Antibióticos sistémicos para el tratamiento de las heridas neoplásicas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011609.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Darshini Ramasubbu: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; checked quality assessment; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; performed previous work that was the foundation of the current review; wrote to study author/experts/companies; provided data; performed economic analysis; performed translations; approved the final review prior to submission and is a guarantor of the review.
Valerie Smith: conceived and designed the review; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review and approved the final review prior to submission.
Fiona Hayden: made an intellectual contribution to the review; advised on the review and approved the final review prior to submission.
Patricia Cronin: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; made an intellectual contribution to the review; performed previous work that was the foundation of the current review; wrote to study author/experts/companies and approved the final review prior to submission.
Contributions of editorial base:
Andrea Nelson (Editor): edited the protocol; advised on methodology interpretation and content; approved the final protocol prior to submission.
Gill Norman (Editor): edited the review; advised on methodology interpretation and content; approved the final review prior to submission.
Gill Rizzello and Sally Bell‐Syer (Managing Editors): co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and the review.
Zipporah Iheozor‐Ejiofor (Methodologist): advised on methodology in the review.
Reetu Child and Naomi Shaw (Information Specialists): designed the search strategy, edited the search methods section and ran the searches for the review.
Ursula Gonthier (Editorial Assistant): edited the plain language summary and reference sections of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Darshini Ramasubbu: none known.
Valerie Smith: none known.
Fiona Hayden: none known.
Patricia Cronin: none known.
Acknowledgements
The review authors would like to thank Cochrane Wounds for their support in undertaking this review, copy editors Elizabeth Royle and Jason Elliot‐Smith for their helpful feedback, and the following peer referees for their comments: Julie Bruce, Jane Nadel (protocol and review); Andrew McKean (review); Mark Rodgers and Gill Worthy (protocol).
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 24 | Systemic antibiotics for treating malignant wounds | Review | Darshini A Ramasubbu, Valerie Smith, Fiona Hayden, Patricia Cronin | |
| 2015 Apr 07 | Systemic antibiotics for treating malignant wounds | Protocol | Darshini A Ramasubbu, Valerie Smith, Fiona Hayden, Patricia Cronin | |
Differences between protocol and review
We updated our search strategies adding terms for smelly and malodorous tumours.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
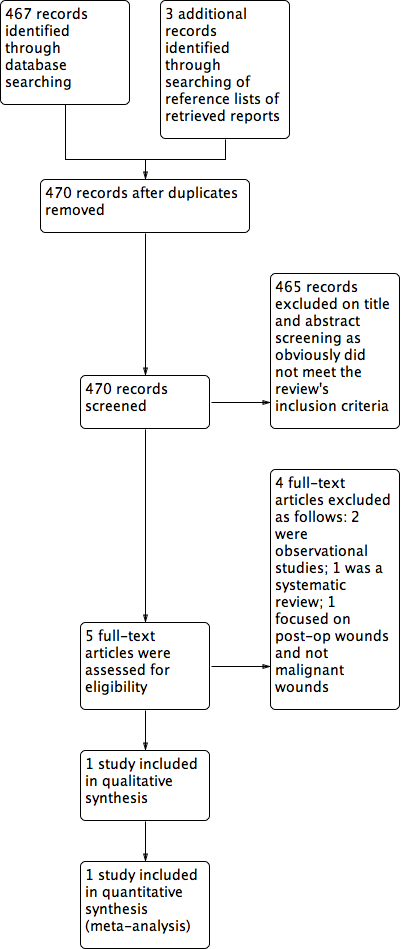
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
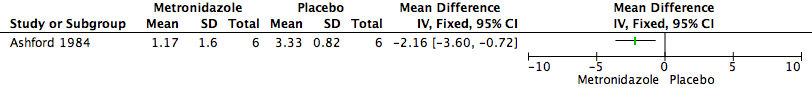
Forest plot of comparison: 1 Metronidazole versus Placebo, outcome: 1.1 Malodour (Smell Score).
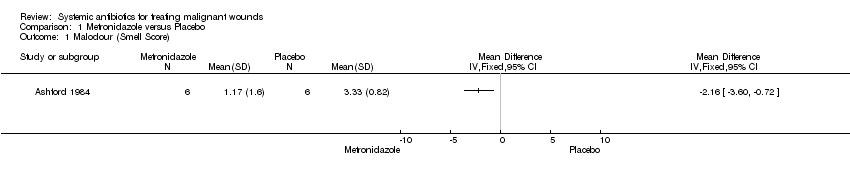
Comparison 1 Metronidazole versus Placebo, Outcome 1 Malodour (Smell Score).
| Metronidazole compared to Placebo for treating malignant wounds | ||||||
| Patient or population: treating malignant wounds | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Placebo | Risk with Metronidazole | |||||
| Malodour (smell score measured on a scale of 0 to 3 with higher scores indicating a more offensive smell) | The mean malodour (smell score) was 3.33 (range 2.0 to 4.0) | MD 2.16 lower | ‐ | 6 | ⊕⊝⊝⊝ | It is uncertain whether metronidazole leads to a reduction in malodour because the quality of the evidence is very low |
| Adverse effects | Study population | not estimable | 6 | NA | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 3 levels: serious limitation — insufficient details provided to make clear judgements on random sequence generation and allocation concealment. There was also a 33% loss to follow‐up; very serious imprecision; a small sample size of 6 participants. 2 Smell was independently assessed at each visit by the patient, doctor, and nurse, who graded the smell as "absent" (0), "not offensive" (1), "offensive but tolerable" (2), or "offensive and intolerable" (3), and an amalgamated score calculated. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Malodour (Smell Score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

