weeks follow‐up postoperatively were 0.00 (95% CI ‐0.39 to 0.39) and 0.00 (95% CI ‐0.37 to 0.37). |
| Wound Inflammation 6 to 12 weeks follow‐up | 370 per 1000 | 843 per 1000 (89 to 1000) | RR 2.28 (0.24 to 21.91) | 95 | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; OCTD: open carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale; CTS‐FSS: Carpal Tunnel Syndome Functional Status Scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice based on study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). 3Based on a rule‐of‐thumb guide to interpretation of SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988 in Higgins 2011). | ||||||
| Absorbable sutures compared with non‐absorbable sutures for endoscopic carpal tunnel decompression | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by endoscopic carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting: secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after ECTD in the non‐absorbable suture groups was 0.81 SMD lower (1.36 to 0.25 lower) | 54 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | SMD ‐0.81 (95% CI ‐1.36 to ‐0.25) A SMD of ‐0.81 represents a large difference between groups.3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Postoperative hand function | ‐ | ‐ | ‐ | ‐ | Not measured | |
| Wound Inflammation 6 to 12 weeks follow‐up | 38 per 1000 | 36 per 1000 (2 to 542) | RR 0.93 (0.06 to 14.09) | 54 (1 RCT) | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | 692 per 1000 | 893 per 1000 (672 to 1000) | RR 1.29 (0.97 to 1.72) | ‐ | ⊕⊝⊝⊝ | |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; ECTD: endoscopic carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice for study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain (10 days) after CTD Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Postoperative pain (6 weeks) after CTD Show forest plot | 4 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.72, 0.84] |
| 3 Postoperative hand function (BCQ‐FSS) after CTD Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 2 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 3.2 6 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.39, 0.39] |
| 3.3 12 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.37, 0.37] |
| 4 Wound inflammation Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain (10 days) after CTD Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Wound inflammation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Scar satisfaction (scar assessed as 'nice') by participant after endoscopic CTD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

