| Absorbable sutures compared with non‐absorbable sutures for carpal tunnel decompression after open carpal tunnel decompression surgery |
| Patients: adults undergoing primary carpal tunnel decompression by open carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting : secondary care |
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after OCTD in the non‐absorbable suture groups was 0.03 SMD higher (0.43 lower to 0.48 higher) | | 137 (3 RCTs) | ⊕⊝⊝⊝
Very low1,2 | SMD 0.03 (95% CI ‐0.43 to 0.48) A SMD of 0.03 represents little or no difference between groups3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. |
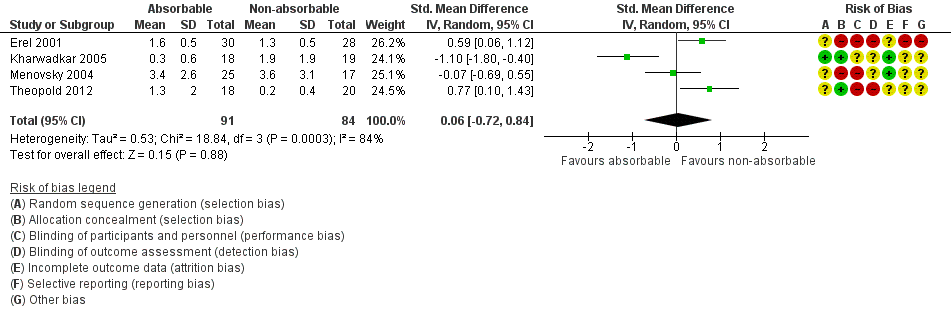
| Postoperative pain: late pain (6 weeks postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative pain (6 weeks) after open CTD in the non‐absorbable suture groups was
0.06 SMD higher
(0.72 lower to 0.84 higher) | | 175 (4 RCTs) | ⊕⊝⊝⊝
Very low1,2 | SMD 0.06 (95% CI ‐0.72 to 0.84) A SMD of 0.06 represents little or no difference between groups. It is uncertain whether there is any difference in postoperative pain scores at 6 weeks because the quality of evidence is very low. |
| Postoperative hand function (2 weeks postoperatively) Mean
CTS‐FSS score (scale 1 to 5, where higher scores indicate worse function) | The mean FSS score in the non‐absorbable suture group 2 weeks postoperatively was 1.6 | The mean FSS score 2 weeks postoperatively was 0.1 lower (0.53 lower to 0.33 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝
Very low1,4 | MD ‐0.10, 95% CI ‐0.53 to 0.33) It is uncertain whether there is any difference between groups in postoperative hand function because the quality of evidence is very low. MD at 6 and 12 weeks follow‐up postoperatively were 0.00 (95% CI ‐0.39 to 0.39) and 0.00 (95% CI ‐0.37 to 0.37). |
| Wound Inflammation 6 to 12 weeks follow‐up | 370 per 1000 | 843 per 1000 (89 to 1000) | RR 2.28 (0.24 to 21.91) | 95
(2 RCTs) | ⊕⊝⊝⊝
Very low1,4 | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; OCTD: open carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale; CTS‐FSS: Carpal Tunnel Syndome Functional Status Scale |
| Quality of Evidence Grades
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |