Ranolazina para la angina de pecho estable
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel‐group trial Total study duration: 3 months Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 40 (20/20 for placebo/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported | |
| Participants | Total number: 40 Country of enrolment: Greece Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and coronary artery disease (CAD) established by coronary stenosis > 70% in one or more vessels documented by angiography (macrovascular angina) Comorbidities: none (non suitability for invasive treatment) Age (mean): 69 ± 7 years Gender (male %): 75% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: optimised anti‐anginal treatment (not further specified) Excluded medications: none Placebo group Intervention: placebo Duration of intervention: 3 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 3 months | |
| Outcomes | Total number of outcomes: 1
OUTCOMES Adverse events incidence
RESULTS Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: It is worth mentioning that two ranolazine group participants and four placebo group participants were not eligible for revascularisation because of complicated coronary anatomy or lack of grafts Source of funding: not stated Notable conflicts of interest: the authors declare that they have no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | No exclusion or withdrawal is reported, it is stated that no patient withdrew from the study because of ranolazine‐related adverse reactions. We assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Exercise capacity and duration and time to appearance of angina results were not reported in spite they were mentioned among the study benchmarks |
| Other bias | Unclear risk | The authors declare that they have no conflict of interest. However, the source of funding is not stated. Furthermore, editorial assistance for the preparation of the manuscript was provided by Luca Giacomelli, PhD, on behalf of Content Ed Net; this assistance was funded by Menarini International, an Italian pharmaceutical company. |
| Methods | Study design: parallel‐group trial Total study duration: 14 weeks plus follow‐up (around 14 months). Patients were enrolled from July 1999 to August 2001 Duration of follow‐up: from August 2001 to 31 October 2002 Method of randomisation: randomisation schedules generated by Quintiles (UK) Limited in SAS version 6.12, stratified by the 3 background anti‐anginal therapies using a block size of 6 Method of concealment of allocation: drug packaging with code break envelopes provided by Brecon Pharmaceuticals Ltd. Blinding: double‐blind, not described but presumably referred to participants and study personnel Power calculation: 90% to detect a difference of 30s in the primary end point Phases of the study: 3 (qualifying phase, treatment phase, open‐label follow‐up phase) Number of patients randomised: 823 (269/279/275 for placebo/ranolazine 750 mg/ranolazine 1000 mg group) Exclusions post‐randomisation (for each phase): Qualifying phase: 32 (11/7/14 from placebo/ranolazine 750 mg/ranolazine 1000 mg group). Treatment phase: 54 (14/18/22 from placebo/ranolazine 750 mg/ranolazine 1000 mg group) Withdrawals (and reasons): not reported (excluded patients were reported only as "dropped out") | |
| Participants | Total number: 823 Country of enrolment: 15 (Canada, Czech Republic, Georgia, Greece, Ireland, Israel, Italy, New Zealand, Poland, Romania, Russia, Spain, United Kingdom, United States) Setting/location: ambulatory outpatient Diagnostic criteria (stable angina pectoris): minimum 3 month history of exertional angina with CAD confirmed by angiography, documented prior myocardial infarction, or a diagnostic stress myocardial imaging study (macrovascular angina) Comorbidities: none Age (mean, SD): 63.7(8.9)/64.3(9.3)/63.9(9.3) for placebo/ranolazine 750 mg/ranolazine 1000 mg group Gender (male %): 75.1/77.8/79.6 for placebo/ranolazine 750 mg/ranolazine 1000 mg group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 3 Concomitant medications: background anti‐anginal treatment (atenolol 50 mg once daily, diltiazem 180 mg once daily, or amlodipine 5m g once daily) Excluded medications: none Placebo group Intervention: placebo twice daily Duration of intervention: 12 weeks Ranolazine 750 mg group Intervention: ranolazine ER 750 mg twice daily Duration of intervention: 12 weeks Ranolazine 1000 mg group Intervention: ranolazine ER 1000 mg twice daily Duration of intervention: 12 weeks | |
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency, survival rate Time points reported: 12 weeks (plus the 14‐day safety follow‐up), 17 months (including follow‐up phase) Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Angina episodes frequency
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics Inc. Notable conflicts of interest: six of the authors have financial relationships with CV Therapeutics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Randomisation schedules generated by Quintiles (UK) Limited in SAS version 6.12, stratified by the 3 background anti‐anginal therapies using a block size of 6 |
| Allocation concealment (selection bias) | Low risk | Drug packaging with code break envelopes made by Brecon Pharmaceuticals Ltd. The medication assignment was provided to the principal investigator in a sealed envelope. |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment phase is declared to be double‐blinded, but no description is provided. Drug packages were made with code break envelopes and provided to the clinical units, so patients and personnel were not aware of the treatment assigned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Exercise treadmill test ECGs were interpreted by a core EGC laboratory blinded to treatment assignment using customised software. Although this is stated only for the single‐blind qualifying phase, we assume it also applies for the double‐blind treatment phase. However, for outcomes such as all‐cause mortality, angina frequency and adverse events incidence blinding measures have not been described |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of patients dropped out during the qualifying and treatment phases are reported, but reasons and explanations are not provided |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported, but results for some additional outcomes (haemodynamics) are also reported. |
| Other bias | High risk | The study was supported by CV Therapeutics Inc. Furthermore, six of the authors have financial relationships with CV Therapeutics. |
| Methods | Study design: parallel‐group trial Total study duration: 9 weeks; recruitment from July 30, 2004 to February 16, 2005 Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: randomisation in a 1:1 ratio, centralised and not stratified by centre Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase), treatment phase made up by 1‐week run‐in phase and 6‐week full‐dose treatment phase Number of patients randomised: 565 (284/281 for placebo/ranolazine group) Exclusions post‐randomisation: run‐in phase: 1 withdrawal before study drug treatment (placebo group), 3 exclusions from placebo group because not beginning full‐dose treatment phase, 4 exclusions from ranolazine group, 1 because not beginning full‐dose treatment phase, 3 because not having any diary data in the full‐dose treatment phase Withdrawals (and reasons): ranolazine group (3 adverse events, 1 death, 3 withdrew consent), placebo group (5 adverse events (4 according to the text), 1 death) | |
| Participants | Total number: 564 Country of enrolment: Eastern Europe, United States, Canada Setting/location: not specified Diagnostic criteria (stable angina pectoris): history of chronic stable angina ≥3 months, documented history of CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 61.3±9.0 / 62.0±8.7 for placebo / ranolazine group Gender (male %): 73% / 72% for placebo / ranolazine group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: amlodipine 10 mg/day; LANs and sublingual nitroglycerin as required Excluded medications: inhibitors of cytochrome P450‐3A4, digitalis preparation, perhexiline, trimetazidine, beta‐blockers, calcium cannel blockers other than amlodipine Placebo group
Ranolazine group
| |
| Outcomes | Total number of outcomes:
All‐cause mortality
Quality of life
Acute myocardial infarction incidence (fatal and non‐fatal)
Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Quality of life
Acute myocardial infarction incidence (fatal and non‐fatal)
Angina episodes frequency
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: given that 4 placebo patients and 3 ranolazine patients discontinued the study because of adverse events but 5 placebo patients are reported not to have terminated the trial because of adverse events and 3 more ranolazine patients are reported not to have terminated the trial because of withdrawing consent, it is not clear which patients were finally included in the efficacy analysis Source of funding: CV Therapeutics Notable conflicts of interest: all the authors have received some kind of reward or support for participating in this trial from several pharmaceutical companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio, centralized but not stratified" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Treatment phase is declared to be double‐blinded, but not description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Treatment phase is declared to be double‐blinded, but not description is provided |
| Incomplete outcome data (attrition bias) | Low risk | Patients who did not complete the trial are described clearly, however, there is an inconsistency in the data provided for terminating patients in the placebo group for only one case and it is not clear which patients were finally included in the efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes mentioned in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by CV Therapeutics Inc. All the authors have received some kind of reward or support for participating in this trial from several pharmaceutical companies |
| Methods | Study design: cross‐over trial Total study duration: 6 weeks plus follow‐up, the study began in December 1997 and ended in May 1999 Duration of follow‐up: about 2 years; to October 15, 2001 Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 3 (qualifying phase, treatment phase, open‐label follow‐up phase) Number of patients randomised: 191 Exclusions post‐randomisation: 16 (treatment phase, those who did not complete at least three treatment periods) Withdrawals (and reasons): 23 patients (12%) discontinued the study before completing all treatment periods: 15 patients (7.9%) for adverse events, 4 patients (2.1%) by elective withdrawal, 2 patients (1.0%) for other reasons, 1 patient (˂ 1%) because of death and 1 patient (˂ 1%) because of inappropriate enrolment | |
| Participants | Total number: 191 Country of enrolment: 4 (Canada, Czech Republic, Poland, United States) Setting/location: not specified Diagnostic criteria (stable angina pectoris): at least a three‐month history of effort angina responding to beta‐blockers, calcium channel blockers and/or long‐acting nitrates with well‐documented CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 64.3 ± 9.4 years Gender (male %): 73.3% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 4 Concomitant medications: sublingual nitroglycerin Excluded medications: anti‐anginal treatment except sublingual nitroglycerin Placebo group Intervention: placebo twice daily Duration of intervention: 1 week Ranolazine 500 mg group Intervention: ranolazine SR 500 mg twice daily Duration of intervention: 1 week Ranolazine 1000 mg group Intervention: ranolazine SR 1000 mg twice daily Duration of intervention: 1 week Ranolazine 1500 mg group Intervention: ranolazine SR 1500 mg twice daily Duration of intervention: 1 week | |
| Outcomes | Total number of outcomes:
Adverse events incidence Outcome definition: number of patients who report any adverse event Method and unit of measurement: percentage Time points reported: 1 week RESULTS Adverse events incidence Sample size: 191 (intention‐to‐treat analysis) Missing participants: none Summary data: 15.6%/16.0%/21.7%/34.2% for placebo/ranolazine 500 mg/ranolazine 1000 mg/ranolazine 1500 mg group. Over a total of 191 participants, the adverse events most frequently reported were dizziness (2/2/10/23), nausea (0/1/2/16), asthenia (4/0/3/12), constipation (0/0/3/8), angina (10/10/3/6), headache (4/1/2/5) and sweating (0/0/0/5) for placebo/ranolazine 500 mg/ranolazine 1000 mg/ranolazine 1500 mg group. Subgroup analyses: not performed | |
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics Notable conflicts of interest: three of the authors have financial relationships with CV Therapeutics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Exercise treadmill test ECGs were interpreted by a core EGC laboratory blinded to treatment assignment using customised software. However, for outcomes such as adverse events incidence, blinding measures have not been described |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients who did not complete the trial are described, but the treatment they were receiving is not specified |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by CV Therapeutics. Three of the authors have financial relationships with CV Therapeutics |
| Methods | Study design: cross‐over trial Total study duration: 10 weeks plus up to 24 months of qualifying phase Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 20 Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported | |
| Participants | Total number: 20 Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): chest pain and abnormal routine stress testing without obstructive CAD (< 50% epicardial coronary stenosis in all epicardial coronary arteries) on clinically indicated coronary angiography (microvascular angina) Comorbidities: none Age (mean): 57 ± 11 years Gender (male %): 0% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: usual anti‐anginal medication Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group Intervention: placebo twice daily Duration of intervention: 4 weeks (plus 2 weeks of washout) Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily (the dose was increased from 500 mg to 1000 mg twice daily during the second half of treatment period if tolerated) Duration of intervention: 4 weeks (plus 2 weeks of washout) | |
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ) Outcome definition: score in the 5 sub‐scales, reported separately (For scales) upper and lower limits and whether a high or low score is good: higher scores are better, upper and lower limits are not described Method and unit of measurement: score Time points reported: 4 weeks 2. Duke Activity Status Index (DASI) Outcome definition: functional capacity scale, not further described (For scales) upper and lower limits and whether a high or low score is good: not described Method and unit of measurement: score Time points reported: 4 weeks RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ) Sample size: 47 (intention‐to‐treat analysis) Missing participants: none Summary data: reported as mean (minimum, maximum) Subgroup analyses: not performed 2. Duke Activity Status Index (DASI) Sample size: 20 (intention‐to‐treat analysis) Missing participants: none Summary data: 8.9 (5.4 minimum, 12.1 maximum) METS / 8.6 (3.7 minimum, 11.5 maximum) METS for placebo/ranolazine group Subgroup analyses: not performed | |
| Notes | Relevant observations for the data provided before: details about the qualifying phase are not provided Source of funding: grants and contracts from several public and private organisations in the United States. Notable conflicts of interest: the authors reported they have no relationships to disclose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated, but no description is provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Study is declared to be double‐blinded. Participants and investigators. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Cardiac magnetic resonance imaging outcomes were measured by readers blinded to treatment assignment. However, for outcomes such as quality of life, blinding measures have not been described |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the study. |
| Selective reporting (reporting bias) | Low risk | There is published protocol in clinical trials. Results for all the outcomes stated in the "Methods" section of the paper are reported. |
| Other bias | Unclear risk | The study received grants and contracts from several public and private organisations in the United States. The authors reported they have no relationships to disclose. |
| Methods | Study design: parallel‐group trial Total study duration: mean of 350 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: via a central computerised telephone system with stratification by the responsible physician’s intended management strategy (early invasive versus conservative) Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: 90% to detect a significant difference between treatment groups at 2‐sided 5% significance level Phases of the study: 1, treatment phase Number of patients randomised: 6560 in the original trial, 3565 in the sub‐study on stable angina patients (1789/1776 for ranolazine/placebo group) Exclusions post‐randomisation: 5 lost to follow‐up Withdrawals (and reasons): 8.1%/4.1% discontinued ranolazine due to an adverse event in ranolazine/placebo group | |
| Participants | Total number: 3565 Country of enrolment: 17 (Australia, France, Germany, Poland, United Kingdom, United States, Spain, Israel, Austria, Switzerland, Italy, The Netherlands, South Africa, Hungary, Czech Republic, Canada, Belgium) Setting/location: hospitalisation Diagnostic criteria (stable angina pectoris): history of prior stable angina before and separate from the presenting ACS Comorbidities: Acute coronary syndrome: clinical presentation consistent with an ACS with at least 1 indicator of moderate to high risk of death or recurrent ischaemic events Age (mean (25th, 75th)): 65 (57,73)/66 (56/73) for ranolazine/placebo group Gender (male %): 1149/1789(64.2%)‐1083/1776(61.0%) for ranolazine‐placebo group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: intravenous ranolazine 12 hours to 96 hours before intervention Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group Intervention: placebo twice daily Duration of intervention: mean of 350 days Ranolazine group Intervention: ranolazine ER 1000 mg twice daily (500 mg twice daily for renal insufficiency patients) Duration of intervention: mean of 350 days | |
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency Time points reported: overall study duration (mean of 350 days) RESULTS All‐cause mortality Sample size: 3560 (1785 + 1775) (intention‐to‐treat analysis) Missing participants: 5 Summary data: 114/1775 ‐ 111/1785 for placebo‐ranolazine group Subgroup analyses: not performed Adverse events The most common adverse effects that were more frequent in the ranolazine group compared with placebo were dizziness (12.4% versus 7.4%), nausea (9.7% versus 6.1%) and constipation (8.5% versus 3,3%). | |
| Notes | Relevant observations for the data provided before: sub‐study of the MERLIN TIMI 36 trial not considered in the protocol Source of funding: CV Therapeutics (CVT), Inc. Notable conflicts of interest: five of the authors have financial relationships with CVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via a central computerised telephone system |
| Allocation concealment (selection bias) | Low risk | Not mentioned. However, given the use of a centralised telephone system for randomization (and allocation), it can be assumed that study personnel was blinded until the moment of assignment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Low risk | All elements of the primary composite and major secondary efficacy end points as well as hospitalisation for new or worsening heart failure were adjudicated by members of a Clinical Events Committee blinded to treatment allocation. Exercise treadmill test results were also interpreted by a core laboratory blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Patients who did not complete the trial are described, but the treatment they were receiving or the reasons for withdrawal are not specified |
| Selective reporting (reporting bias) | High risk | This sub‐study of the MERLIN TIMI 36 trial included in this review was not pre‐specified in the published protocol. Furthermore, results for some outcomes (laboratory abnormalities) included in the protocol are not reported in the paper while results for some other outcomes (worsening angina, need for an increase of anti‐anginal therapy) not mentioned in the protocol are reported in the paper. |
| Other bias | High risk | The study was supported by CV Therapeutics (CVT), Inc. Five of the authors have financial relationships with CVT |
| Methods | Study design: parallel‐group trial Total study duration: 37 days Duration of follow‐up: 30 days Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (treatment phase, 30‐day follow‐up phase) Number of patients randomised: 70 (35/35 for placebo/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported | |
| Participants | Total number: 70 Country of enrolment: Italy Setting/location: not specified Diagnostic criteria (stable angina pectoris): typical stable effort angina with positive stress test, with indication for PCI Comorbidities: none Age (mean ± SD): 60 ± 18/64 ± 17 for placebo/ranolazine group Gender (male %): 57%/63% for placebo/ranolazine group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: aspirin 100 mg/d, loading dose of clopidogrel 600 mg or ticlopidine 250 mg bid (before the procedure), and clopidogrel 75 mg/d or ticlopidine 250 mg bid for 1 or 12 months. Other medications such as β‐blockers, calcium antagonists, statins, and angiotensin‐converting enzyme inhibitors given as appropriate Excluded medications: none Placebo group Intervention: placebo as pretreatment for PCI Duration of intervention: 1 week Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily as pretreatment for PCI Duration of intervention: 1 week | |
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency Time points reported: 37 days Acute myocardial infarction incidence (fatal and non‐fatal) Outcome definition: (periprocedural) postprocedural increase of CK‐MB ≥ 3 times above the upper limit of normal, number of cases Method and unit of measurement: absolute frequency Time points reported: 7 days (periprocedural), 37 days (periprocedural plus spontaneous) Need for revascularisation procedure Outcome definition: target‐vessel revascularisation, number of cases Method and unit of measurement: absolute frequency Time points reported: 37 days RESULTS All‐cause mortality Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 1/35‐0/35 for placebo‐ranolazine group Subgroup analyses: not performed Acute myocardial infarction incidence (fatal and non‐fatal) Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 9/35‐2/35 for placebo/ranolazine group (37 days) Subgroup analyses: not performed Need for revascularisation procedure Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 1/35‐1/35 for placebo‐ranolazine group Subgroup analyses: not performed | |
| Notes | Relevant observations for the data provided before: none Source of funding: no extramural funding Notable conflicts of interest: no conflicts of interest to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Low risk | No exclusion or withdrawal is reported, we assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Low risk | The authors declare that the study was not supported by any external source of funding. Furthermore, there were no conflicts of interest to declare. |
| Methods | Study design: cross‐over trial Total study duration: 8 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind (with "double dummy" technique) Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 318 Exclusions post‐randomisation: 6 (not described) Withdrawals (and reasons): premature withdrawals due to adverse events are declared to have been very similar for all treatments, but no details are provided | |
| Participants | Total number: 312 Country of enrolment: United States, Canada. Setting/location: not specified Diagnostic criteria (stable angina pectoris): chronic (≥ 3 months) stable angina pectoris that had responded to conventional anti‐anginal therapy Comorbidities: none Age (mean, range): 64.3 (33‐85) years Gender (male %): 226 (72%) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 4 Concomitant medications: β‐blockers and/or calcium antagonists (minimum medication needed during qualifying phase) Excluded medications: long‐acting nitrates Placebo group Intervention: placebo Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 400 mg bid group Intervention: ranolazine IR 400 mg twice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 267 mg tid group Intervention: ranolazine IR 267 mg thrice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 400 mg tid group Intervention: ranolazine IR 400 mg thrice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) | |
| Outcomes | Total number of outcomes:
Adverse events incidence Outcome definition: number of patients experiencing an adverse event Method and unit of measurement: percentage Time points reported: 1 week RESULTS Adverse events incidence Sample size: 312 (intention‐to‐treat) Missing participants: 6 Summary data: it is stated that adverse events rates were similar for all ranolazine and placebo regimens and approximately 25%, but data for each group is not reported. It is stated that only minor gastrointestinal complaints tended to occur more often with ranolazine (6.6% to 10.7%) than with placebo (3,2%). Subgroup analyses: not performed | |
| Notes | Relevant observations for the data provided before: "all patients" (intention‐to‐treat) (N = 312) and per‐protocol (N = 260) analysis were performed for ETT variables Source of funding: in part by a grant from Syntex Research Notable conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but no described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions and withdrawals are reported, but no reasons or explanations are provided |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Unclear risk | The study was supported in part by a grant from Syntex Research. The authors did not stated any conflicts of interest. |
| Methods | Study design: cross‐over trial Total study duration: 28 to 40 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind (with 'double‐dummy' technique), not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 158 Exclusions post‐randomisation: 4 (did not perform ≥ 1 exercise test in the treatment phase) Withdrawals (and reasons): 6, 4 withdrawals attributed to adverse events (2 during ranolazine therapy, 1 because of hematologic abnormality, 1 because of asthenia, nausea and chest pain; 2 during placebo therapy, due to exacerbation of angina) | |
| Participants | Total number: 158 Country of enrolment: Europe and Canada Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms and exercise test results that support the diagnosis of chronic angina with evidence of CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 59 ± 8 years Gender (male %): 89% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 3 Concomitant medications: (permitted) short‐acting nitrates, calcium cannel blockers (except those that are cardiodepressants) Excluded medications: β‐blockers, verapamil Placebo group Intervention: Placebo Duration of intervention: 7 to 10 days Atenolol group Intervention: atenolol 100 mg/d Duration of intervention: 7 to 10 days Ranolazine group Intervention: ranolazine IR 400 mg thrice daily Duration of intervention: 7 to 10 days | |
| Outcomes | Total number of outcomes:
Acute myocardial infarction incidence (fatal and non‐fatal) Outcome definition: mentioned as “serious adverse events”, number of cases Method and unit of measurement: absolute frequency Time points reported: 28 to 40 days (total study duration) Angina episodes frequency Outcome definition: average number of episodes per week Method and unit of measurement: number per week Time points reported: 28 to 40 days (total study duration) Adverse events incidence Outcome definition: number of patients that reported ≥ 1 adverse event Method and unit of measurement: absolute frequency Time points reported: 28 to 40 days RESULTS Acute myocardial infarction incidence (fatal and non‐fatal) Sample size: 155 (intention‐to‐treat analysis) Missing participants: 3 Summary data (for each intervention group) (according to type of analysis) (for the largest time point): 1/154 – 0/154 – 0/155 for placebo – atenolol – ranolazine group Subgroup analyses: not performed Angina episodes frequency Sample size: not specified (presumably 154) Missing participants: not specified (presumably 4) Summary data: numerical data not reported Subgroup analyses: not performed Adverse events incidence Sample size: 155 (intention‐to‐treat analysis) Missing participants: 3 Summary data: 26/154 – 39/154 – 45/155 for placebo – atenolol – ranolazine group. The most frequently reported adverse events were asthenia (19/26/4), dizziness (2/9/4), headache (6/0/5), nausea (6/0/2), palpitations (4/2/3), dyspepsia (7/0/2), pain (1/2/2), constipation (5/0/1), malaise (1/1/2) and dyspnoea (0/1/3) for ranolazine/atenolol/placebo group. Subgroup analyses: not performed | |
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics, Inc. Notable conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Low risk | Six patients are reported not to have completed the study. Reasons for withdrawal and treatment assigned are not described for two of them |
| Selective reporting (reporting bias) | High risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported, but results for some additional outcomes (haemodynamics and safety and adverse events) are also reported |
| Other bias | High risk | The study was supported by CV Therapeutics, Inc. Conflicts of interest were not stated. |
| Methods | Study design: parallel‐group trial Total study duration: about 3.5 years Duration of follow‐up: mean of 643 days Method of randomisation: interactive web‐based block randomisation system (block sizes of 10), with randomisation stratified by diabetes history (presence versus absence) and acute coronary syndrome presentation (acute versus non‐acute) Method of concealment of allocation: not described in enough detail, it is just mentioned that investigators and patients were masked to treatment allocation Blinding: double‐blind, referred to participants, clinicians ("masked to treatment allocation"), data collectors (independent Data Safety Monitoring Board, independent on‐site clinical monitors, independent angiographic core laboratory), outcome adjudicators (independent Clinical Endpoint Committee) and data analysts (independent statistical data analysis group, Duke Clinical Research Institute for quality of life and economic analyses) Power calculation: 85% power using a 2‐sided log‐rank test at the 5% significance level, with regard to the primary end point events Phases of the study: 1 (treatment phase, including a 7‐day run‐in period) Number of patients randomised: 2651 (1319/1332 for placebo/ranolazine group) Exclusions post‐randomisation: 32 exclusions in the placebo group (19 not treated, 3 scientific misconduct, 10 no qualifying PCI), 15 exclusions in the ranolazine group (7 not treated, 3 scientific misconduct, 5 no qualifying PCI). Additionally, for the quality of life sub‐study, there were 105 exclusions in the placebo group (97 questionnaires invalid, 8 questionnaires not done) and 110 exclusions in the ranolazine group (103 questionnaires invalid, 7 questionnaires not done) Withdrawals (and reasons): 463/1287 ‐ 529/1317 for placebo ‐ ranolazine group, reasons detailed in the appendix of the study report | |
| Participants | Total number: 2651 Country of enrolment: 15 countries (Europe, Israel, Russia, USA) Setting/location: inpatient and outpatient Diagnostic criteria (stable angina pectoris): symptoms of stable angina Comorbidities: incomplete revascularisation (ICR) post‐PCI Age (mean): 63.3±10 / 63.3±10.4 years for placebo/ranolazine group Gender (male %): 80.3% / 79.7% for placebo/ranolazine group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: per the discretion of the investigator Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: mean (IQR) of 642 (575‐561) days Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: mean (IQR) of 644 (575‐757) days | |
| Outcomes | Total number of outcomes: 1
Cardiovascular mortality
All‐cause mortality
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. Mental Health Inventory‐5 (MHI‐5)
4. European QOL Five Dimension Three‐Level Scale (EuroQOL‐5D‐3 L)
5. Rose Dyspnea Scale (RDS)
Acute myocardial infarction incidence (fatal and non‐fatal)
Need for revascularisation procedure
RESULTS Cardiovascular mortality
All‐cause mortality
Quality of life 1182 1207 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. Mental Health Inventory‐5 (MHI‐5)
4. European QOL Five Dimension Three‐Level Scale (EuroQOL‐5D‐3 L)
5. Rose Dyspnea Scale (RDS)
Acute myocardial infarction incidence (fatal and non‐fatal)
Need for revascularisation procedure
Adverse events Dizziness, constipation, nausea, hypotension, vomiting and vertigo were reported more often in the ranolazine group than in the placebo group. | |
| Notes | Relevant observations for the data provided before: none Source of funding: Gilead Sciences, Menarini Group Notable conflicts of interest: seven of the authors declare current of past financial relationships with Gilead Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Random sequence generated by an interactive web‐based block randomisation system with block sizes of ten |
| Allocation concealment (selection bias) | Low risk | Described for participants and clinicians as "masked to treatment allocation". The use of a web‐based system for randomization (and allocation) can be considered sufficient to mantain blinded the study personnel until the momment of assignment. |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment phase is declared to be double‐blinded, but no description for participants and personnel is provided. However, the subjects idea about study arm was measured, and study data was collected by independent groups, then presumably both participants and investigators were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Data for events and quality of life/economic analyses were collected by independent outcome adjudication groups |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions and withdrawals are described, and reason are reported |
| Selective reporting (reporting bias) | High risk | There are two sub studies (quality of life and health economics) besides the main study for the RIVER‐PCI trial, and all the main endpoints considered in the protocol were reported (the health economics sub study has not yet been published). Of note, for the events under study, time to event occurrence was stated to be the endpoint rather than the rate/incidence of the event; however, results for the secondary endpoint events were reported only as rate/incidence, and no data about time to event occurrence was provided |
| Other bias | High risk | The study was supported by Gilead Sciences and Menarini Group. Seven of the authors declare current or past financial relationships with Gilead Sciences. |
| Methods | Study design: cross‐over trial Total study duration: recruitment was undertaken since 12 May 2011 to 10 Aug 2015, treatment phase duration was of 6 weeks (including 2 periods of treatment of 2 weeks and 1 period of washout of 1 week) Duration of follow‐up: 2 weeks Method of randomisation: performed at a 1:1 ratio blocked by clinical site, not further described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: 90% power to detect a mean difference of 15 in SAQ score using a two‐sided t‐test at the 0.017 Holm‐Bonferroni corrected level of significance Phases of the study: 1 (treatment phase) Number of patients randomised: 142 Exclusions post‐randomisation: 4 participants were excluded because they received incomplete treatment Withdrawals (and reasons): number differ between the text (subject characteristics) and figure 1, data from the later was deemed to be more coherent. Five participants dropped‐out during ranolazine treatment and 4 during placebo washout (no dropouts during ranolazine washout), reasons not described | |
| Participants | Total number: 128 Country of enrolment: USA Setting/location: not specified Diagnostic criteria (stable angina pectoris): chronic angina or its equivalent with coronary angiogram revealing coronary microvascular dysfunction (CMD) with no obstructive CAD (microvascular angina) Comorbidities: none Age (mean ± SD): 55.2 ± 9.2 years Gender (male %): 4% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: (permitted) anti‐anginals, antihypertensives, statins, hormone replacement therapy Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo twice daily Duration of intervention: 2 weeks Ranolazine group Intervention: ranolazine ER 500/1000 mg twice daily Duration of intervention: 2 weeks | |
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ and SAQ‐7)
2. Duke Activity Satuts Index (DASI)
3. QOL
Angina episodes frequency
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. QOL
Angina episodes frequency
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: none Source of funding: unrestricted research grant from Gilead and contracts from several public (USA) entities Notable conflicts of interest: seven of the authors declare financial relationships with private pharmaceutical organisations (Gilead among others) and public (USA) entities | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio, blocked by clinical site" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Exclusions and withdrawals are reported, but no reasons or explanations are provided. Furthermore, inconsistencies among the data provided were observed |
| Selective reporting (reporting bias) | Unclear risk | Protocol published in clinicaltrials.gov and as Online Exhibit adjoined to the results publication. Healthcare costs and biochemical parameters were not reported, adverse events incidence was reported but not included among the study outcomes in protocol |
| Other bias | High risk | The study was supported by Gilead and several public organisations. Seven authors declared financial relationships with private pharmaceutical organisations |
| Methods | Study design: parallel‐group trial Total study duration: from 1 January 2012 to 11 April 2013 Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described, performed in a 1:1 ratio Method of concealment of allocation: drugs distributed using sequentially numbered opaque sealed envelopes Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 47 (24/23 for trimetazidine/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported | |
| Participants | Total number: 40 Country of enrolment: Greece Setting/location: outpatient Diagnostic criteria (stable angina pectoris): history of exertional angina and CAD documented by coronary angiography (macrovascular angina) Comorbidities: diabetes mellitus Age (mean): 57.4 ± 9.1 / 58 ± 8.1 years for trimetazidine/ranolazine group Gender (male %): 83% (87.5/78.3 for trimetazidine/ranolazine group) Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: statins Excluded medications: those mentioned in exclusion criteria Trimetazidine group
Ranolazine group
| |
| Outcomes | Total number of outcomes: 1
Angina episodes frequency
RESULTS Angina episodes frequency
Adverse events The adverse events reported for the ranolazine group included angina, constipation, postural hypotension, headache, dizziness, nausea and weakness; for the trimetazidine group were constipation, weakness, palpitations, angina, dizziness, nausea, dyspepsia, headache, gastric discomfort and joint pain. | |
| Notes | Relevant observations for the data provided before: none Source of funding: no external source of funding Notable conflicts of interest: the authors declare that they have no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | Study drugs were provided in sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Study declared to be double‐blinded, but no description is provided. However, it can be presumed that participants and personnel were blinded since drugs were provided in sealed envelopes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Low risk | No exclusion or withdrawal is reported, we assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Low risk | The authors declare that the study was not supported by external source of funding. Also, they declared that they had no conflict of interest. |
| Methods | Study design: cross‐over trial Total study duration: 16 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind, it is stated that the investigators and the patient remained blinded to the treatment until the completion of the trial, but no details are provided Power calculation: not performed (pilot study) Phases of the study: 1 (treatment phase) Number of patients randomised: 28 Exclusions post‐randomisation: 4 Withdrawals (and reasons): 5 (3 withdrew voluntarily, 1 lost to follow‐up, 1 withdrew involuntarily) (only 4 patients were excluded from the analysis) | |
| Participants | Total number: 28 Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptomatic (exertional angina or dyspnoea) ischaemic cardiomyopathy (ICM) with angiographic documentation of CAD (macrovascular angina) Comorbidities: none (not treatable by further revascularisation) Age (mean ± SD): 71.5 ± 8.4 years Gender (male %): 82.1% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), a beta blocker, and at least one additional anti‐ischaemic drug (amlodipine or long‐acting nitrate) Excluded medications: none Placebo group Intervention: placebo Duration of intervention: 6 weeks (plus 2 weeks of washout) Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg/1000 mg twice daily Duration of intervention: 6 weeks (plus 2 weeks of washout) | |
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Rose Dyspnea Scale (RDS)
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Rose Dyspnea Scale (SAQ)
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: none Source of funding: research grant from Gilead Notable conflicts of interest: Dr Shammas is a speaker for and is on the advisory board of Gilead | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | It is stated that the investigators remained blinded to the treatment (allocation) until the completion of the trial, but no details are provided |
| Blinding of participants and personnel (performance bias) | Low risk | Study declared to be double‐blinded, with blinding corresponding to investigators and patients |
| Blinding of outcome assessment (detection bias) | Low risk | A core laboratory blinded to patient treatment determined the SAQ scores |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals and reasons are described; however, there is an inconsistency in the number of patients who did not complete the trial (5) and the number of patients excluded from the analysis (4) |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by a research grant from Gilead Science. Dr Shammas is a speaker for and is on the advisory board of Gilead. |
| Methods | Study design: parallel‐group trial Total study duration: 8 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 58 (29/29 for placebo/ranolazine group) Exclusions post‐randomisation: none Withdrawals (and reasons): none | |
| Participants | Total number: 58 Country of enrolment: Italy Setting/location: not specified Diagnostic criteria: sings and symptoms of myocardial ischaemia without obstructive CAD (microvascular angina) Comorbidities: none Age (mean ± SD): 66 ± 10 years Gender (male %): 67% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: aspirin Excluded medications: those mentioned in exclusion criteria Placebo group
Ranolazine group
| |
| Outcomes | Total number of outcomes: 3 According to study protocol: no published protocol; according to the "Methods" section: 3 (coronary flow reserve, left ventricular ejection fraction, SAQ score) Reported: 3 Quality of life
RESULTS Quality of life
| |
| Notes | Relevant observations for the data provided before: none Source of funding: not stated Notable conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | Low risk | It is reported that no patient withdrew the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Unclear risk | The source of funding was not stated. Conflicts of interest were not stated. |
| Methods | Study design: parallel‐group trial Total study duration: 12 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: Interactive Voice/Web Response System (IVRS/IWRS) Method of concealment of allocation: blinded study drug bottle assigned by the IVRS/IWRS Blinding: double‐blind, participants and clinicians were blinded by using study drug bottles assigned by the IVRS/IWRS Power calculation: 90% to show a relative reduction of 20% in weekly angina frequency Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 949 (476/473 for placebo/ranolazine group) Exclusions post‐randomisation: 22 (11 in the ranolazine arm, 11 in the placebo arm) Withdrawals (and reasons): 20 (9 withdrawals, 3 deaths in the ranolazine group, 11 withdrawals, 2 deaths in the placebo group) | |
| Participants | Total number: 949 Country of enrolment: 14 (United States, Belarus, Bulgaria, Canada, Czech Republic, Georgia, Germany, Israel, Poland, Russian Federation, Serbia, Slovakia, Slovenia, Ukraine) Setting/location: not specified Diagnostic criteria (stable angina pectoris): at least a three‐month history of chronic stable angina that remain symptomatic despite treatment with 1 or 2 anti‐anginal, with documented history of CAD (macrovascular angina) Comorbidities: type 2 diabetes mellitus Age (mean ± SD): 64 ± 8.5 years Gender (male %): 61% Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 2 Concomitant medications: Anti‐anginal: beta blockers, calcium channel blockers, long‐acting nitrates; other cardiovascular medications: statins, antiplatelet agents, ACE‐I/ARB; antidiabetic medications: glucose‐lowering medications, insulin Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group
Ranolazine group
| |
| Outcomes | Total number of outcomes:
All‐cause mortality
Quality of life 1. Medical Outcomes Short Form‐36 (SF‐36)
2. Patient’s Global Impression of Change (PGIC)
Acute myocardial infarction incidence (non‐fatal)
Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Quality of life 1. Medical Outcomes Short Form‐36 (SF‐36)
2. Patient’s Global Impression of Change (PGIC)
Acute myocardial infarction incidence (non‐fatal)
Angina episodes frequency
Adverse events incidence:
| |
| Notes | Relevant observations for the data provided before: none Source of funding: Gilead Sciences Notable conflicts of interest: all the authors have financial relationships with Gilead Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation undertaken by the IVRS/IWRS |
| Allocation concealment (selection bias) | Low risk | Intervention was provided to personnel in blinded study drug bottles assigned by the IVRS/IWRS |
| Blinding of participants and personnel (performance bias) | Low risk | Study declared to be double‐blinded, but no description is provided. Intervention was provided to personnel and patients in blinded drug bottles, so they were unaware of the treatment assigned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description about the blinding of data collectors/outcome adjudicators is provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals are reported, but no description is provided |
| Selective reporting (reporting bias) | Low risk | According to the protocol published in Clinicaltrials.gov, results for all the outcomes are reported |
| Other bias | High risk | The study was supported by Gilead Sciences. All the authors have financial relationships with Gilead Sciences |
| Methods | Study design: parallel‐group trial Total study duration: 35‐40 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 319 (79, 81, 81 and 78 for placebo, ranolazine 30 mg, ranolazine 60 mg and ranolazine 120 mg groups) Exclusions post‐randomisation: 20 Withdrawals (and reasons): 31 (15 because of adverse events, new intercurrent illnesses, or new laboratory abnormalities, 4 because of unsatisfactory therapeutic response, 2 because of study administration problems, 10 for 'other' reasons (4 did not take study medication in compliance with the protocol, 2 elected to have surgical intervention, 2 declined to finish the study, 1 violated the protocol, and 1 required medication to control ventricular ectopy)). Withdrawals were similarly distributed among the study groups: 9/79, 9/81, 7/81 and 6/78 for placebo, ranolazine 30 mg, ranolazine 60 mg and ranolazine 120 mg groups | |
| Participants | Total number: 319 Country of enrolment: United States Setting/location: not specified Diagnostic criteria: at least a 3‐month history of symptomatic chronic stable angina triggered by physical effort and relieved by rest or nitroglycerin Comorbidities: none Age (mean ± SD): 65 ± 8 years Gender (male %): 74.7%/80.2%/81.5%/79.5% for placebo/ranolazine 30 mg/ranolazine 60 mg/ranolazine 120 mg group Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Number of intervention groups: 4 Concomitant medications: (permitted) sublingual nitroglycerin (0.4mg tablets), taken for anginal pain and not as a prophylactic agent; hydrochlorothiazide and potassium supplementation for the treatment of hypertension Excluded medications: all anti‐anginal medication with the exception of sublingual nitroglycerin Placebo group
Ranolazine 30 mg group
Ranolazine 60 mg group
Ranolazine 120 mg group
| |
| Outcomes | Total number of outcomes: 6 According to study protocol: no published protocol; according to the "Methods" section: 10 (change from baseline in ETT total exercise duration, time to onset of angina and time to 1‐mm ST segment depression (peak and through), change from baseline in the number and duration of ST segment depression episodes (by Holter monitoring), change from baseline in the weekly rate of NTG consumption and anginal attacks, adverse events incidence) Reported: 12 (including circulatory data at peak and through) Angina episodes frequency
Adverse events incidence
RESULTS Angina episodes frequency
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: 'all patients' (intention‐to‐treat) (N = 299) and per‐protocol (N = 258) analysis were performed for ETT variables Source of funding: Syntex Research, Palo Alto, CA, USA Notable conflicts of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals and reasons are provided and similarly distributed among the study groups, however, number of withdrawals is nearly 10% of total number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by Syntex Research. Authors did not state conflicts of interest. |
| Methods | Study design: parallel‐group trial Total study duration: 4 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: computer‐generated table of random numbers Method of concealment of allocation: drugs were given to patients in anonymous drug packages by three of the authors who were not involved in the clinical assessment of patients. Cardiologists involved in the clinical and laboratory assessment of patients and/or analyses of data were blinded to the allocation of treatment Blinding: not mentioned, but presumably involving participants and data collectors/outcome adjudicators Power calculation: 90% to detect a significant difference of 15 points (SD 10) between each active drug versus placebo in any SAQ item and in the EuroQoL score Phases of the study: 1 (treatment phase) Number of patients randomised: 46 Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported | |
| Participants | Total number: 46 Country of enrolment: Italy Setting/location: ambulatory patients Diagnostic criteria (stable angina pectoris): history of typical effort angina with exercise‐induced ST‐segment depression ≥ 1 mm, normal coronary angiography and absence of any specific cardiac disease including vasospastic angina (microvascular angina) which remains symptomatic despite anti‐ischaemic therapy Comorbidities: none Age (mean, interval): 57 ± 12/57 ± 11/60 ± 9 years for ivabradine/ranolazine/placebo group Gender (male %): 2/16, 3/15, 4/15 for ivabradine‐ranolazine‐placebo group Inclusion criteria:
Exclusion criteria: Not described | |
| Interventions | Number of intervention groups: 3 Concomitant medications: anti‐anginals, antihypertensives, anti‐aggregants, statins Excluded medications: none Placebo group
Ivabradine group
Ranolazine group
| |
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. EuroQoL visual analogue scale (VAS)
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. EuroQoL visual analogue scale (VAS)
Adverse events incidence
| |
| Notes | Relevant observations for the data provided before: none Source of funding: not stated Notable conflicts of interest: no conflicts of interest to disclose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Drugs were given to patients in anonymous drug packages by three of the authors who were not involved in the clinical assessment of patients. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding design is not stated. It is mentioned that study drugs were provided in anonymous packages, so it could be assumed that patients and personnel were blinded to the allocation of treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding design is not stated. It is mentioned that the cardiologists involved in the clinical and laboratory assessment of patients and/or analyses of data were blinded to the allocation of treatment. However, for outcomes such as quality of life, blinding measures have not been described |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or withdrawals were reported. We assumed that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There was no published protocol. Results for all outcomes in the 'Methods' section were reported |
| Other bias | Unclear risk | Funding source not stated. The authors declared no conflicts of interest |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Substudy of RCT: substudy of the TERISA 2013 trial on quality of life | |
| Wrong intervention: ranolazine given in single dose | |
| Not RCT: health economics study not conducted alongside a RCT | |
| Not RCT: health economics study not conducted alongside a RCT | |
| Not RCT: three‐period cross‐over trial with only one group of participants, no randomisation method stated | |
| Not RCT: health economics study not conducted alongside a RCT | |
| Not RCT: health economics study not conducted alongside a RCT | |
| No angina population: condition studied did not meet inclusion criteria | |
| Substudy of RCT: subgroup analysis of the CARISA 2004 and ERICA 2006 trials | |
| Not RCT: open‐label follow‐up study of the CARISA 2004 and MARISA 2004 trials, including only participants treated with ranolazine (without comparator) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: parallel‐group trial Duration of follow‐up: 6 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: open‐label Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 160 (estimated) Country of enrolment: USA Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of angina with evidence of stable CAD (macrovascular angina) Comorbidities: metabolic syndrome Inclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: standard medical therapy Excluded medications: those mentioned in exclusion criteria Control group Intervention: no treatment Duration of intervention: 6 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily Duration of intervention: 6 months |
| Outcomes | Total number of outcomes: 6 (ETT parameters, fasting glucose, angina (SAQ scale), concomitant medications, lipid profile, HbA1c) OUTCOMES No outcome meets inclusion criteria |
| Notes |
| Methods | Study design: parallel‐group trial Duration of follow‐up: not reported Method of randomisation: not described Method of concealment of allocation: not described Blinding: single‐blind (outcome assessors) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: Greece Setting/location: inpatient Diagnostic criteria (stable angina pectoris): not described Comorbidities: patients scheduled for elective on‐pump CABG Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Control group Intervention: no treatment Duration of intervention: not reported Ranolazine group Intervention: ranolazine (type of formulation not specified) 375 mg twice daily for 3 days prior to surgery and until discharge Duration of intervention: not reported |
| Outcomes | Total number of outcomes: 3 (post‐operative atrial fibrillation, left atrial diameter, left ventricular ejection fraction) OUTCOMES No outcome meets the inclusion criteria |
| Notes |
| Methods | Study design: parallel‐group trial Duration of follow‐up: Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 86 Country of enrolment: China Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: diltiazem Excluded medications: not described Control group Intervention: no treatment Duration of intervention: not reported Ranolazine group Intervention: ranolazine (type of formulation not specified) (dosage not reported) Duration of intervention: not reported |
| Outcomes | Total number of outcomes: 2 (ECG total effective rate, adverse events incidence) OUTCOMES Adverse events incidence Outcome definition: not described Method and unit of measurement: absolute frequency Time points to report: not reported |
| Notes |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 8 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: China Setting/location: not specified Diagnostic criteria (stable angina pectoris): stable angina with coronary heart disease (macrovascular angina) Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 3 Concomitant medications: conventional therapy (aspirin, cholesterol lowering agents, metoprolol) Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 8 weeks Ranolazine 500 mg group Intervention: ranolazine SR 500 mg twice daily Duration of intervention: 8 weeks Ranolazine 1000 mg group Intervention: ranolazine SR 1000 mg twice daily Duration of intervention: 8 weeks |
| Outcomes | Total number of outcomes: 5 (angina frequency, nitroglycerin consumption frequency, ECG total effective rate, ADR, liver/kidney function) OUTCOMES Angina episodes frequency Outcome definition: not described Method and unit of measurement: not described Time points to report: 8 weeks |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: medical therapy (not described) Excluded medications: not mentioned No treatment group Intervention: none Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 5 (ETT parameters, symptoms, arrhythmia, angina during moderate exercises, re‐hospitalisation) OUTCOMES No outcome appears to meet the inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 3 Concomitant medications: standard therapy (not described) Excluded medications: not mentioned No treatment group Intervention: none Duration of intervention: 30 days Ivabradine group Intervention: ivabradine (dose not reported) Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 3 (ETT parameters, weekly angina during daily moderate exercises, re‐hospitalisation) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 8 weeks Method of randomisation: computer generated randomisation Method of concealment of allocation: sequentially numbered, sealed, opaque envelopes Blinding: not specified ("Investigator Blinded") Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: India Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: sustained STEMI Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: those indicated by the patient's cardiologist Excluded medications: not mentioned Trimetazidine group Intervention: trimetazidine 35 mg twice daily Duration of intervention: 8 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 8 weeks |
| Outcomes | Total number of outcomes: 3 (changes in LV systolic and diastolic function, improvement in angina symptoms, ADR) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | 28/10/2013 |
| Contact information | Dr Melvin George Assistant Professor Cardiac Research SRM Medical College Hospital Dept of Cardiology SRM MCH RC Kattankulathur Kancheepuram 9894133697 |
| Notes | Source of funding: SRM Medical College Hospital |
| Trial name or title | 2011‐001278‐24 / MEIN/10/Ran‐Cad/003 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 24 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 1460 (estimated) Country of enrolment: Greece, Switzerland Setting/location: not specified Diagnostic criteria (stable angina pectoris): exercise angina in patients with CAD (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 24 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 750 mg twice daily Duration of intervention: 24 weeks |
| Outcomes | Total number of outcomes: 5 (ETT parameters, angina frequency, nitroglycerin consumption frequency, adverse events incidence, laboratory findings) OUTCOMES Angina episodes frequency Outcome definition: weekly frequency Method and unit of measurement: number/week Time points to report: 4, 12 and 24 weeks Adverse events incidence Outcome definition: not described Method and unit of measurement: absolute frequency Time points to report: 4, 12 and 24 weeks |
| Starting date | Not reported |
| Contact information | Study Medical Expert (SME) Via Walter Tobagi, 8 Peschiera Borromeo ‐ Italy 003902516555236 |
| Notes | Source of funding: Menarini International Operations Luxembourg SA |
| Trial name or title | EUCTR2012‐001584‐77‐DE / MEIN/10/Ran‐PCI/005 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 5 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: Germany Setting/location: not specified Diagnostic criteria (stable angina pectoris):history of chronic stable angina and coronary stenosis by angiography (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: standard therapy (beta‐blocker and/or calcium channel blocker) Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: film‐coated tablet Duration of intervention: 5 weeks Ranolazine group Intervention: ranolazine ER (dose not reported) Duration of intervention: 5 weeks |
| Outcomes | Total number of outcomes: 2 (changes of the wall motion abnormalities, heart’s perfusion deficit and related variables) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | Not reported |
| Contact information | Dr. Notghi Contract Research GmbH Zimmerstraße 55 ‐ Berlin 004903046064780 |
| Notes | Source of funding: Menarini International Operations Luxembourg S.A. Reported as Prematurely ended |
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group Duration of follow‐up: 6 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: not specified Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 185 Country of enrolment: India Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: type 2 diabetes Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medication: 1 or 2 anti‐anginals Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 6 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 6 weeks |
| Outcomes | Total number of outcomes: 2 (weekly angina frequency, weekly sublingual nitrate use) OUTCOMES Angina episodes frequency Outcome definition: not described Method and unit of measurement: number per week Time points reported: 6 weeks |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
| Trial name or title | |
| Methods | Study design: cross‐over trial Duration of follow‐up: 30 days Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 100 (estimated) Country of enrolment: Italy Setting/location: not specified Diagnostic criteria (stable angina pectoris): coronary artery disease (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) 750 mg twice daily Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 2 (occurrence of symptoms of palpitations, occurrence of arrhythmia in case of symptoms of palpitations) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | January 2014 |
| Contact information | Francesco Pelliccia, MD +39064997 |
| Notes | Source of funding: University of Roma La Sapienza |
| Trial name or title | |
| Methods | Study design: parallel‐group Duration of follow‐up: 3 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: single‐blind (subject) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): history of stable angina Comorbidities: other cardiac conditions (such as atrial fibrillation) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: amiodarone Excluded medications: not described Placebo group Intervention: sugar pill Duration of intervention: 3 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily Duration of intervention: 3 months |
| Outcomes | Total number of outcomes: 6 (ventricular arrhythmia burden, atrial arrhythmia burden, QTc interval measurement, hospitalisation rate, syncope hospitalisation rate, liver function assay) OUTCOMES No outcomes meets inclusion criteria |
| Starting date | Not reported |
| Contact information | Erik J Sirulnick, MD 702‐731‐8224 |
| Notes | Source of funding: Cardiovascular Consultants of Nevada, Gilead Sciences |
| Trial name or title | |
| Methods | Study design: cross‐over trial Duration of follow‐up: 4 weeks Method of randomisation: not described Method of concealment of allocation: "labelled" bottles provided by the sponsor Blinding: double‐blind (Subject, Investigator) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 70 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and CAD (criteria for diagnosis not described) (macrovascular angina) Comorbidities: type 1 and 2 diabetes mellitus Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: anti‐anginals (not described) Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 4 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) (dosage not reported) Duration of intervention: 4 weeks |
| Outcomes | Total number of outcomes: 9 (post‐exercise coronary vasodilator reserve, symptoms of exertional angina and/or dyspnoea (SAQ/RDS scales), left ventricular systolic function, post‐exercise global myocardial blood flow, post‐exercise global coronary vascular resistance, serum biomarkers of myocardial strain, LV diastolic function, correlation between multimodality imaging parameters) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | April 2013 |
| Contact information | Ron Blankstein, MDBrigham and Women's Hospital |
| Notes | Source of funding: Brigham and Women's Hospital |
| Trial name or title | |
| Methods | Study design: parallel‐group Duration of follow‐up: not stated Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 40 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): stable angina for at least 3 months with documented CAD (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
Contraindicated medications
|
| Interventions | Number of intervention groups: 2 Concomitant therapy: aerobic exercise three times per week, 45 minutes per session at an intensity of 10‐20 beats per minute below the angina threshold Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 13 weeks (not explicitly stated) Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: 13 weeks (not explicitly stated) |
| Outcomes | Total number of outcomes: 3 (change in peak oxygen consumption, change in treatment satisfaction (SAQ scale), change in total daily energy expenditure) Adverse events incidence No outcome meets the inclusion criteria |
| Starting date | December 2013 |
| Contact information | Leslie H Willis, MS 9196606782 leslie.willisduke.edu |
| Notes | Source of funding: Duke University, Gilead Sciences |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 4 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 30 (estimated) Country of enrolment: United States Setting/location: emergency patients Diagnostic criteria (stable angina pectoris): microvascular angina Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not mentioned Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 4 weeks Ranolazine group Intervention: ranolazine ER 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 4 weeks |
| Outcomes | Total number of outcomes: 3 (Coronary Flow Reserve, quality of life (SAQ scale), return visits) OUTCOMES Quality of life Outcome definition: not described Method and unit of measurement: score Time points reported: 4 weeks |
| Starting date | April 2014 |
| Contact information | Matthew Naftilan, MS 203‐785‐4676 |
| Notes | Source of funding: Yale University, Gilead Sciences |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and no evidence of CAD (microvascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 12 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: 12 weeks |
| Outcomes | Total number of outcomes: 5 (quality of life (SAQ scale), peak rate of oxygen consumption, ETT parameters, Coronary Flow Velocity Reserve (CFR), Hyperemic Microcirculatory Resistance (HMR)) OUTCOMES Quality of life Outcome definition: Seattle Angina Questionnaire score regarding angina frequency, physical functioning, treatment satisfaction, angina stability, and quality of life Method and unit of measurement: score (change from baseline) Time points reported: 12 weeks |
| Starting date | September 2014 |
| Contact information | Habib Samady, MD (404) 778‐1237 |
| Notes | Source of funding: Emory University, Gilead Sciences |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 4 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 250 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described, patients deferred from having a PCI Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: sugar pill twice daily Duration of intervention: 16 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 16 weeks |
| Outcomes | Total number of outcomes: 3 (quality of life (SAQ scale), subjective well being, ischemia‐driven revascularisation or hospitalisation) OUTCOMES Quality of life Outcome definition: the Seattle Angina Questionnaire is a valid and reliable instrument that measures five clinically important dimensions of health in patients with CAD (physical limitation, anginal stability, anginal frequency, treatment satisfaction, and disease perception) Method and unit of measurement: score (change from baseline) Time points reported: 4 months Need for revascularisation procedure Outcome definition: frequency of the number of reported adverse events for ischaemia driven revascularisation Method and unit of measurement: absolute frequency Time points reported: 4 months |
| Starting date | August 2015 |
| Contact information | Anthony A Bavry, MD MPH 352‐376‐1611 ext 4726 |
| Notes | Source of funding: North Florida Foundation for Research and Education, Gilead Sciences, University of Florida |
| Trial name or title | |
| Methods | Study design: parallel‐group Duration of follow‐up: 24 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 40 (estimated) Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of chronic stable angina and evidence of CAD (macrovascular angina) Comorbidities: Metabolic Syndrome Inclusion criteria:
Exclusion criteria:
Additional exclusion
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 24 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 24 weeks |
| Outcomes | Total number of outcomes: (angina frequency (SAQ scale), biomarkers) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | September 2015 |
| Contact information | Gladys Velarde, MD 904‐244‐43095 |
| Notes | Source of funding: University of Florida |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 16 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: sugar pill twice daily Duration of intervention: 16 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 16 weeks |
| Outcomes | Total number of outcomes: 3 (quality of life (SAQ scale), subjective well being, ischemia‐driven revascularisation or hospitalisation) OUTCOMES Quality of life Outcome definition: the Seattle Angina Questionnaire is a valid and reliable instrument that measures five clinically important dimensions of health in patients with coronary artery disease (physical limitation, anginal stability, anginal frequency, treatment satisfaction, and disease perception) Method and unit of measurement: score (change from baseline) Time points reported: 16 weeks Need for revascularisation procedure Outcome definition: frequency of the number of reported adverse events for ischaemia driven revascularisation Method and unit of measurement: absolute frequency Time points reported: 16 weeks |
| Starting date | September 2014 |
| Contact information | Anthony A Bavry, MD, MPH 352‐376‐1611 ext 4726 |
| Notes | Source of funding: North Florida Foundation for Research and Education, Gilead Sciences |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 9 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: Double‐blind (Subject, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 3 Concomitant medications: standard therapy (not described) Excluded medications: not mentioned Placebo group Intervention:placebo with up‐titration after 1 week Duration of intervention: 9 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated after 1‐week 500 mg twice daily) Duration of intervention: 9 weeks |
| Outcomes | Total number of outcomes: 4 (cardiac MRI strain, dobutamine wall motion scoring index, quality of life, ETT parameters) OUTCOMES Quality of life Outcome definition: measured with 3 scales (SAQ, DASI, SF‐12) Method and unit of measurement: total score Time points to report: 9 weeks |
| Starting date | June 2015 |
| Contact information | Ashesh N Buch, MB.ChB, M.D. [email protected] |
| Notes |
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 52 Country of enrolment: not mentioned Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Trimetazidine group Intervention: trimetazidine 35 mg twice daily Duration of intervention: 12 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 375/500 mg twice daily Duration of intervention: 12 weeks |
| Outcomes | Total number of outcomes: 2 (flow‐mediated (endothelium‐dependent) dilation (FMD) of brachial artery, nitroglycerin‐induced (endothelium‐independent) (GTN) dilation of brachial artery) OUTCOMES No outcomes meets inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.56, 1.88] |
| Analysis 1.1  Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 1 Cardiovascular mortality. | ||||
| 2 All‐cause mortality Show forest plot | 3 | 6249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.25] |
| Analysis 1.2  Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 2 All‐cause mortality. | ||||
| 3 Quality of life Show forest plot | 3 | 2254 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.57, 2.13] |
| Analysis 1.3  Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 3 Quality of life. | ||||
| 4 AMI incidence Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.14, 2.15] |
| Analysis 1.4 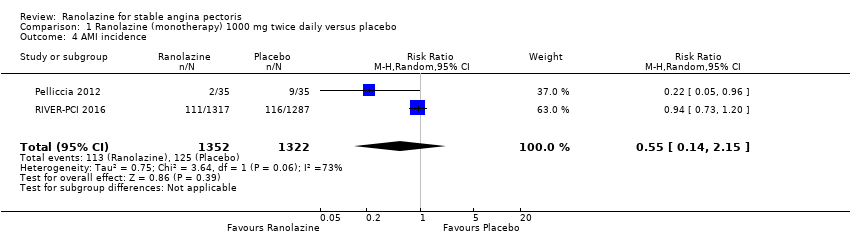 Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence. | ||||
| 5 Need for revascularisation procedure Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| Analysis 1.5  Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 5 Need for revascularisation procedure. | ||||
| 6 Adverse events incidence Show forest plot | 2 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.98] |
| Analysis 1.6 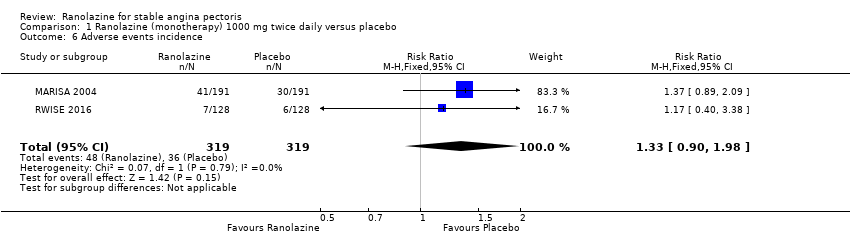 Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMI incidence Show forest plot | 3 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| Analysis 2.1 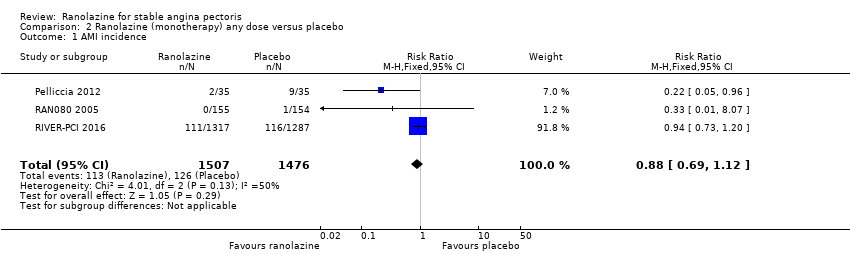 Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 1 AMI incidence. | ||||
| 2 Angina episodes frequency Show forest plot | 2 | 402 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.85, 1.01] |
| Analysis 2.2  Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 2 Angina episodes frequency. | ||||
| 3 Adverse events incidence Show forest plot | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.12, 2.00] |
| Analysis 2.3  Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 3 Adverse events incidence. | ||||
| 3.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.13, 2.07] |
| 3.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| Analysis 3.1  Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 1 All‐cause mortality. | ||||
| 2 Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| Analysis 3.2  Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 2 Quality of life. | ||||
| 3 AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| Analysis 3.3 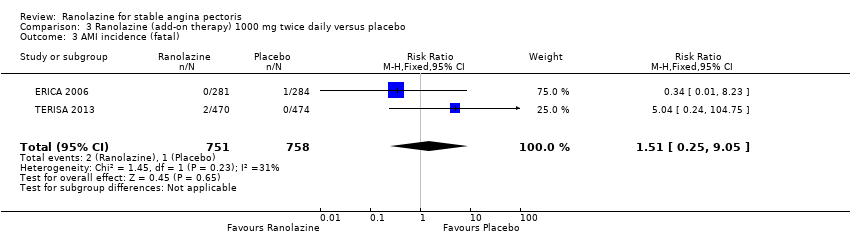 Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 3 AMI incidence (fatal). | ||||
| 4 AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| Analysis 3.4  Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence (non‐fatal). | ||||
| 5 Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| Analysis 3.5  Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 5 Angina episodes frequency. | ||||
| 6 Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
| Analysis 3.6  Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 4 | 1563 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.01, 0.52] |
| Analysis 4.1 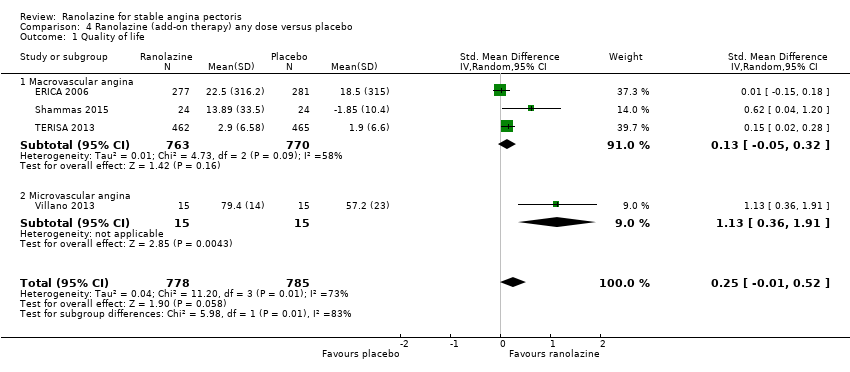 Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 1 Quality of life. | ||||
| 1.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 1.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 2 Time to 1‐mm ST‐segment depression Show forest plot | 3 | 1165 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] |
| Analysis 4.2  Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 2 Time to 1‐mm ST‐segment depression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| Analysis 5.1  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 1 Comparison 1 ‐ All‐cause mortality. | ||||
| 2 Comparison 1 ‐ Quality of life Show forest plot | 2 | 1998 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.86, 2.05] |
| Analysis 5.2  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 2 Comparison 1 ‐ Quality of life. | ||||
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| Analysis 5.3  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 3 Comparison 1 ‐ AMI incidence. | ||||
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| Analysis 5.4 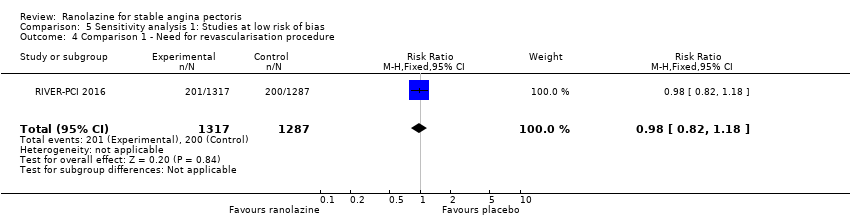 Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure. | ||||
| 5 Comparison 2 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| Analysis 5.5 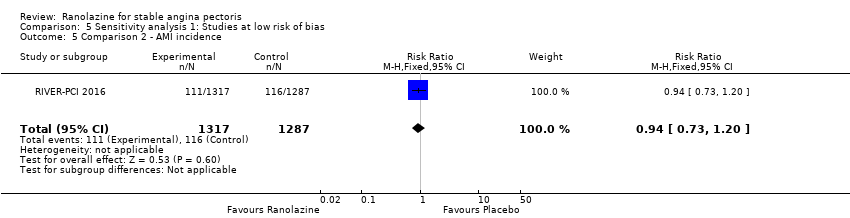 Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 5 Comparison 2 ‐ AMI incidence. | ||||
| 6 Comparison 3 ‐ All‐cause mortality Show forest plot | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.95] |
| Analysis 5.6 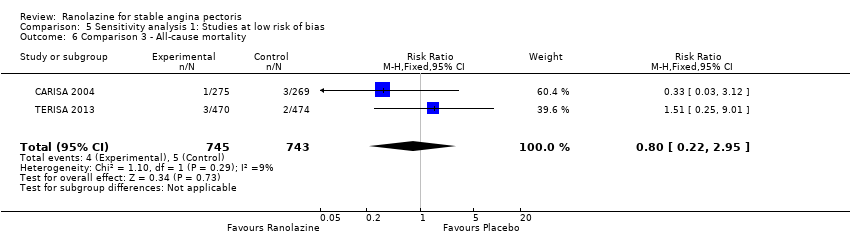 Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 6 Comparison 3 ‐ All‐cause mortality. | ||||
| 7 Comparison 3 ‐ Quality of life Show forest plot | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| Analysis 5.7  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 7 Comparison 3 ‐ Quality of life. | ||||
| 8 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [0.24, 104.75] |
| Analysis 5.8  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 8 Comparison 3 ‐ AMI incidence (fatal). | ||||
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.22] |
| Analysis 5.9 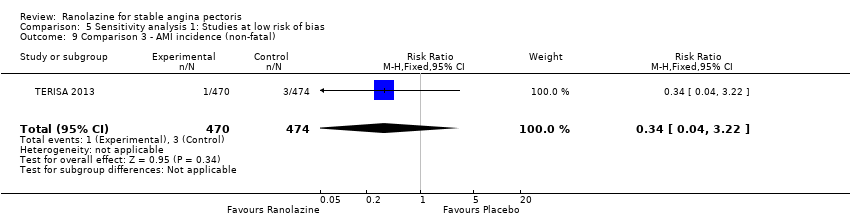 Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal). | ||||
| 10 Comparison 3 ‐ Angina episodes frequency Show forest plot | 2 | 1446 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.96, ‐0.32] |
| Analysis 5.10 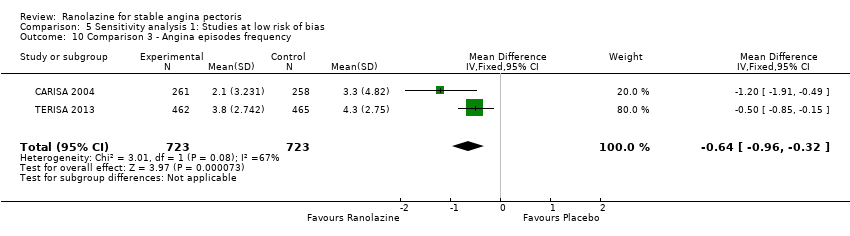 Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 10 Comparison 3 ‐ Angina episodes frequency. | ||||
| 11 Comparison 3 ‐ Adverse events incidence Show forest plot | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.06, 1.53] |
| Analysis 5.11  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 11 Comparison 3 ‐ Adverse events incidence. | ||||
| 12 Comparison 4 ‐ Quality of life Show forest plot | 3 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.03, 1.10] |
| Analysis 5.12  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 12 Comparison 4 ‐ Quality of life. | ||||
| 12.1 Macrovascular angina | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| 12.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] | |
| Analysis 5.13  Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 3 | 6249 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.25] |
| Analysis 6.1  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 1 Comparison 1 ‐ All‐cause mortality. | ||||
| 2 Comparison 1 ‐ Quality of life Show forest plot | 3 | 2254 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.57, 2.13] |
| Analysis 6.2  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 2 Comparison 1 ‐ Quality of life. | ||||
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| Analysis 6.3 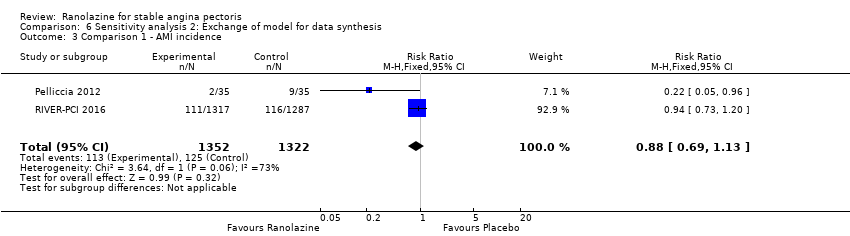 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 3 Comparison 1 ‐ AMI incidence. | ||||
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| Analysis 6.4  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure. | ||||
| 5 Comparison 1 ‐ Adverse events incidence Show forest plot | 2 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.90, 1.99] |
| Analysis 6.5  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 5 Comparison 1 ‐ Adverse events incidence. | ||||
| 6 Comparison 2 ‐ AMI incidence Show forest plot | 3 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.63] |
| Analysis 6.6 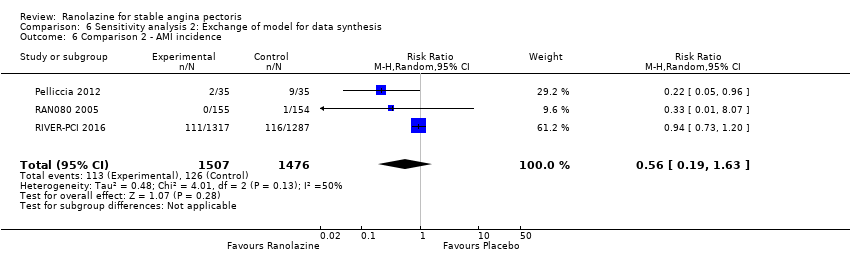 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 6 Comparison 2 ‐ AMI incidence. | ||||
| 7 Comparison 2 ‐ Angina episodes frequency Show forest plot | 2 | 402 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.85, 1.01] |
| Analysis 6.7  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 7 Comparison 2 ‐ Angina episodes frequency. | ||||
| 8 Comparison 2 ‐ Adverse events incidence Show forest plot | 3 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.12, 2.01] |
| Analysis 6.8 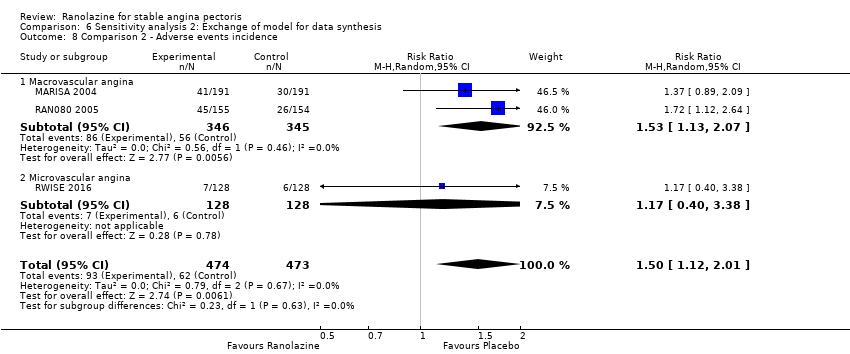 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 8 Comparison 2 ‐ Adverse events incidence. | ||||
| 8.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.13, 2.07] |
| 8.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.40, 3.38] |
| 9 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.25, 3.04] |
| Analysis 6.9  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 9 Comparison 3 ‐ All‐cause mortality. | ||||
| 10 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| Analysis 6.10  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 10 Comparison 3 ‐ Quality of life. | ||||
| 11 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.10, 19.46] |
| Analysis 6.11 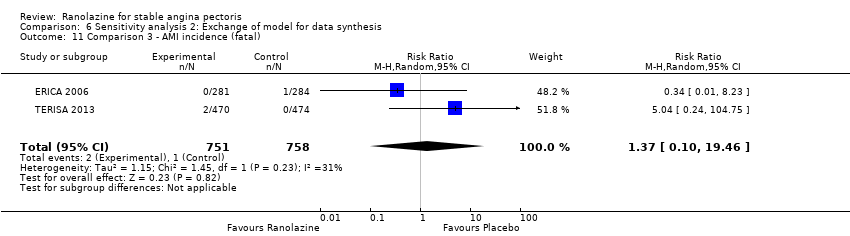 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 11 Comparison 3 ‐ AMI incidence (fatal). | ||||
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.11] |
| Analysis 6.12  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal). | ||||
| 13 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.28, ‐0.27] |
| Analysis 6.13 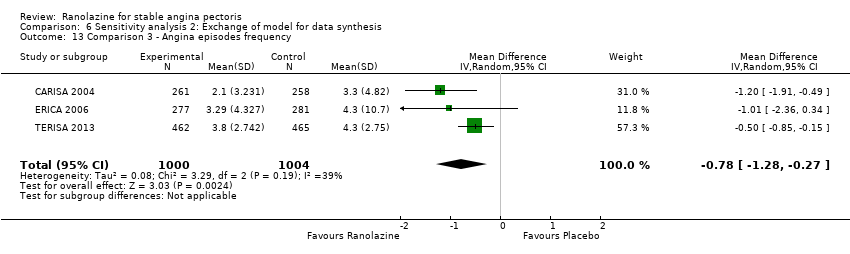 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 13 Comparison 3 ‐ Angina episodes frequency. | ||||
| 14 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.06, 1.39] |
| Analysis 6.14  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 14 Comparison 3 ‐ Adverse events incidence. | ||||
| 15 Comparison 4 ‐ Quality of life Show forest plot | 4 | 1563 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.23] |
| Analysis 6.15 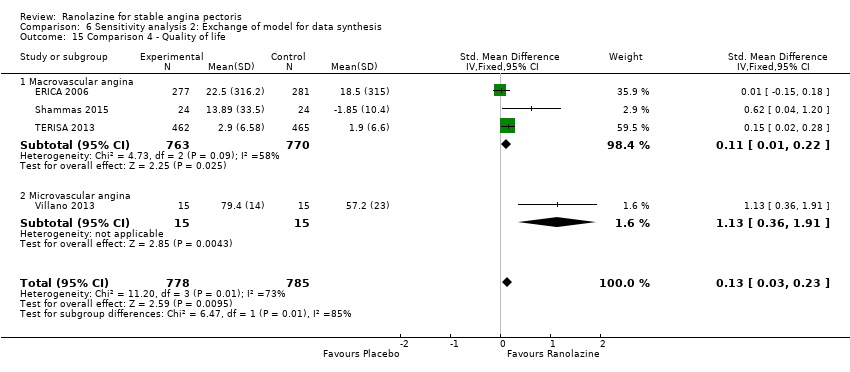 Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 15 Comparison 4 ‐ Quality of life. | ||||
| 15.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| 15.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.13 [0.36, 1.91] |
| 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 3 | Mean Difference (Random, 95% CI) | 51.05 [4.05, 98.04] | |
| Analysis 6.16  Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 3 | 6249 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.27] |
| Analysis 7.1  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 1 Comparison 1 ‐ All‐cause mortality. | ||||
| 2 Comparison 1 ‐ Quality of life Show forest plot | 3 | 2254 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| Analysis 7.2  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 2 Comparison 1 ‐ Quality of life. | ||||
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 2 | 2674 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.41] |
| Analysis 7.3 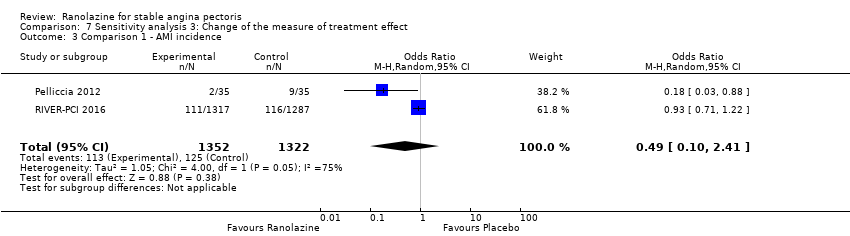 Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 3 Comparison 1 ‐ AMI incidence. | ||||
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 2 | 2674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| Analysis 7.4  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure. | ||||
| 5 Comparison 1 ‐ Adverse events incidence Show forest plot | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.88, 2.26] |
| Analysis 7.5  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 5 Comparison 1 ‐ Adverse events incidence. | ||||
| 6 Comparison 2 ‐ AMI incidence Show forest plot | 3 | 2983 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| Analysis 7.6  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 6 Comparison 2 ‐ AMI incidence. | ||||
| 7 Comparison 2 ‐ Angina episodes frequency Show forest plot | 2 | 402 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.19, 0.20] |
| Analysis 7.7  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 7 Comparison 2 ‐ Angina episodes frequency. | ||||
| 8 Comparison 2 ‐ Adverse events incidence Show forest plot | 3 | 947 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.15, 2.35] |
| Analysis 7.8  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 8 Comparison 2 ‐ Adverse events incidence. | ||||
| 8.1 Macrovascular angina | 2 | 691 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.17, 2.49] |
| 8.2 Microvascular angina | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.60] |
| 9 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.73] |
| Analysis 7.9  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 9 Comparison 3 ‐ All‐cause mortality. | ||||
| 10 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| Analysis 7.10  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 10 Comparison 3 ‐ Quality of life. | ||||
| 11 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.09] |
| Analysis 7.11 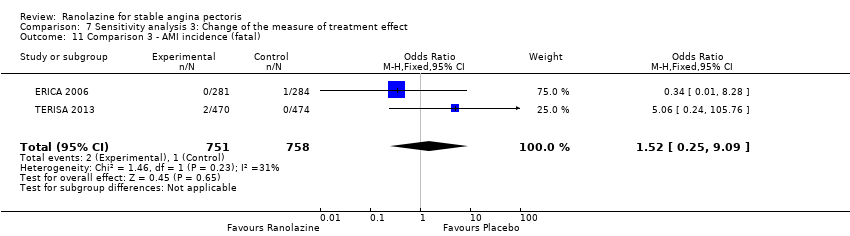 Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 11 Comparison 3 ‐ AMI incidence (fatal). | ||||
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.08] |
| Analysis 7.12  Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal). | ||||
| 13 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.28, ‐0.11] |
| Analysis 7.13 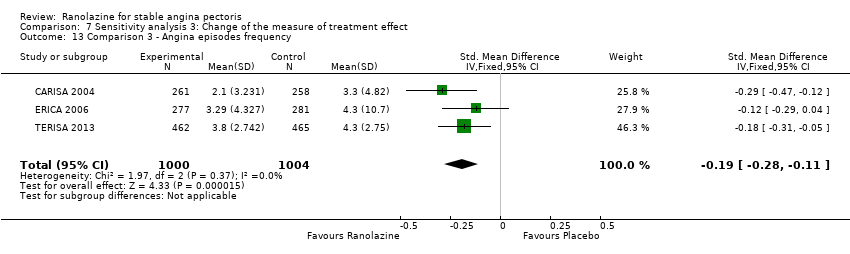 Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 13 Comparison 3 ‐ Angina episodes frequency. | ||||
| 14 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.09, 1.61] |
| Analysis 7.14 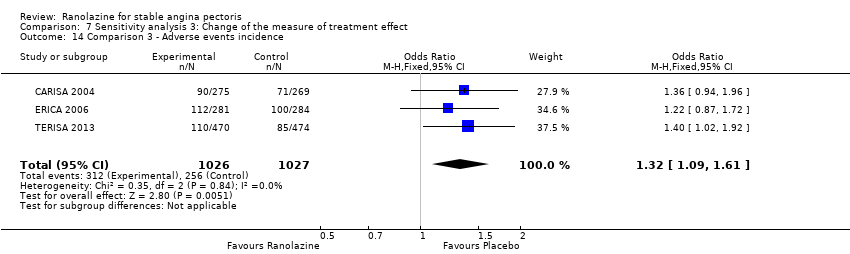 Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 14 Comparison 3 ‐ Adverse events incidence. | ||||
| 15 Comparison 4 ‐ Quality of life Show forest plot | 4 | 1563 | Mean Difference (IV, Random, 95% CI) | 11.17 [‐2.54, 24.87] |
| Analysis 7.15 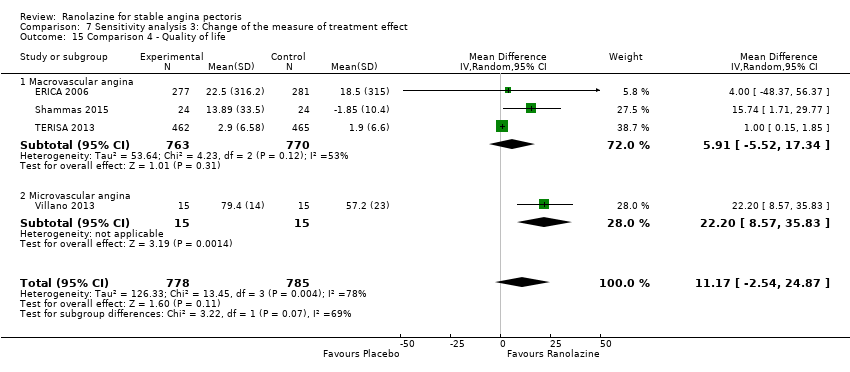 Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 15 Comparison 4 ‐ Quality of life. | ||||
| 15.1 Macrovascular angina | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| 15.2 Microvascular angina | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.20 [8.57, 35.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| Analysis 8.1 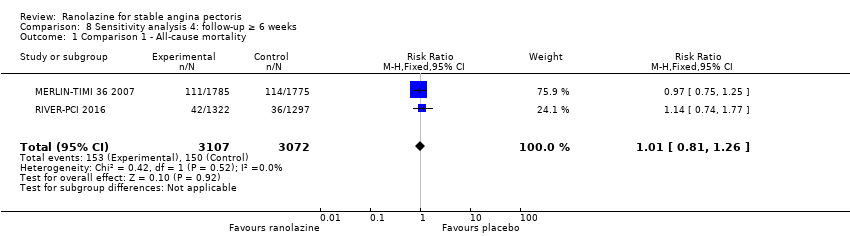 Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 1 Comparison 1 ‐ All‐cause mortality. | ||||
| 2 Comparison 1 ‐ Quality of life Show forest plot | 1 | 1958 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.08, 1.88] |
| Analysis 8.2  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 2 Comparison 1 ‐ Quality of life. | ||||
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| Analysis 8.3  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 3 Comparison 1 ‐ AMI incidence. | ||||
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| Analysis 8.4 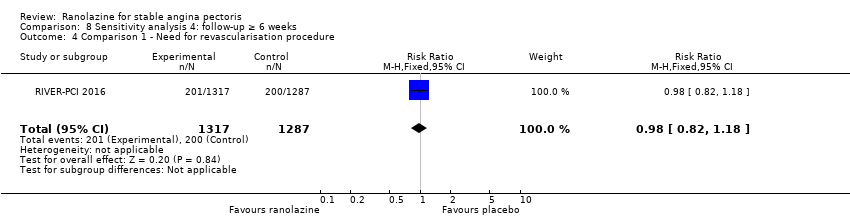 Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure. | ||||
| 5 Comparison 2 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| Analysis 8.5  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 5 Comparison 2 ‐ AMI incidence. | ||||
| 6 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| Analysis 8.6  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 6 Comparison 3 ‐ All‐cause mortality. | ||||
| 7 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| Analysis 8.7  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 7 Comparison 3 ‐ Quality of life. | ||||
| 8 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| Analysis 8.8  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 8 Comparison 3 ‐ AMI incidence (fatal). | ||||
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| Analysis 8.9  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal). | ||||
| 10 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| Analysis 8.10 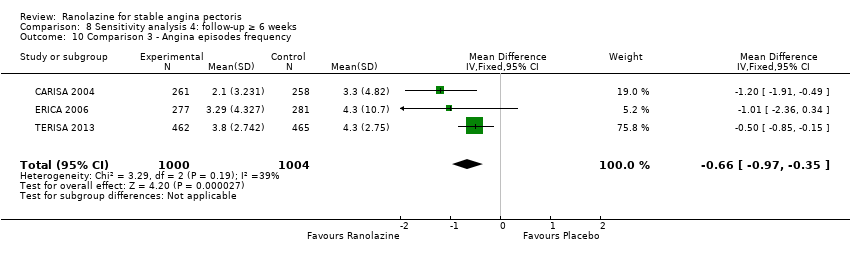 Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 10 Comparison 3 ‐ Angina episodes frequency. | ||||
| 11 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
| Analysis 8.11 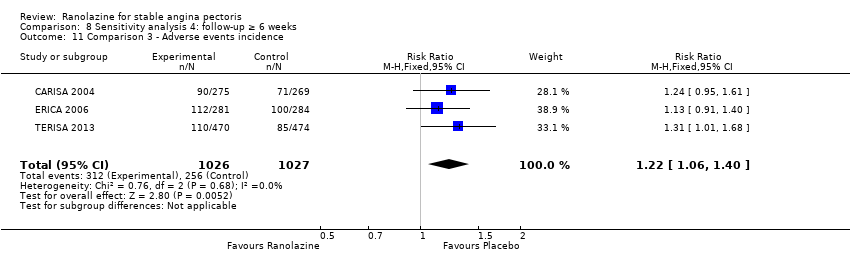 Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 11 Comparison 3 ‐ Adverse events incidence. | ||||
| 12 Comparison 4 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| Analysis 8.12 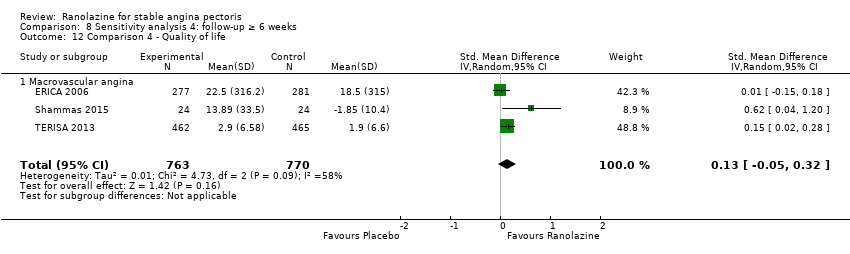 Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 12 Comparison 4 ‐ Quality of life. | ||||
| 12.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 34.6 [33.06, 36.14] | |
| Analysis 8.13  Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression. | ||||
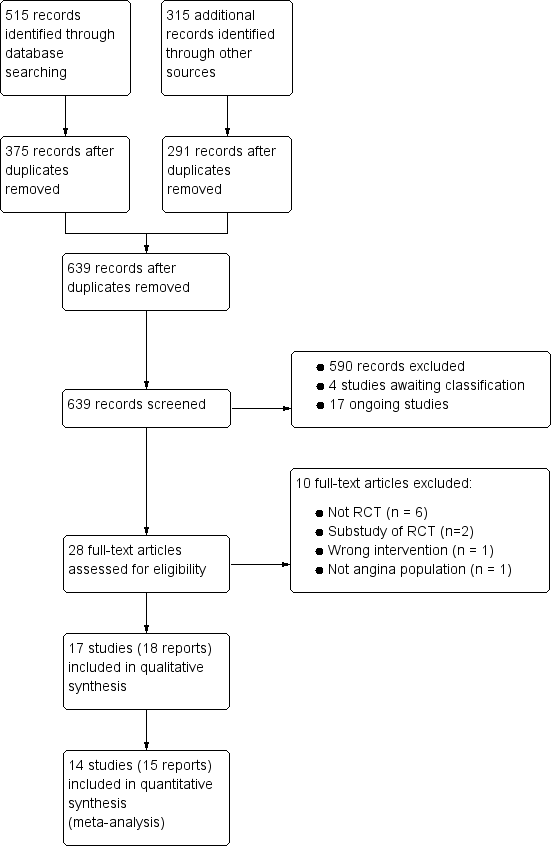
Study flow diagram
†Two included articles report data from the RIVER‐PCI trial
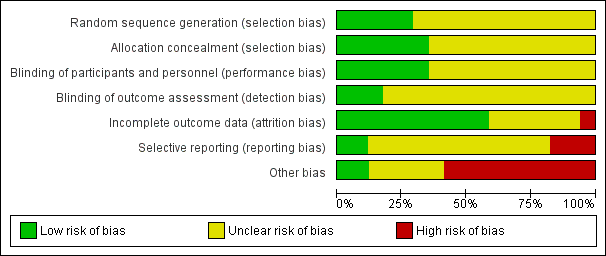
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
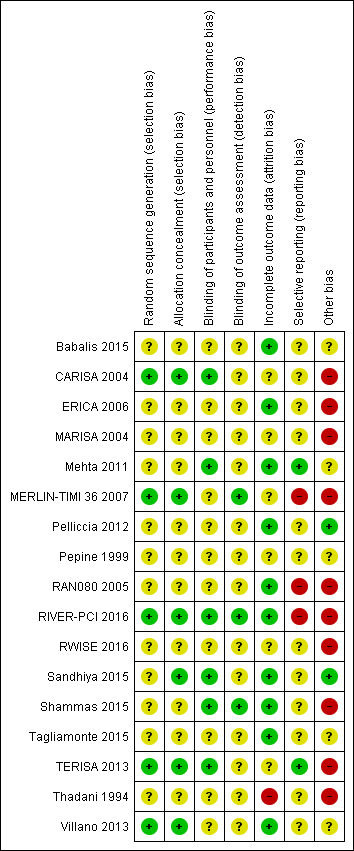
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Other bias criteria: we considered the source of funding in this section, we scored high risk of bias if the source of funding was solely from private organisations, unclear risk of bias if it was mixed (private and public) and low risk of bias if it was solely not external or from public organisations.

Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 1 Cardiovascular mortality.

Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 2 All‐cause mortality.

Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 3 Quality of life.

Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence.
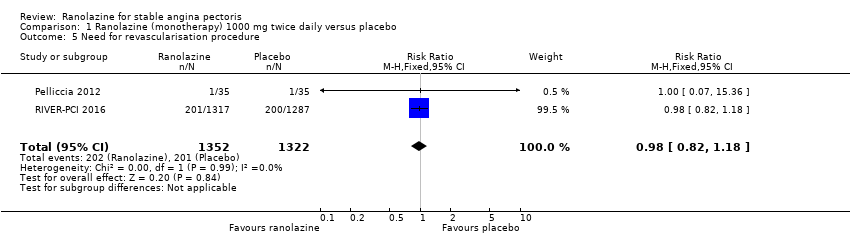
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 5 Need for revascularisation procedure.
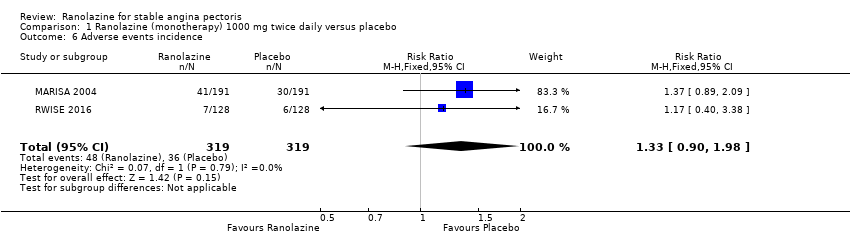
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence.

Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 1 AMI incidence.

Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 2 Angina episodes frequency.

Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 3 Adverse events incidence.

Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 1 All‐cause mortality.

Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 2 Quality of life.

Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 3 AMI incidence (fatal).

Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence (non‐fatal).
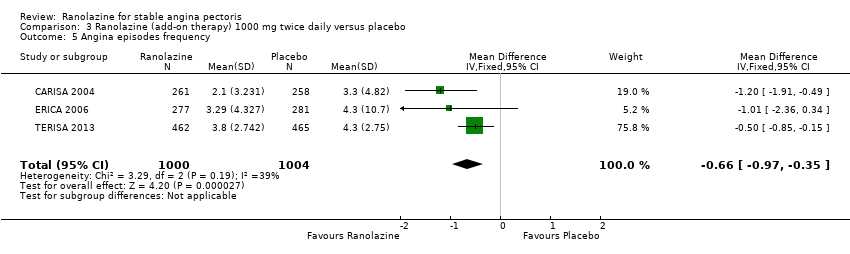
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 5 Angina episodes frequency.
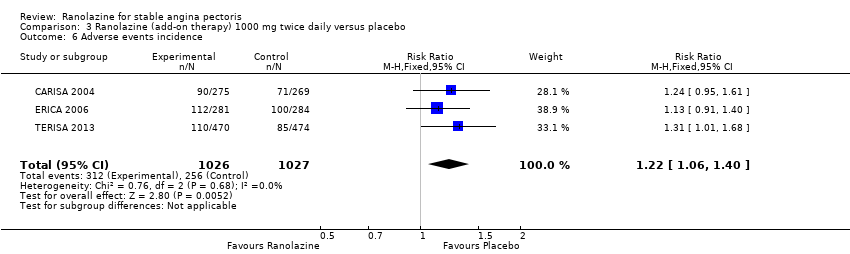
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence.

Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 1 Quality of life.
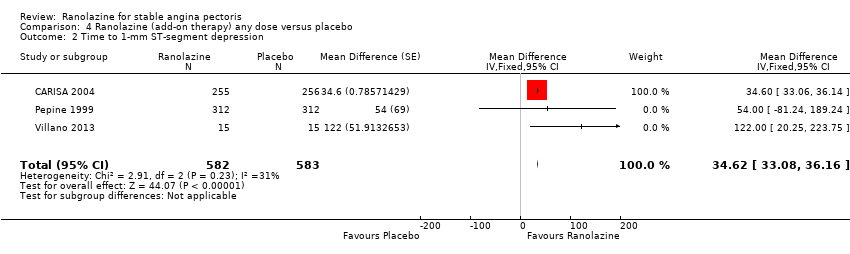
Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 2 Time to 1‐mm ST‐segment depression.
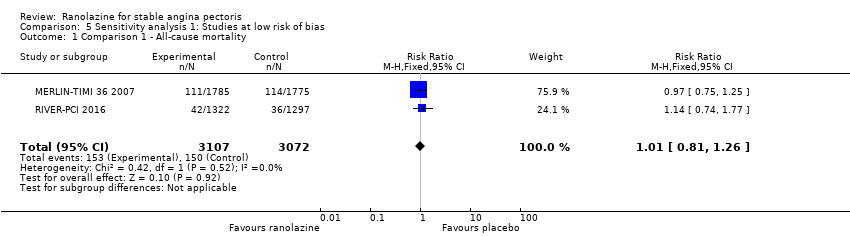
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 1 Comparison 1 ‐ All‐cause mortality.
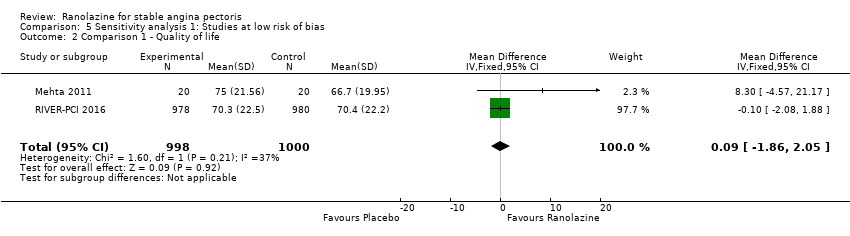
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 2 Comparison 1 ‐ Quality of life.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 3 Comparison 1 ‐ AMI incidence.
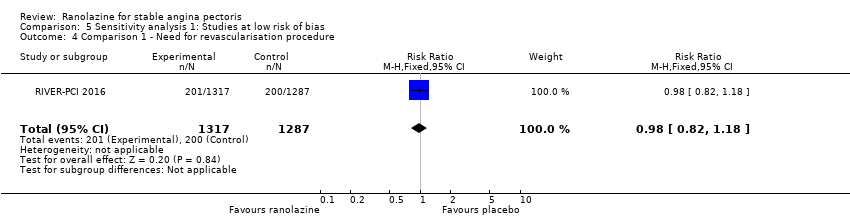
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 5 Comparison 2 ‐ AMI incidence.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 6 Comparison 3 ‐ All‐cause mortality.
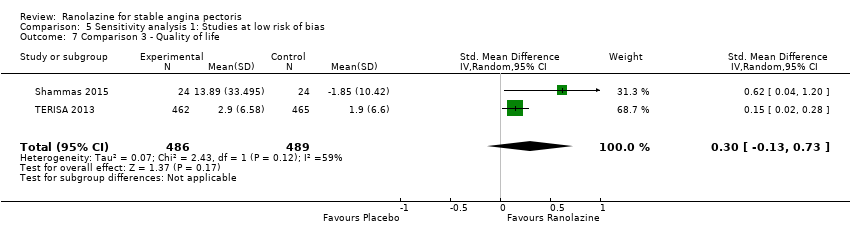
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 7 Comparison 3 ‐ Quality of life.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 8 Comparison 3 ‐ AMI incidence (fatal).

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal).

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 10 Comparison 3 ‐ Angina episodes frequency.
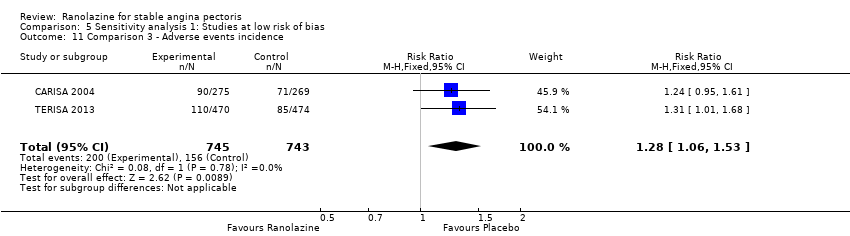
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 11 Comparison 3 ‐ Adverse events incidence.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 12 Comparison 4 ‐ Quality of life.

Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.
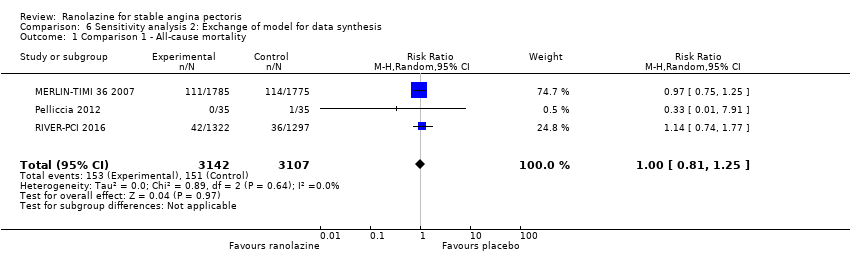
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 1 Comparison 1 ‐ All‐cause mortality.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 2 Comparison 1 ‐ Quality of life.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 3 Comparison 1 ‐ AMI incidence.
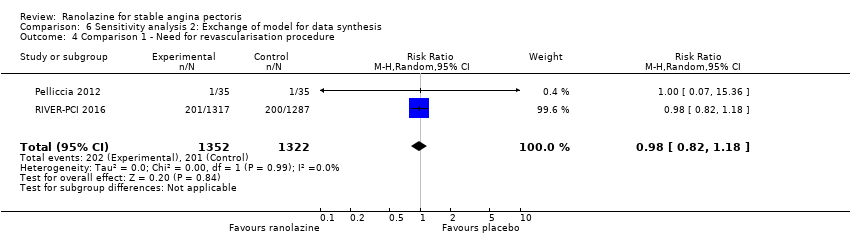
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 5 Comparison 1 ‐ Adverse events incidence.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 6 Comparison 2 ‐ AMI incidence.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 7 Comparison 2 ‐ Angina episodes frequency.
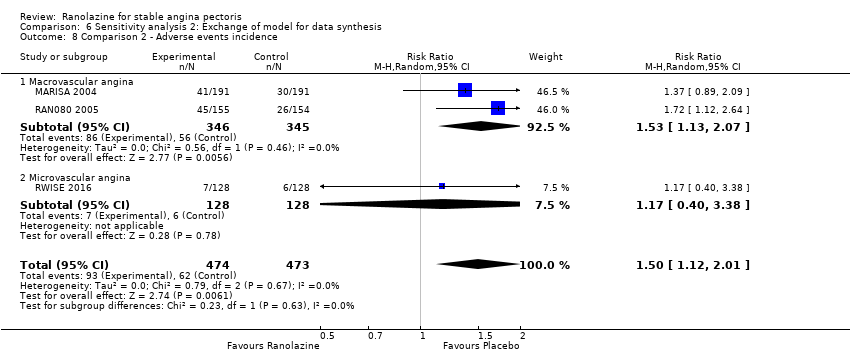
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 8 Comparison 2 ‐ Adverse events incidence.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 9 Comparison 3 ‐ All‐cause mortality.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 10 Comparison 3 ‐ Quality of life.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 11 Comparison 3 ‐ AMI incidence (fatal).
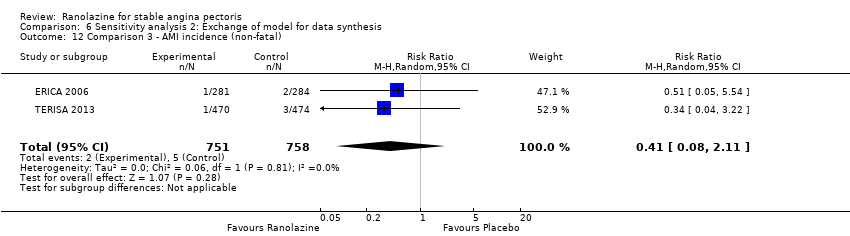
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal).
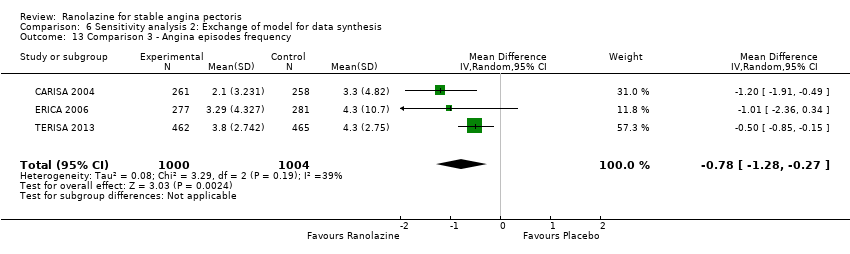
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 13 Comparison 3 ‐ Angina episodes frequency.
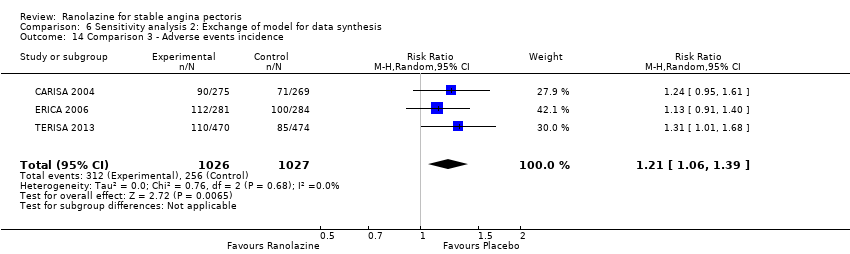
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 14 Comparison 3 ‐ Adverse events incidence.
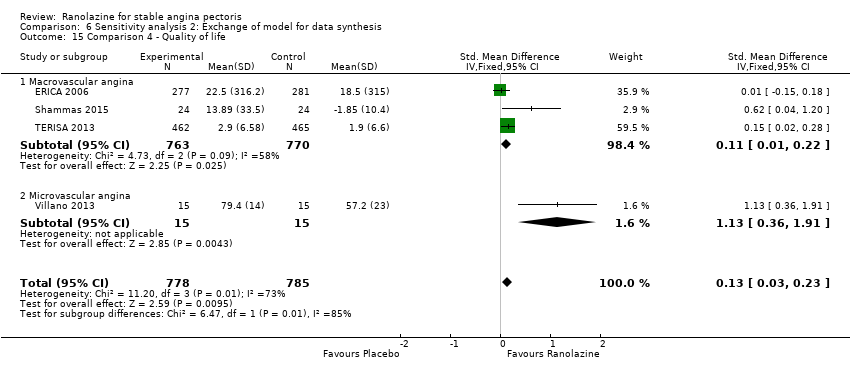
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 15 Comparison 4 ‐ Quality of life.

Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 1 Comparison 1 ‐ All‐cause mortality.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 2 Comparison 1 ‐ Quality of life.
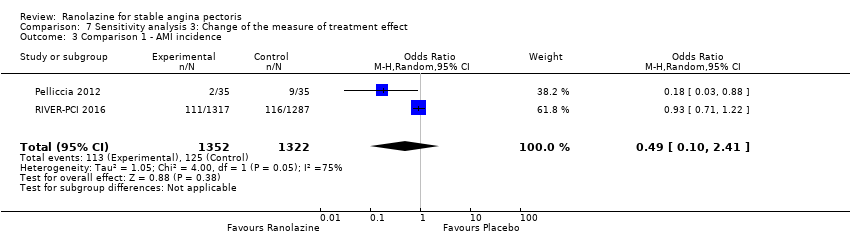
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 3 Comparison 1 ‐ AMI incidence.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 5 Comparison 1 ‐ Adverse events incidence.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 6 Comparison 2 ‐ AMI incidence.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 7 Comparison 2 ‐ Angina episodes frequency.
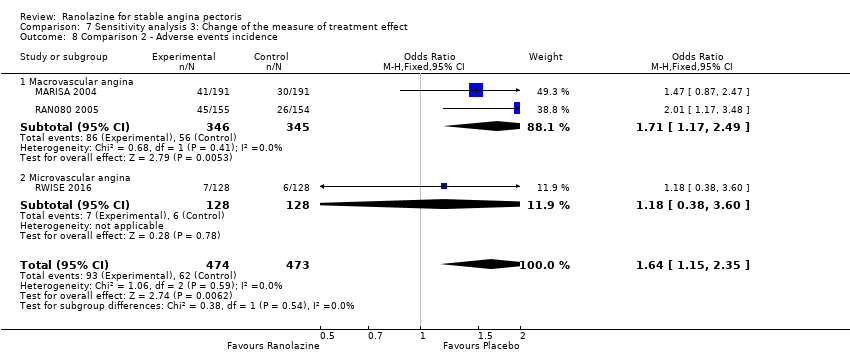
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 8 Comparison 2 ‐ Adverse events incidence.
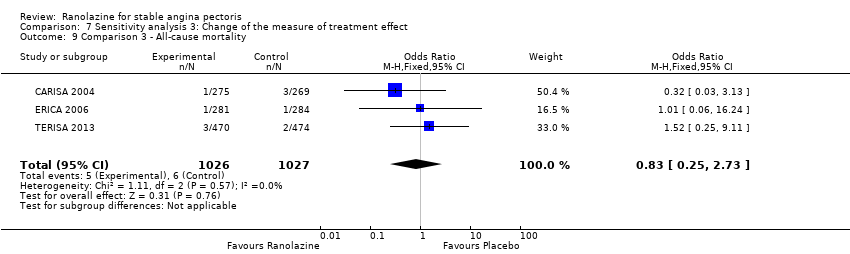
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 9 Comparison 3 ‐ All‐cause mortality.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 10 Comparison 3 ‐ Quality of life.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 11 Comparison 3 ‐ AMI incidence (fatal).
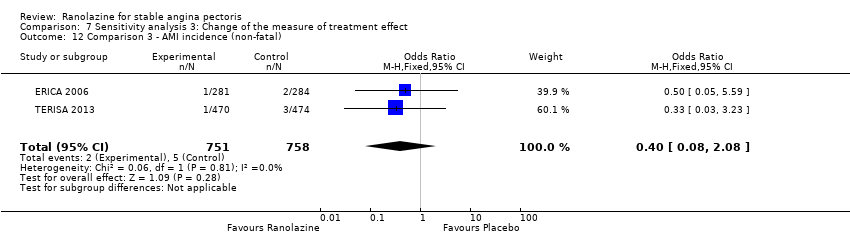
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal).
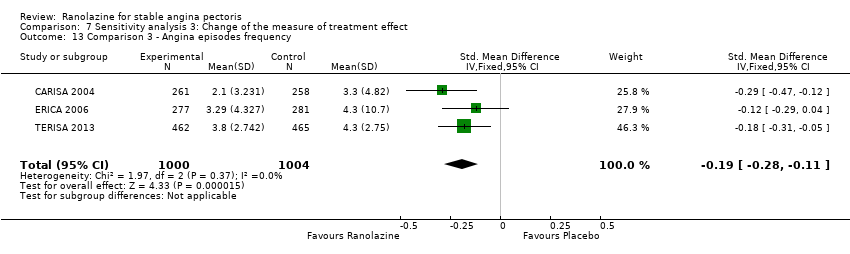
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 13 Comparison 3 ‐ Angina episodes frequency.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 14 Comparison 3 ‐ Adverse events incidence.

Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 15 Comparison 4 ‐ Quality of life.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 1 Comparison 1 ‐ All‐cause mortality.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 2 Comparison 1 ‐ Quality of life.
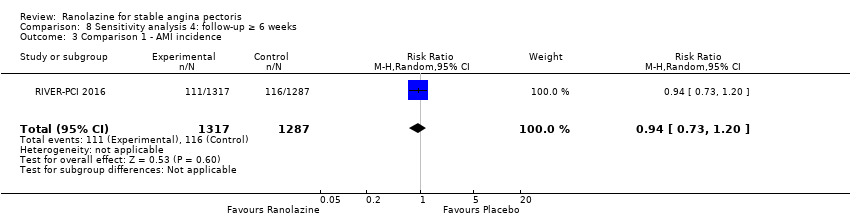
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 3 Comparison 1 ‐ AMI incidence.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.
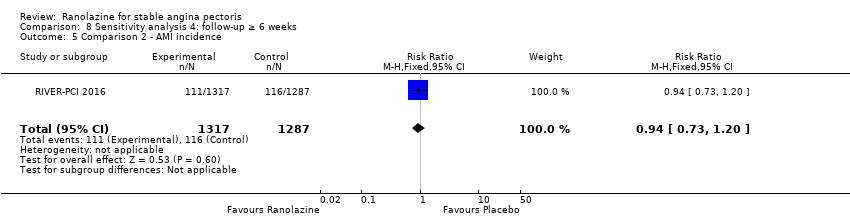
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 5 Comparison 2 ‐ AMI incidence.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 6 Comparison 3 ‐ All‐cause mortality.
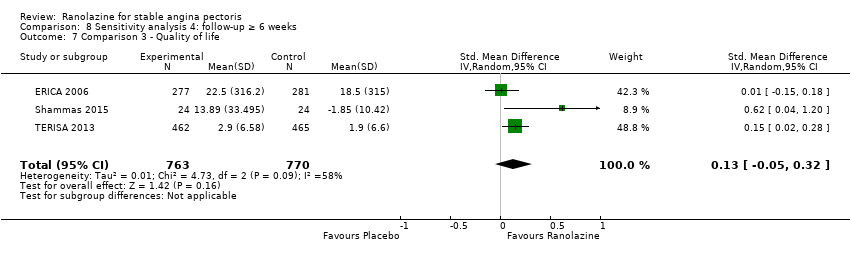
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 7 Comparison 3 ‐ Quality of life.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 8 Comparison 3 ‐ AMI incidence (fatal).

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal).

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 10 Comparison 3 ‐ Angina episodes frequency.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 11 Comparison 3 ‐ Adverse events incidence.
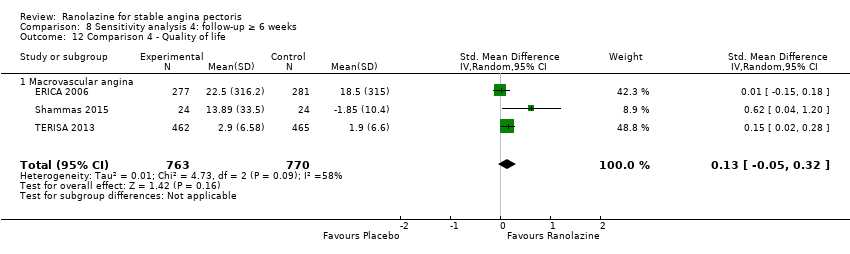
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 12 Comparison 4 ‐ Quality of life.

Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.
| Ranolazine (add‐on therapy) versus placebo for stable angina pectoris* | ||||||
| Patient or population: patients with stable angina pectoris | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ranolazine | |||||
| Cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| Non‐cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| All‐cause mortality | 6 per 1000 | 5 per 1000 | RR 0.83 | 2053 | ⊕⊕⊝⊝ | Ranolazine 1000 mg twice daily |
| Quality of life | Mean quality of life in control group participants was | Mean quality of life in intervention group participants was | 1563 | ⊕⊕⊕⊝ | Ranolazine any dose (SMD 0.25, 95% CI ‐0.01 to 0.52) | |
| AMI incidence | 7 per 1000 | 3 per 1000 | RR 0.40 | 1509 | ⊕⊕⊝⊝ | Ranolazine 1000 mg twice daily |
| Angina episodes frequency | Mean angina episode frequency in control group participants was | Mean angina episodes frequency in intervention group participants was | 2004 | ⊕⊕⊕⊝ | Ranolazine 1000 mg twice daily (MD ‐0.66, 95% CI ‐0.97 to ‐0.35) | |
| Adverse events incidence | 241 per 1000 | 294 per 1000 | RR 1.22 | 2123 | ⊕⊕⊕⊝ | Ranolazine 1000 mg twice daily |
| *Add‐on therapy: refers to the addition of ranolazine to an antianginal regimen already in course. The results reported correspond to the comparisons (data and analyses) 3 and 4 of the review (involving ranolazine given at 1000mg twice daily or any dosage); this is specified in the Comments column. **The assumed risk is based on the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ Quality of evidence was downgraded one level due to unclear risk of bias regarding blinding of outcome assessment and incomplete outcome data | ||||||
| Ranolazine (monotherapy) versus placebo for stable angina pectoris* | ||||||
| Patient or population: patients with stable angina pectoris | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ranolazine | |||||
| Cardiovascular mortality | 16 per 1000 | 16 per 1000 | RR 1.03 | 2604 | ⊕⊕⊝⊝ | Ranolazine 1000 mg twice daily |
| Non‐cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| All‐cause mortality | 49 per 1000 | 49 per 1000 | RR 1.00 | 6249 | ⊕⊕⊝⊝ | Ranolazine 1000 mg twice daily |
| Quality of life | Mean quality of life in control group participants was | Mean quality of life in intervention group participants was | 2256 | ⊕⊕⊕⊝ | Ranolazine 1000 mg twice daily (MD 0.28. 95% CI ‐1.57 to 2.13) | |
| AMI incidence | 85 per 1000 | 75 per 1000 | RR 0.88 | 2983 | ⊕⊕⊝⊝ | Ranolazine any dose |
| Angina episodes frequency | Mean angina episode frequency in control group participants was | Mean angina episode frequency in intervention group participants was | 402 | ⊕⊕⊝⊝ | Ranolazine any dose (MD 0.08, 95% CI ‐0.85 to 1.01) | |
| Adverse events incidence | 131 per 1000 | 197 per 1000 | RR 1.50 | 947 | ⊕⊝⊝⊝ | Ranolazine any dose |
| *Monotherapy: refers to the administration of ranolazine as the only antianginal drug. The results reported correspond to the comparisons (data and analyses) 1 and 2 of the review (involving ranolazine given at 1000mg twice daily or any dosage); this is specified in the Comments column. **The assumed risk is based on the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ Quality of evidence was downgraded one level due to unclear risk of bias regarding allocation concealment and high risk of bias regarding selective reporting | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.56, 1.88] |
| 2 All‐cause mortality Show forest plot | 3 | 6249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.25] |
| 3 Quality of life Show forest plot | 3 | 2254 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.57, 2.13] |
| 4 AMI incidence Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.14, 2.15] |
| 5 Need for revascularisation procedure Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 6 Adverse events incidence Show forest plot | 2 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMI incidence Show forest plot | 3 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 2 Angina episodes frequency Show forest plot | 2 | 402 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.85, 1.01] |
| 3 Adverse events incidence Show forest plot | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.12, 2.00] |
| 3.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.13, 2.07] |
| 3.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| 2 Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 3 AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| 4 AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 5 Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 6 Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 4 | 1563 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.01, 0.52] |
| 1.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 1.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 2 Time to 1‐mm ST‐segment depression Show forest plot | 3 | 1165 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 2 Comparison 1 ‐ Quality of life Show forest plot | 2 | 1998 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.86, 2.05] |
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 2 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 6 Comparison 3 ‐ All‐cause mortality Show forest plot | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.95] |
| 7 Comparison 3 ‐ Quality of life Show forest plot | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| 8 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [0.24, 104.75] |
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.22] |
| 10 Comparison 3 ‐ Angina episodes frequency Show forest plot | 2 | 1446 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.96, ‐0.32] |
| 11 Comparison 3 ‐ Adverse events incidence Show forest plot | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.06, 1.53] |
| 12 Comparison 4 ‐ Quality of life Show forest plot | 3 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.03, 1.10] |
| 12.1 Macrovascular angina | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| 12.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 3 | 6249 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.25] |
| 2 Comparison 1 ‐ Quality of life Show forest plot | 3 | 2254 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.57, 2.13] |
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 1 ‐ Adverse events incidence Show forest plot | 2 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.90, 1.99] |
| 6 Comparison 2 ‐ AMI incidence Show forest plot | 3 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.63] |
| 7 Comparison 2 ‐ Angina episodes frequency Show forest plot | 2 | 402 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.85, 1.01] |
| 8 Comparison 2 ‐ Adverse events incidence Show forest plot | 3 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.12, 2.01] |
| 8.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.13, 2.07] |
| 8.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.40, 3.38] |
| 9 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.25, 3.04] |
| 10 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| 11 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.10, 19.46] |
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.11] |
| 13 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.28, ‐0.27] |
| 14 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.06, 1.39] |
| 15 Comparison 4 ‐ Quality of life Show forest plot | 4 | 1563 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.23] |
| 15.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| 15.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.13 [0.36, 1.91] |
| 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 3 | Mean Difference (Random, 95% CI) | 51.05 [4.05, 98.04] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 3 | 6249 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.27] |
| 2 Comparison 1 ‐ Quality of life Show forest plot | 3 | 2254 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 2 | 2674 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.41] |
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 2 | 2674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 5 Comparison 1 ‐ Adverse events incidence Show forest plot | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.88, 2.26] |
| 6 Comparison 2 ‐ AMI incidence Show forest plot | 3 | 2983 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| 7 Comparison 2 ‐ Angina episodes frequency Show forest plot | 2 | 402 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.19, 0.20] |
| 8 Comparison 2 ‐ Adverse events incidence Show forest plot | 3 | 947 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.15, 2.35] |
| 8.1 Macrovascular angina | 2 | 691 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.17, 2.49] |
| 8.2 Microvascular angina | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.60] |
| 9 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.73] |
| 10 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| 11 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.09] |
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.08] |
| 13 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.28, ‐0.11] |
| 14 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.09, 1.61] |
| 15 Comparison 4 ‐ Quality of life Show forest plot | 4 | 1563 | Mean Difference (IV, Random, 95% CI) | 11.17 [‐2.54, 24.87] |
| 15.1 Macrovascular angina | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| 15.2 Microvascular angina | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.20 [8.57, 35.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1 ‐ All‐cause mortality Show forest plot | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 2 Comparison 1 ‐ Quality of life Show forest plot | 1 | 1958 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.08, 1.88] |
| 3 Comparison 1 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 4 Comparison 1 ‐ Need for revascularisation procedure Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 2 ‐ AMI incidence Show forest plot | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 6 Comparison 3 ‐ All‐cause mortality Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| 7 Comparison 3 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 8 Comparison 3 ‐ AMI incidence (fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) Show forest plot | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 10 Comparison 3 ‐ Angina episodes frequency Show forest plot | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 11 Comparison 3 ‐ Adverse events incidence Show forest plot | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
| 12 Comparison 4 ‐ Quality of life Show forest plot | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 12.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 34.6 [33.06, 36.14] | |

