遗传性血色素沉着症的干预措施
摘要
研究背景
遗传性血色素沉着症是一种与参与铁转运的蛋白质有关的遗传性疾病,导致铁负荷和铁在身体各个组织中的沉积。铁负载导致的并发症包括肝硬化(及相关并发症,如肝功能衰竭和肝细胞癌)、心力衰竭、心律失常、阳痿、糖尿病、关节炎和皮肤色素沉着。放血疗法(静脉切开术或“放血”)是目前推荐的遗传性血色素沉着症治疗方法。遗传性血色素沉着症的最佳治疗方法仍存在争议。
研究目的
通过网状meta分析评价治疗遗传性血色素沉着症的不同干预措施的相对利弊,并根据安全性和有效性对现有治疗方法进行排序。但是,我们发现只有一个比较。因此,我们没有报告网状Meta分析的结果,而是报告了使用标准Cochrane方法评价的不同干预措施获益和危害的比较。
检索策略
我们检索了截至2016年3月Cochrane对照试验的中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)、MEDLINE、Embase、科学引文索引扩展(Science Citation Index Expanded, SCIE)、世界卫生组织国际临床试验注册平台(World Health Organization International Clinical Trials Registry Platform, WHOICTRP)和随机对照试验注册库上的关于遗传性血色素沉着症治疗方法的随机对照试验。
纳入排除标准
我们只纳入了包含遗传性血色素沉着症受试者的随机对照试验(不限语言、盲法或出版状态)。排除了包含曾接受过肝移植的受试者的临床试验。我们比较了各种不同干预措施之间的差异,或干预措施与仅进行支持治疗之间的差异。
资料收集与分析
我们使用了Cochrane推荐的标准方法学程序。基于Review Manager 5软件的现有被试分析,采用固定效应模型和随机效应模型,我们用95%置信区间(confidence intervals, CI)计算出了比值比(odds ratio, OR)和比率比。我们采用试验序贯分析,评价风险偏倚,随机误差的控制风险,并使用GRADE评价试验证据的质量。
主要结果
总共有3项试验(146名受试者)符合本综述纳入标准。其中2项平行小组试验(100名受试者)提供了一个或多个结局信息。其余试验为交叉试验,没有可用资料可供分析。所有试验都存在高偏倚风险。总体而言,所有证据的质量都极低。所有三项试验都比较了红细胞分离术(仅去除红细胞,而不是全血)与静脉切开术。其中两项试验具有相同的第一作者。这三项试验的受试者的平均年龄或年龄中位数在42岁至55岁之间。没有一项试验报告纳入的受试者是有症状、无症状或两者兼有。有两项试验是在未接受过血色素沉着症治疗的人群中进行的。为本综述提供大部分资料的试验排除了患有恶性肿瘤、心力衰竭和严重心律失常的患者。我们没有发现评价铁螯合剂的试验。
只有一项有38名受试者参加的试验报告称,在短期随访结束时(8个月)没有出现死亡和严重不良事件。两项试验报告了出现不良事件的人数比例:红细胞分离术组为10/49(20.4%),而放血组为11/51(21.6%)。这两项试验中,有一项提供了不良事件发生率的资料(每100名接受红细胞分离术的受试者发生42.1起事件,而每100名接受放血疗法的受试者发生52.6起事件)。没有证据表明两组之间发生不良事件的人数比例以及不良事件(严重和非严重)的数量存在差异(发生不良事件的人数比例:OR=0.93, 95%CI [0.36, 2.43],受试者=100,试验=2;不良事件数量:RR=0.80, 95%CI [0.32, 2.03],受试者=38,试验=1)。在短期健康相关生活质量方面,两组之间没有差异(MD=1.00, 95%CI [‐10.80, 12.80],受试者=38,试验=1)。该结局使用EQ‐VAS进行测量(范围:0到100,分数越高表示健康相关的生活质量越好)。所有试验均未报告一年后的死亡率、一年后的健康相关生活质量、肝移植、失代偿性肝病、肝硬化、肝细胞癌、糖尿病或长期随访期间的心血管并发症。
为本综述提供资料的两项试验均由对结果没有既得利益的各方资助;第三项试验的资金来源没有报告。
作者结论
目前没有足够的证据来确定,与放血疗法相比,红细胞分离术是有益还是有害。放血疗法对设备的要求较低,仍然是需要某种形式放血的遗传性血色素沉着症患者的首选治疗方法。然而,应该指出的是,随机临床试验没有证据表明任何形式的放血对遗传性血色素沉着症患者有益。尽管如此,仍不太可能进行包括无治疗在内的试验。未来的试验应该比较不同频率的放血疗法和红细胞分离术,以及使用或不使用不同铁螯合剂的放血疗法之间及与安慰剂的比较。此类试验应纳入对受试者的长期随访(例如使用国家记录链接数据库),以确定治疗对于临床结局(如死亡、与健康相关的生活质量、肝损伤及其后果、心脏损伤及其后果以及对遗传性血色素沉着病患者至关重要的其他结局)是有益还是有害。
PICO
简语概要
遗传性血色素沉着症的干预措施
系统综述背景
遗传性血色素沉着症是一种遗传性疾病(源自父母),导致体内铁积聚过多。有些人会因铁积累过多而导致肝脏和心脏受损,进而导致肝功能衰竭和心力衰竭、阳痿(男性无法勃起或性高潮)、糖尿病、关节炎(关节疼痛和肿胀)以及皮肤色素沉着(着色)。治疗遗传性血色素沉着症有多种方法,但最佳方法尚不明确。本综述纳入了截至2016年3月所发表的所有随机对照临床试验(受试者被随机分配到两个治疗组之一或多个治疗组之一的精心设计的临床试验)。我们纳入了受试者未接受肝移植的试验。除了使用只能同时比较两种治疗方案差异的标准Cochrane方法(直接比较)外,我们还计划采用更高级的方法,即能同时比较在试验中相互独立的多种不同治疗方案差异的方法(网状meta分析)。但是,因为只有一个比较,所以我们只能使用标准的Cochrane方法。
研究特点
我们纳入了三项研究。其中2项平行小组试验(100名受试者)提供了一个或多个结局信息。这些试验比较了放血疗法(移除血液或“放血”)与红细胞分离术(移除血液、分离红细胞(在血液中携带氧气)并将血液的剩余部分送回)。两项试验是在未曾接受过血色素沉着症治疗的人群中进行的。为本综述提供大部分资料的试验排除了患有恶性肿瘤、心力衰竭和严重心律失常的患者。
资金来源: 为本综述提供资料的两项试验均由对结果没有既得利益的各方资助;第三项试验的资金来源没有报告。
主要研究结果
在唯一一项报告了相关信息的试验中,两组均未出现死亡或严重并发症。没有证据表明两种疗法在出现任何并发症的比例、人均并发症数量以及短期健康相关生活质量(衡量个人对生活和健康满意度的指标)方面存在任何差异。所有试验均未报告长期的一年以上的死亡、一年以上与健康相关的生活质量、肝移植、严重肝损伤、肝衰竭、肝癌、糖尿病、心力衰竭或中风。目前没有足够的证据来确定,与放血疗法相比,红细胞分离术是有益还是有害。红细胞去除术需要特殊设备,而静脉切开术则不需要。然而,应该指出的是,随机临床试验没有证据表明任何形式的放血对遗传性血色素沉着症患者有益。尽管如此,仍不太可能进行包括无治疗在内的试验。
证据质量
证据的总体质量极低,因为试验存在很高的偏倚风险,这意味着由于研究的进行方式,有可能做出错误的结论,高估治疗的获益或低估治疗的危害。需要进一步开展高质量的随机临床试验,以确定放血疗法的频率,并对红细胞分离术和放血疗法进行比较。此类试验应纳入对受试者的长期监测(或许可以通过链接某些国家的健康记录实现)。
Authors' conclusions
Summary of findings
| Erythrocytapheresis versus phlebotomy for hereditary haemochromatosis | |||||
| Patient or population: people with hereditary haemochromatosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Phlebotomy | Therapeutic erythrocytapheresis | ||||
| Long‐term mortality | None of the included trials reported mortality beyond 1 year. | ||||
| Mortality Follow‐up period: 8 months | There was no mortality in either group in the short‐term in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Serious adverse events Follow‐up period: 8 months | There were no serious adverse events in either group in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Health‐related quality of life Follow‐up period: 8 months | The mean health‐related quality of life in the control groups was | The mean health‐related quality of life in the intervention groups was | ‐ | 38 | ⊕⊝⊝⊝ |
| Health‐related quality of life beyond one year | None of the included trials reported health‐related quality of life beyond one year | ||||
| *The basis for the assumed risk is the mean control group proportion or control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level for risk of bias. | |||||
Background
Description of the condition
Hereditary haemochromatosis is a genetic disorder related to proteins involved in iron transport, resulting in iron load and deposition of iron in various tissues of the body (Adams 2007). The most common mutation causing hereditary haemochromatosis is substitution of cysteine with tyrosine at position 282 (C282Y) of the HFE gene (Feder 1996; Pietrangelo 2004; Adams 2007; Bardou‐Jacquet 2014). This is an autosomal‐recessive genetic disorder (i.e. it can manifest itself only when both alleles (copies of the gene in both chromosomes) carry the mutation) (Feder 1996; Pietrangelo 2004; Adams 2007; Bardou‐Jacquet 2014). However, compound heterozygosity with another allele H63D (substitution of histamine with aspartic acid at position 63) (i.e. one copy of the C282Y mutated human haemochromatosis protein (HFE) gene and one copy of the H63D mutated HFE gene) can also result in manifestation of haemochromatosis (Feder 1996; van Bokhoven 2011). Several other mutations related to the HFE protein and other proteins involved in iron transport, namely ferroportin, hepcidin, transferrin receptor‐2, haemojuvelin, and ceruloplasmin, can lead to hereditary haemochromatosis (Pietrangelo 2004; Adams 2007; van Bokhoven 2011). Carriers of the autosomal‐recessive mutated HFE gene (either the C282Y or H63D allele in one of the chromosomes) varies globally and depends on ethnic origin. In the US, the prevalence of the carrier state is about 5.4% for C282Y and 13.5% for H63D alleles (Steinberg 2001). Approximately 0.3% of the general population in the US are homozygous for C282Y, 1.9% are homozygous for H63D, and 2% have C282Y/H63D compound heterozygosity (Steinberg 2001). The frequencies of C282Y and H63D alleles are more common in non‐Hispanic white people compared to non‐Hispanic black people and Mexican‐American people (Steinberg 2001). In Europe, there is significant variation in different countries with the frequency of C282Y more common in countries such as Ireland and the UK (Lucotte 2003). Overall, 0% to 28% of people carry at least one C282Y allele in different countries in Europe (Mercier 1998; Cassanelli 2001; Lucotte 2003; Ropero 2006; Voicu 2009); and 23% to 30% carry at least one H63D allele (Cassanelli 2001; Ropero 2006; Voicu 2009). However, on average, only 0.3% of people are homozygous for the C282Y allele. In Australia, screening of people of Northern Europe ancestry revealed that 0.7% of people were homozygous for the C282Y mutation and an additional 2.4% had C282Y/H63D compound heterozygosity (Allen 2008).
Diagnosis of hereditary haemochromatosis is suspected by abnormal serum iron studies such as serum ferritin and transferrin saturation, and established by the presence of C282Y homozygous gene products and the presence of other known rarer mutations (van Bokhoven 2011). Liver iron stores measured by magnetic resonance imaging or liver biopsy may be helpful in identifying elevated iron stores in the liver (Bacon 2011; van Bokhoven 2011). The proportion of people with haemochromatosis‐predisposing mutations who develop clinical symptoms of iron overload is very controversial. Asymptomatic elevation of serum ferritin and transferrin saturation (i.e. screen‐detected hereditary haemochromatosis) is a common mode of clinical presentation (Bardou‐Jacquet 2014). In symptomatic people, common symptoms related to hereditary haemochromatosis at the time of diagnosis are poor general health, fatigue, malaise, diabetes, and arthralgia (Pietrangelo 2004; Allen 2008; van Bokhoven 2011). Complications related to hereditary haemochromatosis include liver cirrhosis (and related complications such as liver failure and hepatocellular carcinoma), cardiac failure, cardiac arrhythmias, impotence, diabetes, arthritis, and skin pigmentation (Pietrangelo 2004; Schmitt 2005; van Bokhoven 2011; Bardou‐Jacquet 2014). While some researchers state that 28% to 50% of men and 1.4% to 44% of women homozygous for haemochromatosis‐predisposing mutations develop symptoms (Bradley 1996; Allen 2008), other researchers point out that the frequency of symptoms commonly attributed to haemochromatosis such as poor general health, fatigue, malaise, diabetes, and arthralgia were similar between people homozygous for haemochromatosis‐predisposing mutations and the general population (Beutler 2002). Therefore, it is not clear whether these symptoms are related to haemochromatosis at all. However, it should be pointed out that people homozygous for haemochromatosis‐predisposing mutations had more frequent liver disorders compared to the general population (Beutler 2002). Overall, the odds of developing hepatocellular carcinoma and porphyria cutanea tarda (skin blisters in areas of the body exposed to sunlight) were higher in C282Y homozygotes and C282Y/H63D compound heterozygotes compared to people in control groups (Ellervik 2007). Approximately one‐third of symptomatic people with C282Y homozygosity and a mean age of 50 years referred to a tertiary care centre die over 20 years (Wojcik 2002; Schmitt 2005). Although the symptoms related to hereditary haemochromatosis are thought to be due to iron overload and some studies have indicated a relationship between symptoms and a serum ferritin level of 1000 µg/L (Allen 2008), there is currently no firm evidence for a relationship between symptoms and the degree of iron overload (Beutler 2002; van Bokhoven 2011). While screening of family members and the general population have been advocated by some researchers (Pietrangelo 2004; Bacon 2011; de Graaff 2015), other researchers found no evidence of any tangible benefit of screening based on systematic reviews of clinical effectiveness (Schmitt 2005; Whitlock 2006). However, asymptomatic elevation of serum ferritin and transferrin saturation (i.e. screen‐detected hereditary haemochromatosis) is a common mode of clinical presentation (Bardou‐Jacquet 2014).
Description of the intervention
The main treatments for hereditary haemochromatosis include phlebotomy (venesection or blood letting), erythrocytapheresis (removal of red cells only instead of removal of whole blood), and administration of iron‐chelating agents such as desferrioxamine (van Bokhoven 2011). Removal of 500 mL of blood per week guided by serum transferrin levels and haemoglobin levels is recommended (van Bokhoven 2011). The major problems with regular phlebotomy are venous access and the requirement to visit a healthcare facility for treatment (van Bokhoven 2011). While there are no absolute contraindications for phlebotomy, the relative contraindications include severe heart disease and anaemia (Assi 2014), and possibly hypoproteinaemia.
Erythrocytapheresis involves removal of red cells only instead of whole blood and requires specialist equipment. However, the number of treatments can be reduced compared to regular phlebotomy as more iron can be removed per session (van Bokhoven 2011; Rombout‐Sestrienkova 2012). Desferrioxamine is usually administered subcutaneously, intramuscularly, or intravenously (Martindale 2011). A starting dose of 500 mg is recommended and the drug may be administered three to seven times a week (Martindale 2011). Adverse reactions of desferrioxamine include severe allergy, arthralgia, pain at injection site, gastrointestinal symptoms, tachycardia, and thrombocytopenia (Martindale 2011; van Bokhoven 2011). Newer iron‐chelating agents such as deferasirox have also been used for the treatment of primary hereditary haemochromatosis (Cancado 2015). Deferasirox is an oral chelating agent and appears to have equivalent efficacy and safety profiles as desferrioxamine (Vichinsky 2007; Pennell 2014). This review will not cover lifestyle modifications such as reduced alcohol consumption and dietary changes.
How the intervention might work
Since red blood cells contain iron as a component of haemoglobin, their removal (by erythrocytapheresis or phlebotomy) reduces body iron content, which could potentially diminish iron deposition in tissues and the subsequent complications. Desferrioxamine is an iron‐chelating agent that might work by removing iron deposition from the tissues (Martindale 2011).
Why it is important to do this review
The optimal treatment of hereditary haemochromatosis is not known. Currently, both the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend phlebotomy as the treatment of choice (EASL 2010; Bacon 2011). One randomised clinical trial reported that erythrocytapheresis required fewer sessions than venesection to decrease iron overload (Rombout‐Sestrienkova 2012). It is also not clear whether any of these measures decrease the development of complications. So, there is clearly discordance between the evidence and recommendations. Network meta‐analysis allows combination of the direct evidence and indirect evidence, and allows ranking of different treatments in terms of the different outcomes (Salanti 2011; Salanti 2012). There has been no network meta‐analysis on the comparative effectiveness of different interventions in the treatment of hereditary haemochromatosis. This systematic review and attempted network meta‐analysis provides evidence from randomised clinical trials on the role of different medical interventions in the treatment of people with hereditary haemochromatosis.
Objectives
To assess the comparative benefits and harms of different interventions in the treatment of hereditary haemochromatosis through meta‐analysis and to generate rankings of the available treatments according to their safety and efficacy. However, we found only one comparison. When more trials become available, we will attempt to conduct network meta‐analysis in order to generate rankings of the available treatments according to their safety and efficacy. This is why we retain the planned methodology for network meta‐analysis in our Appendix 1. Once data appear allowing for the conduct of network meta‐analysis, this Appendix 1 will be moved back into the Methods section.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this network meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other design because of the risk of bias in such studies. We are all aware that such exclusions make us focus much more on potential benefits and not fully assess the risks of serious adverse events as well as risks of adverse events.
Types of participants
We included participants with hereditary haemochromatosis irrespective of the method of diagnosis of the disease or the presence of symptoms. We exclude randomised clinical trials in which participants had undergone liver transplantation previously.
Types of interventions
We planned to include the following interventions that are possible treatments for hereditary haemochromatosis and can be compared with each other or with no active treatment.
The interventions that we considered were:
-
phlebotomy;
-
desferrioxamine;
-
erythrocytapheresis.
The above list was not exhaustive. If we identified any other interventions that we were not aware of, we planned to consider them as eligible and include them in the review if they were used primarily for the treatment of hereditary haemochromatosis. We excluded trials that did not include at least two or more of the included interventions.
Types of outcome measures
We planned to assess the comparative benefits and harms of available pharmacological interventions aimed at treating people with hereditary haemochromatosis for the following outcomes.
Primary outcomes
-
Long‐term mortality (time to death; maximal follow‐up).
-
Mortality:
-
short‐term mortality (up to one year);
-
medium‐term mortality (one to five years).
-
-
Adverse events (within three months after cessation of treatment). Depending on the availability of data, we attempted to classify adverse effects as serious or non‐serious. We defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (any time after commencement of treatment) (ICH‐GCP 1997). We defined a serious adverse event as any event that would increase mortality; was life threatening; required hospitalisation; resulted in persistent or significant disability; was a congenital anomaly/birth defect; or any important medical event that might have jeopardised the person or required intervention to prevent it. We used the definition used by trial authors for non‐serious and serious adverse events:
-
proportion of participants with serious adverse events;
-
number of serious adverse events;
-
proportion of participants with any type of adverse event;
-
number of any type of adverse event.
-
-
Health‐related quality of life as defined in the included trials using a validated scale such as EQ‐5D or 36‐item Short Form (SF‐36) (EuroQol 2014; Ware 2014):
-
short‐term (up to one year);
-
medium‐term (one to five years);
-
long‐term (beyond five years).
-
We planned to consider long‐term health‐related quality of life more important than short‐term or medium‐term health‐related quality of life, although short‐term and medium‐term health‐related quality of life are also important primary outcomes.
Secondary outcomes
-
Liver transplantation (maximal follow‐up):
-
proportion of participants with liver transplantation;
-
time to liver transplantation.
-
-
Decompensated liver disease (maximal follow‐up):
-
proportion of participants with decompensated liver disease;
-
time to liver decompensation.
-
-
Cirrhosis (any cirrhosis with or without clinical symptoms and with or without decompensation) (maximal follow‐up):
-
proportion of participants with cirrhosis;
-
time to cirrhosis.
-
-
Hepatocellular carcinoma (maximal follow‐up).
-
Diabetes (maximal follow‐up).
-
Cardiovascular complications such as cardiac failure, myocardial infarction, and stroke (maximal follow‐up).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 3), MEDLINE (OvidSP), Embase (OvidSP), and Science Citation Index Expanded (Web of Knowledge) (Royle 2003) from inception to 29 March 2016 for randomised clinical trials comparing two or more of the above interventions. We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched the World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/ictrp/en), which searches various trial registers, including ISRCTN and ClinicalTrials.gov on 29 March 2016. Appendix 2 shows the search strategies used.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Reviews on hereditary haemochromatosis to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (EB and MK) independently identified the trials for inclusion by screening the titles and abstracts. We sought full‐text articles for any references that at least one of the review authors identified for potential inclusion. We selected the trials for inclusion based on the full‐text articles. We planned to list the excluded full‐text references with reasons for their exclusion in the 'Characteristics of excluded studies' table. We listed any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved discrepancies through discussion and by arbitration with KG, DT, and ET.
Data extraction and management
Two review authors (EB and MK) independently extracted the following data.
-
Outcome data (for each outcome and for each treatment arm whenever applicable):
-
number of participants randomised;
-
number of participants included for the analysis;
-
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events for count outcomes, and the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
-
definition of outcomes or scale used if appropriate.
-
-
Data on potential effect modifiers:
-
participant characteristics such as age, sex, comorbidities, proportion of symptomatic participants, method of diagnosis, proportion of people with C282Y homozygosity, previous use of treatments;
-
details of the intervention and control (including dose (in the case of desferrioxamine) or target reduction (in the case of phlebotomy and erythrocytapheresis, frequency, and duration);
-
details of any cointerventions;
-
risk of bias (assessment of risk of bias in included studies).
-
-
Other data:
-
year and language of publication;
-
country in which the participants were recruited;
-
year(s) in which the trial was conducted;
-
inclusion and exclusion criteria;
-
follow‐up time points of the outcome.
-
If available, we planned to obtain the data separately for symptomatic participants and asymptomatic participants from the report. We attempted to contact trial authors when there was unclear or missing information, or when there was doubt whether trials shared the same participants, completely or partially (by identifying common authors and centres), or if we needed clarification whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in the Cochrane Hepato‐Biliary Module (Gluud 2015) to assess the risk of bias in included studies. Specifically, we assessed the risk of bias in included trials for the following domains (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
-
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
-
Unclear risk of bias: the study authors did not specify the method of sequence generation.
-
High risk of bias: the sequence generation method was not random. We planned to only include such studies for assessment of harms.
Allocation concealment
-
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
-
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
-
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We planned to only include such studies for assessment of harms.
Blinding of participants and personnel
-
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
-
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
-
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
-
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
-
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
-
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
-
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
-
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
-
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
-
Low risk of bias: the trial reported the following predefined outcomes: mortality, decompensated liver disease, requirement for transplantation, and treatment‐related adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
-
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
-
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
For‐profit bias
-
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial.
-
Uncertain risk of bias: the trial may or may not have been free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
-
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
-
Low risk of bias: the trial appeared to be free of other components (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping) that could put it at risk of bias.
-
Uncertain risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
-
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
We considered a trial at low risk of bias if we assessed the trial as at low risk of bias across all domains. We considered a trial at low risk of bias for an outcome if we assessed the trial as at low risk of bias across all study level domains. Otherwise, we considered the trials at uncertain risk of bias or at high risk of bias regarding one or more domains as at high risk of bias.
Measures of treatment effect
For dichotomous variables (e.g. short‐term and medium‐term mortality or liver transplantation, proportion of participants with adverse events, decompensated liver disease, cirrhosis, hepatocellular carcinoma, or diabetes), we calculated the odds ratio (OR) with 95% confidence intervals (CI). For continuous variables (e.g. quality of life reported on the same scale), we calculated the mean difference (MD) with 95% CI. We planned to use standardised mean differences with 95% CI for quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events), we calculated the rate ratio with 95% CI. For time‐to‐event data (e.g. long‐term mortality or requirement for liver transplantation, time to liver decompensation, and time to cirrhosis), we planned to use the hazard ratio with 95% CIs. We also calculated Trial Sequential Analysis‐adjusted CI to control random errors (Thorlund 2011).
Unit of analysis issues
The unit of analysis was the person with hereditary haemochromatosis according to the intervention group to which they were randomly assigned.
Cluster randomised clinical trials
We found no cluster randomised clinical trials. If we found them, we planned to include them provided that the effect estimate adjusted for cluster correlation was available.
Cross‐over randomised clinical trials
We found one cross‐over randomised clinical trial. We planned to only include the outcomes after the period of first treatment since hereditary haemochromatosis is a chronic disease and the treatments could potentially have a residual effect. However, the cross‐over trial did not report any outcomes prior to the cross‐over.
Trials with multiple treatment groups
We collected data for all trial treatment groups that met the inclusion criteria.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). Otherwise, we planned to use the data that were available to us (e.g. a trial might have reported only per‐protocol analysis results). As such 'per‐protocol' analyses may be biased, we planned to conduct best‐worst case scenario analyses (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analyses (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible but did not perform this because there were no post‐randomisation dropouts in either trial that provided data.
For continuous outcomes, we planned to impute the standard deviation from P values according to guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation may decrease the weight of the study for calculation of MDs and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We planned to assess the presence of clinical heterogeneity by comparing effect estimates in the presence or absence of symptoms, different targets of iron reduction, and different doses of desferrioxamine or different methods of erythrocytapheresis or phlebotomy, and the doses of the pharmacological treatments. Different study designs and risk of bias may contribute to methodological heterogeneity. If we identified substantial heterogeneity, clinical, methodological, or statistical, we explored and address heterogeneity in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity). We used the I2 test and Chi2 test for heterogeneity, and overlapping of CIs to assess heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials that could be included for a direct comparison (Egger 1997; Macaskill 2001). In the presence of heterogeneity that could be explained by subgroup analysis, we planned to produce a funnel plot for each subgroup in the presence of the adequate number of trials. We planned to use the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
We also considered selective reporting and non‐reporting of trials (identified from searching the trial registers) as evidence of reporting bias.
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011), using the software package Review Manager 5 (RevMan 2014). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In the case of a discrepancy between the two models, we have reported both results; otherwise, we have reported only the results from the fixed‐effect model.
Calculation of required information size and Trial Sequential Analysis
For calculation of the required information size, see Appendix 3. We performed Trial Sequential Analysis to control the risks of random errors (Wetterslev 2008; Thorlund 2011; TSA 2011) when there were at least two trials included in the meta‐analysis. We used an alpha error of 2.5% (Jakobsen 2014), power of 90% (beta error of 10%), a relative risk reduction of 20%, a control group proportion observed in the trials, and the diversity observed in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups.
-
Trials with low risk of bias compared to trials with high risk of bias.
-
Participants with symptomatic compared to participants with asymptomatic hereditary haemochromatosis.
-
Different targets of iron reduction.
-
Different doses of desferrioxamine or different methods of erythrocytapheresis or phlebotomy.
We planned to use the chi2 test for subgroup differences to identify subgroup differences.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to re‐analyse the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. However, we did not perform this because both trials that provided data for this review had no post‐randomisation dropouts.
Presentation of results and GRADE assessments
We reported mortality, serious adverse events, and health‐related quality of life in a 'Summary of findings' table format, downgrading the quality of evidence for risk of bias, inconsistency, indirectness, imprecision, and publication bias using GRADE (Guyatt 2011).
Results
Description of studies
Results of the search
We identified 3852 references through electronic searches of CENTRAL (N = 312), MEDLINE (N = 2245), Embase (N = 489), Science Citation Index Expanded (N = 690), World Health Organization International Clinical Trials Registry Platform (N = 30), and randomised controlled trials registers (N = 86). After the removal of 814 duplicates, we obtained 3038 references. We then excluded 3033 clearly irrelevant references through screening titles and reading abstracts. We retrieved five references for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Four of the references were reports of three trials which fulfilled the inclusion criteria of our review (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016; Characteristics of included studies table). One reference is an ongoing trial without any interim data, comparing erythrocytapheresis versus plasmapheresis (Ong 2015; Characteristics of ongoing studies table). The reference flow is summarised in the study flow diagram (Figure 1).
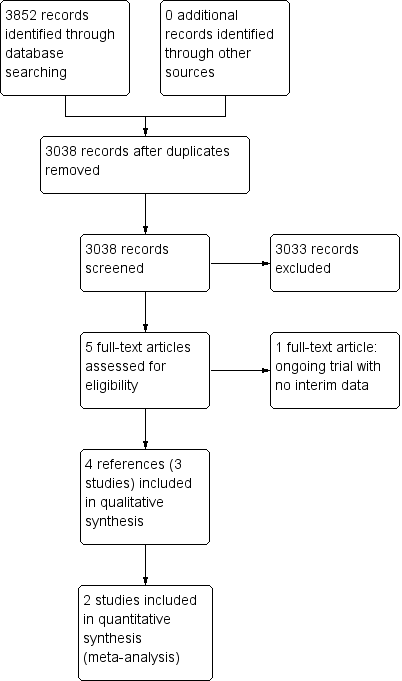
Study flow diagram.
Included studies
Three trials included 146 participants (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016). All the three trials were two‐armed and compared erythrocytapheresis versus phlebotomy. Two trials were simple parallel randomised clinical trials (Rombout‐Sestrienkova 2012; Sundic 2014). The remaining trial was a cross‐over randomised clinical trial in which participants were randomised to receive therapeutic erythrocytapheresis or phlebotomy (Rombout‐Sestrienkova 2016). After one year, the participants were crossed‐over to receive the opposite treatment (Rombout‐Sestrienkova 2016). Two trials with 100 participants provided data for analyses (Rombout‐Sestrienkova 2012; Sundic 2014).
None of the trials reported whether they included symptomatic or asymptomatic participants, or a mixture of both. Two trials which provided data for this review were conducted in people who had not undergone previous treatment for haemochromatosis (Rombout‐Sestrienkova 2012; Sundic 2014). The trial which did not provide data for this review included only people on maintenance therapy for haemochromatosis (Rombout‐Sestrienkova 2016). The trial that provided most data for this review excluded people with malignancy, heart failure, and serious cardiac arrhythmias (Rombout‐Sestrienkova 2012).
One trial carried out erythrocytapheresis bi‐weekly (not clear whether the authors meant this to be once every two weeks or twice weekly) (Sundic 2014), one trial once every two weeks (Rombout‐Sestrienkova 2012), and on trial variably depending upon serum ferritin level (Rombout‐Sestrienkova 2016). Two trials carried out phlebotomy once a week (Rombout‐Sestrienkova 2012; Sundic 2014), and one trial variably depending upon serum ferritin level (Rombout‐Sestrienkova 2016). The amount of red blood cells withdrawn during each treatment of erythrocytapheresis was 350 mL to 800 mL (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016). The amount of blood withdrawn during each treatment of phlebotomy was 450 mL to 500 mL (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016). The treatment duration in one trial was 12 weeks (Sundic 2014), was one year (after which the people crossed‐over) in one trial (Rombout‐Sestrienkova 2016), and was variable depending upon the amount of iron to be removed in one trial (Rombout‐Sestrienkova 2012). All the trials used serum ferritin level of 50 μg/L or less as the target for treatment.
The mean or median age in the trials ranged from 42 to 55 years. The proportion of females was 9.7% in Sundic 2014 and 26.3% in Rombout‐Sestrienkova 2012. Rombout‐Sestrienkova 2016 did not report this information.
Two trials that provided data for this review were funded by parties with no vested interest in the results (Rombout‐Sestrienkova 2012; Sundic 2014); the other trial did not report the source of funding (Rombout‐Sestrienkova 2016).
Excluded studies
We excluded no studies that we sought full text for.
Risk of bias in included studies
The risk of bias is summarised in Figure 2 and Figure 3. As shown in Figure 3, all the trials were at overall high risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial was at low risk of selection bias due to random sequence generation (Sundic 2014). The remaining trials were at unclear risk of random sequence generation (Rombout‐Sestrienkova 2012; Rombout‐Sestrienkova 2016). One trial was at low risk of selection bias due to allocation concealment (Rombout‐Sestrienkova 2012). The remaining trials were at unclear risk of allocation concealment (Sundic 2014; Rombout‐Sestrienkova 2016).
Blinding
None of the trials were at low risk of performance or detection bias due to blinding of participants, personnel, and outcome assessors. All the trials were at high risk of performance bias (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016). One trial was at high risk of detection bias (Rombout‐Sestrienkova 2016); the remaining two trials were at unclear risk of detection bias (Rombout‐Sestrienkova 2012; Sundic 2014).
Incomplete outcome data
Two trials were at low risk of attrition bias due to incomplete outcome data (Rombout‐Sestrienkova 2012; Sundic 2014). The remaining trial was at high risk of attrition bias due to incomplete outcome data (Rombout‐Sestrienkova 2016).
Selective reporting
One trial was at low risk of reporting bias due to selective outcome reporting (Rombout‐Sestrienkova 2012). The remaining two trials were at high risk of reporting bias due to selective outcome reporting (Sundic 2014; Rombout‐Sestrienkova 2016).
Other potential sources of bias
Two trials were at low risk of for‐profit bias (Rombout‐Sestrienkova 2012; Sundic 2014). One trial was at unclear risk of for‐profit bias since the source of funding was not reported (Rombout‐Sestrienkova 2016). All the trials were at low risk of 'other' bias.
Effects of interventions
Long‐term mortality
None of the trials reported long‐term mortality.
Mortality
One trial (38 participants) reported short‐term mortality at eight months (Rombout‐Sestrienkova 2012). There was no mortality in either group. None of the trials reported mortality beyond one year.
Serious adverse events
One trial (38 participants) reported serious adverse events (Rombout‐Sestrienkova 2012). There were no serious adverse events in either group.
All adverse events
Two trials with 100 participants reported proportion of participants with any adverse events (Rombout‐Sestrienkova 2012; Sundic 2014). The proportion of people with any adverse events in the erythrocytapheresis group was 10/49 (20.4%) versus 11/51 (21.6%) in the phlebotomy group. There was no evidence of difference in all adverse events between the groups (OR 0.93, 95% CI 0.36 to 2.43; participants = 100; trials = 2; I2 = 0%). There was no alteration in the results by using the random‐effects model.
One of these two trials with 38 participants also reported the number of adverse events (Rombout‐Sestrienkova 2012). The adverse event rate was 42.1 events per 100 participants in the erythrocytapheresis group and 52.6 events per 100 participants in the phlebotomy groups. There was no evidence of difference in all adverse events between the groups (rate ratio 0.80, 95% CI 0.32 to 2.03; participants = 38; trials = 1).
Health‐related quality of life
One trial with 38 participants reported short‐term health‐related quality of life (up to one year) (Rombout‐Sestrienkova 2012) using EQ‐VAS (EuroQol 2014) on a scale of 0 to 100 with higher numbers indicating better health‐related quality of life. There was no significant difference in health‐related quality of life between the groups (MD 1.00, 95% CI ‐10.80 to 12.80; participants = 38; trials = 1). None of the trials reported health‐related quality of life beyond one year.
Liver transplantation
None of the trials reported liver transplantation.
Decompensated liver disease
None of the trials reported decompensated liver disease.
Cirrhosis
None of the trials reported cirrhosis.
Hepatocellular carcinoma
None of the trials reported hepatocellular carcinoma.
Diabetes
None of the trials reported diabetes.
Cardiovascular complications
None of the trials reported cardiovascular complications such as cardiac failure, myocardial infarction, and stroke at maximal follow‐up.
Subgroup analysis
We did not perform any of the subgroup analyses because none of the trials were at low risk of bias, the trials did not report whether the participants were symptomatic or asymptomatic, all the trials used serum ferritin level of 50 μg/L or less as the target, and the trials used similar methods of erythrocytapheresis and phlebotomy.
Trial Sequential Analysis
Only one comparison had more than one trial and was eligible for Trial Sequential Analysis. As shown in Figure 4, the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS); therefore, the boundaries could not be drawn. There was a high risk of random errors. The TSA‐adjusted CI could not be calculated as there was too little information to be used.
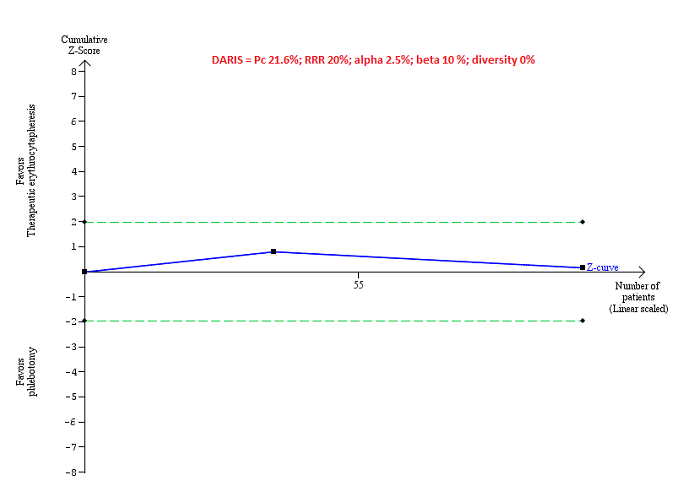
Trial Sequential Analysis of adverse events (proportion) performed using an alpha error of 2.5%, power of 90% (beta error of 10%), relative risk reduction of 20%, control group proportion (Pc) observed in trials (21.6% for proportion of people with adverse events), and observed diversity (0%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS) that the boundaries could not be drawn. The Z‐curve (blue line) does not cross the conventional boundaries (dotted green line). There was a high risk of random errors.
Reporting bias
We did not explore reporting bias using a funnel plot because of few trials included in the review.
Quality of evidence
All the evidence available was downgraded to very low quality of evidence because of the risk of bias in the trials (downgraded one level for risk of bias) and imprecision (downgraded one level for small sample size and one more level for wide CIs). There was no evidence of indirectness, heterogeneity, or publication bias. So, we did not downgrade for these domains (summary of findings Table for the main comparison).
Discussion
Summary of main results
In this review, we included three trials (146 participants) (Rombout‐Sestrienkova 2012; Sundic 2014; Rombout‐Sestrienkova 2016). However, one of these trials provide no information for this review (Rombout‐Sestrienkova 2016). Only one trial was included in most of the outcomes (Rombout‐Sestrienkova 2012). The only outcome of interest for this review and reported in more than one trial was proportion of people with adverse events (Rombout‐Sestrienkova 2012; Sundic 2014). There was no short‐term mortality or serious adverse events in either group in the one trial that reported these outcomes (Rombout‐Sestrienkova 2012). There were no statistically significant differences between erythrocytapheresis and phlebotomy in the proportion of people with adverse events, number of adverse events, and short‐term health‐related quality of life. None of the trials reported mortality beyond one year, health‐related quality of life beyond one year, liver transplantation, decompensated liver disease, cirrhosis, hepatocellular carcinoma, diabetes, or cardiovascular complications in the long‐term. In summary, there was no evidence of a difference between erythrocytapheresis and phlebotomy in people with hereditary haemochromatosis.
Overall completeness and applicability of evidence
We planned to include all treatments used for hereditary haemochromatosis but found that the only comparison reported was erythrocytapheresis versus phlebotomy. The current recommended treatment for hereditary haemochromatosis is phlebotomy and the trials were conducted relatively recently. Therefore, the findings of this review are applicable in the current clinical setting. The trials did not report whether the participants were symptomatic or asymptomatic. They probably included participants who required blood letting of some form regardless of symptoms based on the ferritin and transferrin levels. Therefore, the findings of the review are likely to be applicable in symptomatic and asymptomatic people. Both the trials that contributed data for this review included only treatment‐naive people, that is, people who had not received previous treatments for hereditary haemochromatosis. Therefore, the findings of the review are applicable only in people with hereditary haemochromatosis who had not received treatment previously. Finally, the trial that provided most of the information for this review excluded people with malignancy, serious cardiac arrhythmias, heart failure, and epilepsy. Therefore, the findings of this review are not applicable in such people.
Phlebotomy requires minimal equipment while erythrocytapheresis requires special equipment to perform the procedure. Since there is no evidence to suggest that erythrocytapheresis is beneficial versus phlebotomy, there is no need for hospitals to buy special equipment, based on currently available evidence.
Quality of the evidence
The overall quality of evidence was very low for all the outcomes. All the trials were at high risk of bias, mainly because blinding of participants and healthcare providers was not performed in any of the trials. The sample size was small for all the comparisons. The only outcome in which Trial Sequential Analysis was attempted showed that the sample size was less than 5% of the required information size to identify a relative risk reduction of 20%. There were also wide CIs for all the comparisons.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions with two review authors independently selecting studies and extracting data. We performed a thorough search of literature. However, the search period included the pre‐mandatory trial registration era and it is possible that we missed some trials on treatments that were not effective or were harmful or were not reported at all.
We only included randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. According to our choice of studies (i.e. only randomised clinical trials), we might have missed a large number of studies that address reporting of harms. Accordingly, this review is biased towards benefits ignoring harms. We did not search for interventions and trials registered at regulatory authorities (e.g., FDA (US Food and Drug Administration); EMA (European Medicines Agency), etc). This may have overlooked trials and as such trials usually are unpublished, the lack of inclusion of such trials may make our comparisons look more advantageous than they really are.
Agreements and disagreements with other studies or reviews
There have been no previous systematic reviews on this topic. The authors of the trial that provided most information for this review concluded that erythrocytapheresis is a highly effective treatment to reduce iron overload, and that from a societal perspective, it might potentially also be a cost‐saving therapy (Rombout‐Sestrienkova 2012). Our findings show that it is too early to know whether erythrocytapheresis is beneficial or harmful compared with phlebotomy. While we did not collect the cost information for this review, we noted that the costs of purchasing the equipment and maintenance of the equipment was not included in the cost calculations. This is likely to alter the conclusions about the difference between treatment costs. It is also not clear whether blinding of participants was performed. Lack of blinding of participants to treatment may cause biased estimate of the costs related to productivity loss. Therefore, the existing evidence did not allow us to support the trial authors' conclusions.
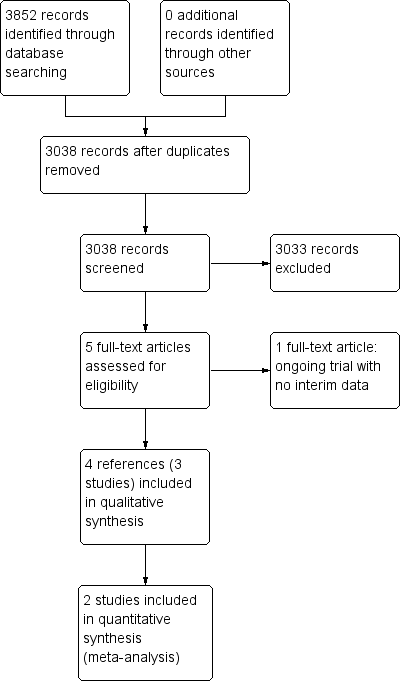
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
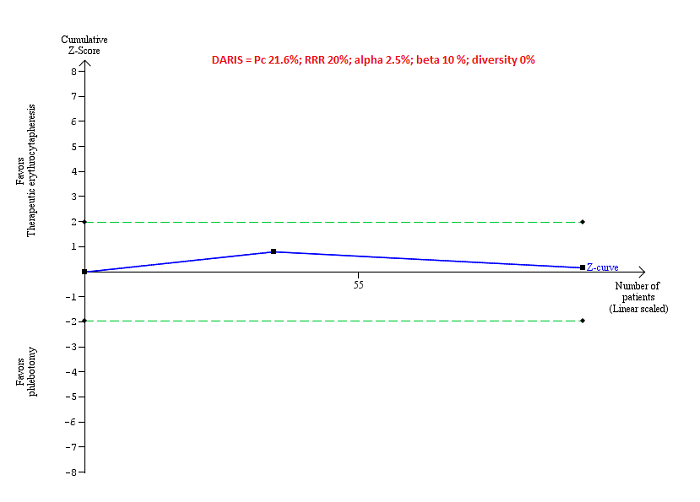
Trial Sequential Analysis of adverse events (proportion) performed using an alpha error of 2.5%, power of 90% (beta error of 10%), relative risk reduction of 20%, control group proportion (Pc) observed in trials (21.6% for proportion of people with adverse events), and observed diversity (0%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS) that the boundaries could not be drawn. The Z‐curve (blue line) does not cross the conventional boundaries (dotted green line). There was a high risk of random errors.
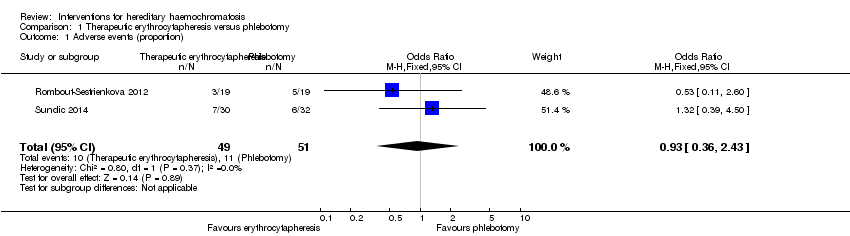
Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 1 Adverse events (proportion).
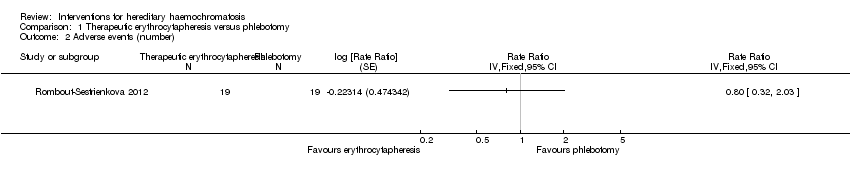
Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 2 Adverse events (number).

Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 3 Health‐related quality of life (EQ‐VAS).
| Erythrocytapheresis versus phlebotomy for hereditary haemochromatosis | |||||
| Patient or population: people with hereditary haemochromatosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Phlebotomy | Therapeutic erythrocytapheresis | ||||
| Long‐term mortality | None of the included trials reported mortality beyond 1 year. | ||||
| Mortality Follow‐up period: 8 months | There was no mortality in either group in the short‐term in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Serious adverse events Follow‐up period: 8 months | There were no serious adverse events in either group in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Health‐related quality of life Follow‐up period: 8 months | The mean health‐related quality of life in the control groups was | The mean health‐related quality of life in the intervention groups was | ‐ | 38 | ⊕⊝⊝⊝ |
| Health‐related quality of life beyond one year | None of the included trials reported health‐related quality of life beyond one year | ||||
| *The basis for the assumed risk is the mean control group proportion or control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level for risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events (proportion) Show forest plot | 2 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.43] |
| 2 Adverse events (number) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 Health‐related quality of life (EQ‐VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

