Contenido relacionado
Revisiones y protocolos relacionados
Sonam Vadera, Charles Wei Kit Yong, Lise Lotte Gluud, Marsha Y Morgan | 20 junio 2019
Athanasios Tyraskis, Christopher Parsons, Mark Davenport | 14 mayo 2018
Anna K Nowak, Michael Findlay, Gordana Culjak, Martin R Stockler | 3 agosto 2020
Ee Teng Goh, Caroline S Stokes, Sandeep S Sidhu, Hendrik Vilstrup, Lise Lotte Gluud, Marsha Y Morgan | 15 mayo 2018
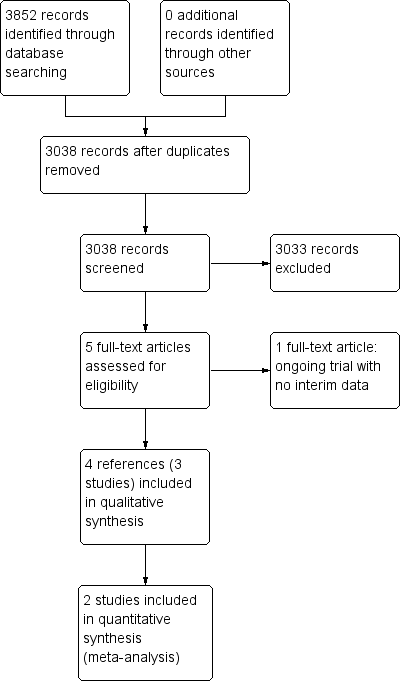
Kurinchi Selvan Gurusamy, Emmanuel Tsochatzis, Clare D Toon, Elias Xirouchakis, Andrew K Burroughs, Brian R Davidson | 4 diciembre 2013
Francesca Saffioti, Kurinchi Selvan Gurusamy, Leonardo Henry Eusebi, Emmanuel Tsochatzis, Brian R Davidson, Douglas Thorburn | 28 marzo 2017
Rosa Lombardi, Simona Onali, Douglas Thorburn, Brian R Davidson, Kurinchi Selvan Gurusamy, Emmanuel Tsochatzis | 30 marzo 2017
Chavdar S Pavlov, Daria L Varganova, Giovanni Casazza, Emmanuel Tsochatzis, Dimitrinka Nikolova, Christian Gluud | 9 abril 2019
Lior H Katz, Hadar Goldvaser, Anat Gafter‐Gvili, Ran Tur‐Kaspa | 12 septiembre 2012
De Zhao Kong, Ning Liang, Guan Lin Yang, Zhe Zhang, Yue Liu, Ye Yang, Yu Xi Liu, Qi Ge Wang, Fan Zhang, Hui Yong Zhang, Dimitrinka Nikolova, Janus C Jakobsen, Christian Gluud, Jian Ping Liu | 22 agosto 2019