Intervenciones para la hemocromatosis hereditaria
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011647.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 marzo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hepatobiliar
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
EB and MK extracted data and provided them in a format that could be analysed.
KG wrote the review.
ET, DT, and BD critically commented on the review.
All review authors approved this version before publication.
Sources of support
Internal sources
-
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Cochrane Hepato‐Biliary and Upper Gastrointestinal and Pancreatic Diseases Groups. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health. The funding covered salary, equipment, and other resources to complete this review.
-
Elena Buzzetti, Italy.
Dr Elena Buzzetti was supported by an educational grant from AIGO (Associazione Italiana Gastroenterologi Ospedalieri) and M.I.M.I (Montecatini Interactive Medicine International).
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
EB: has no financial disclosures.
MK: has no financial disclosures.
DT: funded by Astellas for his attendance at the International Liver Transplantation Society meeting in 2014; received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013.
BD: has no financial disclosures.
ET: has no financial disclosures.
KG: has no financial disclosures.
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice. We also thank the copy editors who improved the clarity of the review.
Peer reviewers of the review: Barbara de Graaff, Australia; Dario Conte, Italy; Ronald Koretz, USA; Theis Lange, Denmark.
Contact editor: Janus Christian Jakobsen, Denmark.
Sign‐off editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Mar 08 | Interventions for hereditary haemochromatosis | Review | Elena Buzzetti, Maria Kalafateli, Douglas Thorburn, Brian R Davidson, Emmanuel Tsochatzis, Kurinchi Selvan Gurusamy | |
| 2015 Apr 14 | Treatments for hereditary haemochromatosis: a network meta‐analysis | Protocol | Kurinchi Selvan Gurusamy, Emmanuel Tsochatzis, Douglas Thorburn, Brian R Davidson | |
Differences between protocol and review
-
There was only one comparison. So we did not perform the network meta‐analysis, and assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. The methodology that we plan to use if we conduct a network meta‐analysis in future is available in Appendix 1.
-
We performed Trial Sequential Analysis in addition to conventional method of assessing the risk of random errors using P‐value.
Notes
There was considerable overlap between the 'Methods' of this review and those of several other reviews written by the same group of authors.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans; Middle Aged;
PICO
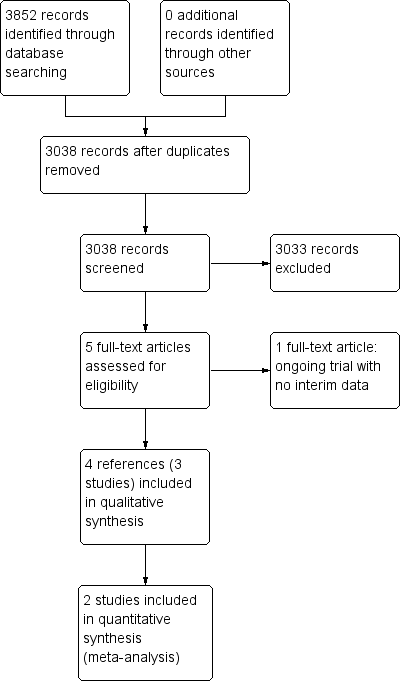
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
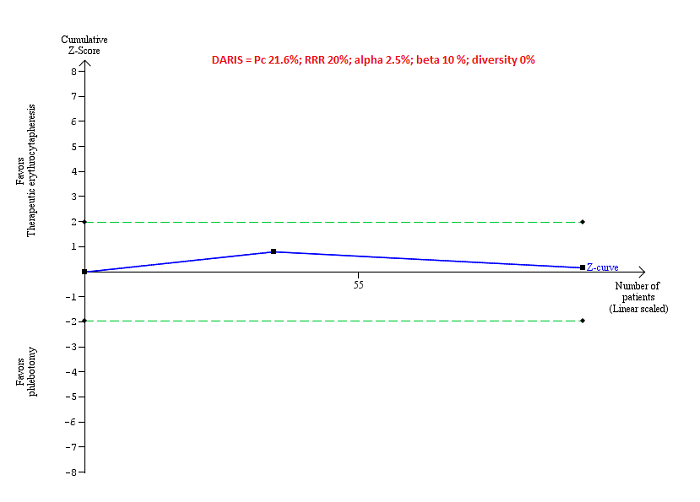
Trial Sequential Analysis of adverse events (proportion) performed using an alpha error of 2.5%, power of 90% (beta error of 10%), relative risk reduction of 20%, control group proportion (Pc) observed in trials (21.6% for proportion of people with adverse events), and observed diversity (0%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS) that the boundaries could not be drawn. The Z‐curve (blue line) does not cross the conventional boundaries (dotted green line). There was a high risk of random errors.
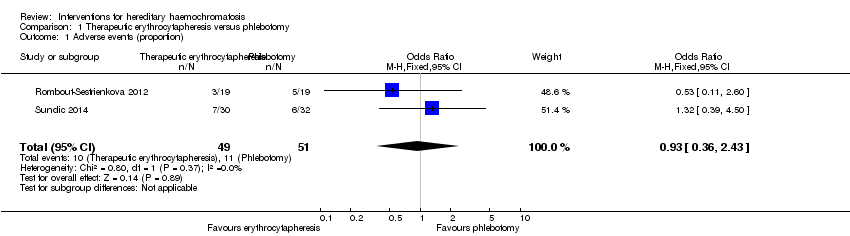
Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 1 Adverse events (proportion).
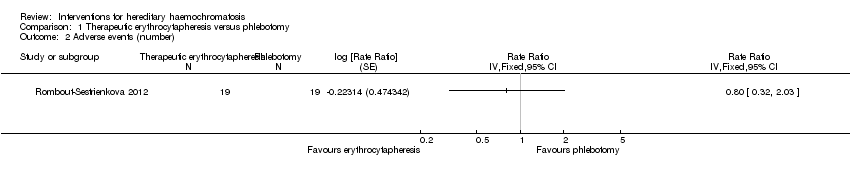
Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 2 Adverse events (number).

Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 3 Health‐related quality of life (EQ‐VAS).
| Erythrocytapheresis versus phlebotomy for hereditary haemochromatosis | |||||
| Patient or population: people with hereditary haemochromatosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Phlebotomy | Therapeutic erythrocytapheresis | ||||
| Long‐term mortality | None of the included trials reported mortality beyond 1 year. | ||||
| Mortality Follow‐up period: 8 months | There was no mortality in either group in the short‐term in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Serious adverse events Follow‐up period: 8 months | There were no serious adverse events in either group in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Health‐related quality of life Follow‐up period: 8 months | The mean health‐related quality of life in the control groups was | The mean health‐related quality of life in the intervention groups was | ‐ | 38 | ⊕⊝⊝⊝ |
| Health‐related quality of life beyond one year | None of the included trials reported health‐related quality of life beyond one year | ||||
| *The basis for the assumed risk is the mean control group proportion or control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level for risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events (proportion) Show forest plot | 2 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.43] |
| 2 Adverse events (number) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 Health‐related quality of life (EQ‐VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

