Antibióticos sistémicos para el tratamiento de las heridas neoplásicas
Resumen
Antecedentes
Las heridas neoplásicas son una complicación devastadora del cáncer. Se desarrollan generalmente en los últimos seis meses de vida, en la mama, la pared torácica o las regiones de la cabeza y el cuello. Son muy difíciles de tratar de forma exitosa, y los síntomas comúnmente asociados del dolor, el exudado, el mal olor y el riesgo de hemorragia son sumamente angustiantes para las pacientes con cáncer avanzado. El tratamiento y la atención de las heridas neoplásicas son principalmente paliativos, y se enfoca en el alivio del dolor, el control de la infección y el olor de la herida, el control del exudado y la protección de la piel circundante para evitar el deterioro adicional. En las heridas neoplásicas, con deterioro tisular y muerte, hay proliferación de bacterias tanto anaerobias como aerobias. El objetivo del tratamiento con antibióticos es eliminar con éxito dichas bacterias, reducir los síntomas asociados, como el olor, y promover la cicatrización de la herida.
Objetivos
Evaluar los efectos de los antibióticos sistémicos para el tratamiento de las heridas neoplásicas.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos electrónicas el 8 de marzo de 2017: registro especializado del Grupo Cochrane de Heridas (Cochrane Wounds Specialised Register), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; the Cochrane Library, 2017, número 3), Ovid MEDLINE, Ovid Embase y EBSCO CINAHL Plus. También se hicieron búsquedas en registros de ensayos clínicos de la World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch) y ClinicalTrials.gov el 20 marzo 2017; y OpenSIGLE (para identificar la literatura gris) y ProQuest Dissertations & Theses Global (para recuperar las tesis de disertaciones relacionadas con el tema de interés) el 13 de marzo de 2017.
Criterios de selección
Los ensayos controlados aleatorios que evaluaron los efectos de cualquier antibiótico sistémico sobre las heridas neoplásicas reunieron los requisitos para la inclusión.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron y seleccionaron los ensayos para la inclusión, evaluaron el riesgo de sesgo y extrajeron los datos de los estudios. Un tercer autor de la revisión examinó los datos extraídos en cuanto a la exactitud antes del análisis.
Resultados principales
Sólo se identificó un estudio para su inclusión en esta revisión. Este estudio fue un ensayo prospectivo cruzado (cross‐over) y doble ciego que comparó el efecto del metronidazol sistémico con un placebo en el olor en las heridas neoplásicas. Se incluyeron nueve participantes con una herida fungiforme y para las que el olor era molesto y seis de las mismas completaron los estadios tanto de intervención como de control (placebo) del ensayo. Cada estadio se prolongó durante catorce días, con una brecha de catorce días (período de lavado) entre la administración del metronidazol y el placebo.
El estudio, al comparar metronidazol y placebo, informó dos de los resultados primarios predeterminados de esta revisión (mal olor y efectos adversos del tratamiento) y ninguno de los resultados secundarios predeterminados de la revisión.
Mal olor
Las puntuaciones medias del mal olor para el grupo de metronidazol fueron de 1,17 (desviación estándar [DE] 1,60) y la media para el grupo de placebo fue de 3,33 (DE 0,82). No está claro si los antibióticos sistémicos se asociaron con una diferencia en el mal olor (un estudio con seis participantes; MD —2.16, 95% CI —3.6 to —0.72) debido a que la calidad de la evidencia (GRADE) fue muy baja para este resultado. La calidad del estudio se disminuyó debido al alto riesgo de sesgo de deserción (pérdidas durante el seguimiento del 33%) y la imprecisión muy grave debido al tamaño de la muestra pequeño.
Efectos adversos
Los autores del ensayo no informaron efectos adversos del tratamiento en el grupo de intervención ni de control.
Conclusiones de los autores
No se conoce si el metronidazol sistémico da lugar a una reducción del mal olor en las pacientes con heridas neoplásicas. Este hecho se debe a que sólo fue posible incluir un único estudio en riesgo alto de sesgo con un tamaño de la muestra muy pequeño, que se centró sólo en las pacientes con cáncer de mama. Se necesita más investigación para fundamentar estos hallazgos y para investigar los efectos del metronidazol sistémico y otros antibióticos en la calidad de vida, el alivio del dolor, el exudado y la contención tumoral en pacientes con heridas neoplásicas.
PICOs
Resumen en términos sencillos
Antibióticos sistémicos para el tratamiento de las heridas neoplásicas
Pregunta de la revisión
Se examinó la evidencia acerca del efecto de los antibióticos sistémicos sobre las heridas neoplásicas. Se realizaron búsquedas de evidencia en relación con los posibles efectos secundarios de este tratamiento, y el impacto sobre la calidad de vida y otros síntomas.
Antecedentes
Las heridas neoplásicas ocurren en los pacientes con cáncer avanzado. Por lo general se desarrollan en los últimos seis meses de vida, en el sitio del tumor o cerca del mismo. Ocurren cuando un tumor se propaga e invade la piel y los vasos sanguíneos circundantes, y causa que estos últimos se rompan. El área pierde nutrición debido al flujo sanguíneo deficiente, y con el tiempo los tejidos mueren, lo cual da lugar a una herida neoplásica. Este tipo de herida puede ser muy dolorosa, puede tener mal olor y puede sangrar o exudar líquido. Estos síntomas pueden ser muy difíciles para los pacientes con cáncer avanzado. El tratamiento para las heridas neoplásicas normalmente no procura cicatrizar la herida, sino limitar los síntomas que afectan la calidad de vida del paciente.
Los antibióticos son fármacos que combaten las infecciones bacterianas. Los antibióticos sistémicos afectan el cuerpo entero. Pueden administrarse por vía oral en forma de comprimidos, o de otras maneras como a través de inyecciones. Se realizaron búsquedas de evidencia sobre si los antibióticos sistémicos pueden prevenir el empeoramiento de las heridas neoplásicas, y ayudar a reducir el mal olor, el dolor y otras complicaciones asociadas con estas heridas.
Características de los estudios
En marzo de 2017 se realizaron búsquedas de ensayos controlados aleatorios que consideraban los efectos de los antibióticos sistémicos sobre las heridas neoplásicas. Se encontró sólo un ensayo, realizado en 1984; que comparaba la efectividad del antibiótico metronidazol con un placebo (pastilla de azúcar) en seis participantes con heridas neoplásicas causadas por el cáncer de mama. El ensayo era un ensayo cruzado (cross‐over) que significa que los participantes reciben tanto el tratamiento que se está evaluando como el tratamiento de comparación, en diferentes puntos temporales, con una interrupción entre los tratamientos para asegurar que los efectos del primer tratamiento hayan desaparecido antes del segundo tratamiento. Este período se denomina "de lavado". En el único ensayo de esta revisión, la mitad los participantes recibieron primero el antibiótico, durante 14 días, y la mitad recibió placebo. Luego ambos grupos tuvieron 14 días sin medicación antes de intercambiar (cruzamiento) y probar el tratamiento alternativo durante 14 días.
Resultados clave
No está claro si el metronidazol reduce el mal olor en las heridas neoplásicas cuando se lo administra por vía oral en forma de comprimidos, sin que ocurran efectos secundarios. Su efectividad con relación a otros resultados como el dolor o la calidad de vida no se midió en este ensayo. No se informó un cambio en el tamaño ni la apariencia de los tumores de los participantes.
Calidad de la evidencia
No se conoce si el metronidazol reduce el mal olor en las heridas neoplásicas cuando se lo administra por vía oral en forma de comprimido debido a que la calidad de la evidencia es muy baja. Esta evidencia provino de un estudio muy pequeño con fallas graves en el diseño de estudio, y se necesita más investigación que incluya a más pacientes con diferentes tipos de cáncer. También se necesitan ensayos que consideren cómo los antibióticos pueden afectar otros resultados, como la calidad de vida, el alivio del dolor y la reducción de cualquier hemorragia o exudado de la herida.
Este resumen en términos sencillos está actualizado hasta marzo de 2017.
Authors' conclusions
Summary of findings
| Metronidazole compared to Placebo for treating malignant wounds | ||||||
| Patient or population: treating malignant wounds | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Placebo | Risk with Metronidazole | |||||
| Malodour (smell score measured on a scale of 0 to 3 with higher scores indicating a more offensive smell) | The mean malodour (smell score) was 3.33 (range 2.0 to 4.0) | MD 2.16 lower | ‐ | 6 | ⊕⊝⊝⊝ | It is uncertain whether metronidazole leads to a reduction in malodour because the quality of the evidence is very low |
| Adverse effects | Study population | not estimable | 6 | NA | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 3 levels: serious limitation — insufficient details provided to make clear judgements on random sequence generation and allocation concealment. There was also a 33% loss to follow‐up; very serious imprecision; a small sample size of 6 participants. 2 Smell was independently assessed at each visit by the patient, doctor, and nurse, who graded the smell as "absent" (0), "not offensive" (1), "offensive but tolerable" (2), or "offensive and intolerable" (3), and an amalgamated score calculated. | ||||||
Background
Description of the condition
Malignant wounds, which are also referred to as fungating wounds, ulcerating tumours or neoplastic (new growth) lesions (Adderley 2014), are a devastating complication of cancer that most often arises in the last six months of life (McDonald 2006). These wounds arise when tumour cells infiltrate the skin and its blood and lymph vessels from a local or distant primary tumour. Carcinomas of the breast in women and lung cancer in men are the most common sources of primary tumours that lead to these cutaneous (skin) metastases. They are also caused by primary cutaneous tumours, or by direct invasion of a primary tumour into the cutaneous structure (Alexander 2009). Globally, malignant wounds occur most frequently on the breast or chest wall (39% to 62%), with head and neck (24% to 33.8%), back, trunk or abdomen (1% to 3%), axilla or groin (3% to 7%) and genitals (3% to 5%) also being common sites (McDonald 2006). Although accurate statistics on, or reports of, their prevalence are not available, it is estimated that malignant wounds are present in 5% to 10% of people with cancer (Seaman 2006).
Malignant wounds present a particularly challenging clinical scenario, as they are very difficult to treat successfully. Alexander 2009 claims that reports of healed malignant wounds are very rare. In addition, the commonly associated symptoms of pain, copious exudate, malodour, and the risk of haemorrhage are extremely distressing for people with advanced cancer.
Initial presentation varies depending on whether tumour invasion is as a result of the extension of a local tumour to the skin surface or as a metastasis (spread) of a primary tumour. In the former, inflammation with induration (hardening), and redness or tenderness — or both — may be present. When skin is affected as a result of metastasis, well‐defined nodules that vary in size, colour and consistency but are generally painless, become visible. These nodules may be fixed to underlying tissue. In both cases, as the tumour spreads and extends above the skin surface, further tissue destruction occurs. The wound takes on an erosive and ulcerative appearance, or a fungus/cauliflower‐like appearance, or both (McDonald 2006). These wounds are thought to interfere with oxygenation of the tissues, lymphatic drainage and haemostasis (maintenance of the healthy state of tissue). Tissue hypoxia (low oxygen levels) leads to lack of oxygen in the local cells, which sometimes leads to cell death (Adderley 2014; Mortimer 2003). The resulting wound bed can vary in colour from pale to pink, with the tissue being very friable (easily torn, fragmented or made to bleed) or necrotic (dead cells or tissue), or a combination of both (Seaman 2006). The surrounding skin can be macerated (i.e. softened and broken down due to prolonged exposure to wetness), fragile and very tender to the touch.
The necrotic tissue of the wound bed becomes an ideal medium for anaerobic bacteria, which grow and flourish in areas of low oxygen concentrations. Their metabolic activities may account, in part, for the odour that patients find extremely distressing. Cell growth in malignant wounds can be very rapid and may affect the pH of extracellular fluid (Adderley 2014). This interferes with coagulation of blood, and vessel occlusion and necrosis may result. Disruption of the lymphatic system can lead to vascular collapse, hypoxia, necrosis, build‐up of waste and oedema (fluid retention). Excessive wound exudate, which can amount to over a litre per day in some individuals, results from a combination of vascular, bacterial and lymphatic factors.
Unless the underlying malignancy can be treated effectively, medically or surgically, the fungation in a malignant wound will continue to grow; and, through loss of vascularity, proliferative growth and ulceration, further damage to the surrounding skin occurs (EONS 2015). Wound‐related symptoms commonly include odour, haemorrhage, pain and exudate, with odour, pain and exudate, in particular, presenting significant challenges for wound management (Gethin 2014). Care and treatment of these wounds is primarily palliative, with care and management focusing on problem solving to control and reduce the overall impact of wound symptoms and provision of physical, psychosocial and psychological support to patients and their families (EONS 2015). There is currently a lack of evidence, overall, to direct the clinical management of wound odour and local colonisation, with a ‘trial and error’ approach most frequently adopted (Gethin 2014). Nonetheless, a recent publication by the European Oncology Nursing Society recommends that wound cleansing and irrigation, debridement (non‐surgical), metronidazole topically or orally, activated charcoal and antimicrobial (silver) dressings, frequent dressing changes (twice a day) and the use of opiates for pain management can help with effectively managing the most distressing symptoms (EONS 2015).
Description of the intervention
Antibiotics are agents that are used to kill or prevent the growth of disease‐causing micro‐organisms without causing damage to the host (patient). This selective toxicity depends on targeting differences between the host cell and that of the infecting micro‐organism. While the term 'micro‐organisms' includes bacteria, viruses and fungi, bacteria account for most infectious disease. Most antibiotics work by exploiting differences between bacterial and host cells — such as synthesis of proteins, DNA, RNA (ribonucleic acid), and peptidoglycan in bacterial cell walls (Rang 2003).
Systemic antibiotics, which affect the whole body, can be used to improve symptom management, reduce further deterioration and improve quality of life for those with malignant wounds (Alexander 2009). Systemic administration of antibiotic agents, rather than topical (surface) application to the infected area, can take place through a variety of routes. The route selected for systemic administration depends on factors relating to the infection, drugs and the patient.
Systemic antibiotics can be administered orally, rectally, transdermally (through the skin), sublingually (under the tongue) and via the buccal route (between gums and inner face of the cheek). In addition they can be delivered parenterally directly into veins, muscles and joints (intravenous, intramuscular and intra‐articular routes, respectively). Topical antibiotics are applied to the wound site, acting directly on the site of application. Thus use of topical antibiotics will not lead to blood or tissue drug concentration levels. In this sense, topical agents are 'surface' agents rather than systemic agents. Transdermal antibiotics are applied to intact skin away from the wound site, and are absorbed into the body through the skin and mucous membranes, leading to drug levels in circulating blood. They are intended to act on an area of the body away from the site of application. The most common routes of administration for systemic antibiotics are the oral, intravenous, intramuscular and rectal routes. The oral route is suitable for well‐absorbed drugs, and is generally used for less severe infections; it is suitable if the patient can swallow and has no vomiting, nausea or gastrointestinal contraindications (conditions that would make the drug or route unsuitable). Generally, more severe infections require intravenous treatment. The intravenous route is also used if the drug is poorly absorbed when taken orally. If a patient cannot swallow or is vomiting, then parenteral or rectal routes of administration may be more suitable (British National Formulary 2017).
How the intervention might work
In malignant wounds, tissue degradation can lead to proliferation of both anaerobic and aerobic bacteria. Successful elimination of bacteria may reduce associated symptoms, such as odour, and promote wound healing. Antibiotics may be described as bacteriostatic (halting the growth of bacteria) or bactericidal (toxic to bacteria). Antibiotics achieve this effect by exploiting differences between the structure and biochemistry of microbial cells and human cells.
Anaerobic proliferation (rapid increase in bacteria that do not grow or live when oxygen is present) is common in malignant wounds and, although many antibiotics target anaerobic bacteria, metronidazole — a bactericidal antibiotic — is the most commonly used topical antibiotic for these wounds. It may also be used systemically against these bacteria, and appears to be the antibiotic of choice. When given systemically, it is reduced to its active form, which binds to bacterial DNA, inactivating it and preventing DNA synthesis. Metronidazole is highly active against anaerobic bacteria (Löfmark 2010), the growth of which may be facilitated by tissue hypoxia (deficiency in oxygen) in wounds. Metronidazole is used systemically by the oral, intravenous and rectal routes. Adverse reactions to metronidazole include nausea, vomiting, anorexia (loss of appetite) and oral mucositis (inflammation of the lining of the digestive tract). It interacts with other drugs including alcohol, warfarin and phenytoin. It should be used with caution in those with hepatic impairment (British National Formulary 2017). Other antibiotics may also be used systemically, depending on the type of bacteria present in the wound.
In malignant wounds, loss of vascularity (blood supply) and subsequent necrosis (death of the tissue) caused by tumour invasion may compromise the distribution, penetration and efficacy of systemic antibiotics such as metronidazole (Grocott 2001a).
Why it is important to do this review
A Cochrane Review on malignant wounds, titled ‘Topical agents and dressings for fungating wounds’, concluded that there was weak evidence to suggest that a 6% solution of miltefosine might slow progression of small, superficial fungating wounds on the breast, but that ultimately more research was needed in this area (Adderley 2014). Systemic treatments for malignant wounds have focused on various regimens of antibiotics, primarily metronidazole, aimed at slowing disease progression, alleviating symptoms and optimising the quality of life of the patient. We hoped that this review would expand the current evidence base regarding treatment options and, ideally, contribute to clinical decision making for those with malignant wounds and potentially identify the most effective treatment and management regime.
Objectives
To assess the effects of systemic antibiotics for treating malignant wounds.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that assessed the effects of systemic antibiotics on malignant wounds were eligible for inclusion. In the absence of RCTs, we would have considered including controlled clinical trials (CCTs) that assessed the effects of systemic antibiotics in the treatment of malignant wounds, with a concurrent control group and prospective baseline assessment of the malignant wound in the review, but no eligible CCTs were identified.
Types of participants
Studies involving people of any age with a clinically diagnosed malignant wound resulting from any type of cancer were eligible for inclusion. Where differences in the definitions of a malignant wound existed, we accepted the study authors’ diagnosis as definitive.
Types of interventions
Studies evaluating the effects of any systemic antibiotic used in the treatment of any type of malignant wound were eligible for inclusion. This would have involved including studies that compared systemic antibiotics with a placebo, no treatment, or a topical antibiotic; and studies that compared one systemic antibiotic with another systemic antibiotic.
Types of outcome measures
Primary outcomes
-
Malodour (measured by a validated assessment tool, e.g. the TELER system (Grocott 2001b) which assesses malignant wounds using a 0 to 5 ordinal scale on a number of indicators, for example skin condition, wound exudate and odour. For odour, a score of 0 indicates odour that is obvious in the house/hospital ward. A score of 5 indicates no odour).
-
Adverse effects of treatment (e.g. nausea, vomiting, rash or any other adverse effect as reported by the trial authors).
Secondary outcomes
-
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D (Brooks 1996), SF‐36 (Jenkinson 1996), or a disease‐specific questionnaire).
-
Exudate/haemorrhage (number of dressing changes).
-
Pain relief (measured using visual analogue scale or other validated pain‐measuring scale).
-
Cost (including measurements of resource use, where reported).
-
Malignant tumour containment or regression as measured by time to progression (WHO 1979).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
-
the Cochrane Wounds Specialised Register (searched 8 March 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) in the Cochrane Library (searched 8 March 2017);
-
MEDLINE Ovid including In‐Process & Other Non‐Indexed Citations (1946 to 8 March 2017);
-
Embase Ovid (1974 to 8 March 2017);
-
CINAHL EBSCO Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 8 March 2017).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2017). There were no restrictions with respect to language, date of publication or study setting. We also searched the following clinical trials registries:
-
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 20 March 2017);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx) (searched 20 March 2017).
Search strategies for clinical trial registries can be found in Appendix 1.
Searching other resources
We reviewed the reference lists of all included papers to identify any additional potentially eligible studies that were not captured by the electronic searches as well as relevant systematic reviews, meta‐analyses and Health Technology Assessment reports. We searched the OpenSIGLE database to identify grey literature and the ProQuest Dissertations & Theses Global database to retrieve dissertation theses related to our topic of interest. We also contacted experts in the field to identify any unpublished studies. We searched for conference abstracts or publications from the European Wound Management Association (1991 to 2016), the European Wound Management Association Journal since its inception in 2001, the Wound Care UK (www.wounds‐uk.com) website and journal (2005 to 2016) and the International Conference on Wound Management (1999 to 2016).
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Ramasubbu 2015), which are based on methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Independently, two review authors (DR and PC) assessed the titles and abstracts of citations identified by the search strategy. The review authors were not blinded to the study authors or to the name of the publication. Full reports of all potentially relevant studies were retrieved for further assessment of eligibility based on the inclusion criteria. Had any disagreements occurred, we had planned to resolve them through discussion and consensus, or, where necessary, in consultation with a third review author (VS); however, this was not required.
Data extraction and management
Two review authors (DR and PC) extracted data independently using a pre‐designed agreed data extraction form. A third review author (VS) checked the extracted data for accuracy. Where information regarding data was unclear (one study), we contacted the lead author of the original trial report for further details; however, we did not receive a response.
Assessment of risk of bias in included studies
Independently, two review authors (DR and PC) assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other biases (e.g. extreme baseline imbalance; see Appendix 2 for details of criteria on which the judgement was based). Blinding and completeness of outcome data were assessed for each outcome separately. We completed a ‘Risk of bias’ table for the included study. We had planned to discuss any disagreements amongst all review authors to achieve a consensus should they have arisen; however, this was not required. We present the assessment of risk of bias using a ‘Risk of bias’ summary figure that presents the judgements for each risk of bias criterion.
Measures of treatment effect
Dichotomous data
For dichotomous data, (e.g. specific adverse events that are present or absent), we planned to present the results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we present the results as mean difference (MD) with 95% CI. When pooling data across studies, we had planned to estimate the MD if the outcome(s) were measured in the same way between trials, and use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods. However, as only one trial was included in the review, this was not applicable.
Time‐to‐event data
For time‐to‐event data such as time to wound progression, we had planned to use methods of survival analysis and present the results as a hazard ratio (HR) with 95% CI. As time‐to‐event data were not reported in the one included study in the review, this was not applicable.
Unit of analysis issues
Multiple ulcers in the same participant
Where included studies evaluated the effect of the treatment on multiple ulcers in the same participant we had planned to seek further statistical advice on how to handle and analyse these data; or, where we were unable to include these data in a meta‐analysis, we would have reported the results using narrative synthesis. As one trial only was included in the review and focused on one wound per participant only, this was not applicable. We will consider this in future updates of the review, if indicated.
Dealing with missing data
We noted the levels of attrition for the included study. Using sensitivity analysis, we had planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect. For all outcomes we planned to carry out analyses as far as possible on an intention‐to‐treat basis. For dichotomous data, where participant outcome data were missing, we had planned to make an assumption that the outcome concerned was likely not to have occurred. For continuous outcome data (e.g. quality of life), the denominator for each outcome in each trial would have been the number randomised minus any participants whose outcomes were known to be missing; we had planned to use the numbers reported in the study. Dealing with missing data was not necessary in the review; we will consider this, if indicated, in future updates.
Assessment of heterogeneity
Had more than one study been included in this review, we would have assessed statistical heterogeneity in each meta‐analysis, where performed, using the I² statistic (Higgins 2011b). We planned to interpret the I² statistic according to the following thresholds:
-
0% to 40%: might not be important;
-
40% to 60%: may represent moderate heterogeneity;
-
more than 60%: may represent substantial heterogeneity.
We will apply this interpretation to future updates should additional studies be included in future meta‐analyses.
We had planned to explore identified heterogeneity visually using forest plots and analytically using sub‐group analyses (e.g. for clinical variation such as type of cancer) and random‐effects meta‐analyses; we will apply this, if indicated, in future updates.
Assessment of reporting biases
To address the potential for publication of positive, statistically significant results, we searched the grey literature comprehensively for unpublished data; however, no additional unpublished studies meeting the review's inclusion criteria were found. Had data from unpublished studies been identified, we would have included this data cautiously, recognising unpublished reports as a possible source of bias, and would have contacted the original trialists for further information as necessary; we will consider this, if indicated, in future updates.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). Had more than one study been included in the review, we would have synthesised data only where we thought it was appropriate to do so, that is where trials were examining similar interventions, with similar populations and methods. We would have used a fixed‐effect approach for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is where trials were examining the same intervention, and the trials’ populations and methods were sufficiently similar. Where there was clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may have differed between trials, random‐effects meta‐analysis would have been used; we will apply this data synthesis plan to future updates.
Subgroup analysis and investigation of heterogeneity
If sufficient studies on diverse populations had been identified as eligible for inclusion, we would have attempted to conduct the following subgroup analyses for systematic antibiotics for malignant wounds according to:
-
organ affected by cancer;
-
drug used;
-
dose of drug;
-
duration of treatment with drug.
We will continue to consider these subgroup analyses in future updates.
Sensitivity analysis
Had more than one study been included in the review, we would have carried out sensitivity analyses to explore whether analysing studies stratified by quality (low risk of bias and high risk of bias) produces similar or different results. We would have carried out a sensitivity analysis by excluding those studies at an overall high risk of bias from the meta‐analysis (as defined in Assessment of risk of bias in included studies); we will consider this analysis, if indicated, in future updates.
'Summary of findings' tables
We assessed the quality of the results for the primary outcomes of the review in a 'Summary of findings' table (summary of findings Table for the main comparison). A 'Summary of findings' table presents key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' table:
-
malodour
-
adverse effects of the treatment.
Results
Description of studies
See Characteristics of included studies table
Results of the search
The search yielded a total of 470 citations (467 from database searching and 3 from searching of additional sources), of which 415 were excluded on the basis of title or title and abstract as they did not investigate the use of systemic antibiotics in the treatment of malignant wounds (Figure 1). This resulted in the retrieval of five full‐text reports for possible inclusion in the review (Ashford 1984; Dankert 1981; De Castro 2015; McGregor 1982; Sparrow 1980).

Study flow diagram.
Included studies
One study only met the inclusion criteria (Ashford 1984). This study was a prospective, double‐blind cross‐over trial that compared the effect of systemic metronidazole with a placebo on odour in malignant wounds. Nine participants with a fungating wound and for whom the smell was troublesome were recruited and six of these completed both the intervention and control (placebo) stages of the trial. Each stage lasted fourteen days, with a fourteen day gap (washout period) between administration of the metronidazole and the placebo. Swabs of the tumour were taken before and after each treatment stage, and tumour odour was assessed independently by the participant, a doctor and a nurse using an ordinal grading scale of 0 (no smell) to 3 (offensive and intolerable). All of the six participants that completed the trial stages had recurrent breast cancer and completed the trial with little change in tumour appearance, but had a reduction in malodour and anaerobic (in the absence of air) isolates (level of bacteria that grew in a laboratory test from a swab of the wound). Although it is not entirely clear where the research took place, the researchers, who were clinical oncologists, worked in a hospital in London at the time of the study. Details of study funding are also not provided in the study report; however, it is noted that the placebo was provided by a pharmaceutical company.
Excluded studies
Three studies were excluded as they were not RCTs or CCTs (Dankert 1981; De Castro 2015; Sparrow 1980); and one study was excluded as it evaluated treatment of surgical rather than malignant wounds (McGregor 1982). See Characteristics of excluded studies table.
Risk of bias in included studies
The included study had high risk of bias. See Figure 2, Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study was described as a cross‐over, double‐blind randomised trial (Ashford 1984). The authors did not report the method of allocation concealment or how the randomisation sequence was generated, and the trial was assessed as unclear risk of bias on allocation.
Blinding
The trial was described as double blind, whereby both participants and personnel were blind to the intervention and control through the use of ‘identical tablets’.
Incomplete outcome data
Three participants withdrew from the study. The reasons for withdrawal are provided by the authors in the study report: one participant died; a second required antibiotics for septicaemia; and a third responded unexpectedly to radiotherapy. This loss of three participants represents a loss of one‐third of the total study participants, with six only remaining for data analysis. For this reason, other potential sources of bias were assessed as high risk of bias.
Selective reporting
Trial registration was not available and because the outcomes of interest were not clearly specified, only inferred, we assessed the risk of bias for selective reporting as unclear.
Other potential sources of bias
The cross‐over design was considered suitable because of the difficulty of recruitment in this population. The wash‐out period of 14 days was deemed appropriate to eliminate the carry‐over effect and was not considered a potential source of bias in the study.
Data from the six patients who completed both arms of the trial were presented.
The analysis of the available data was appropriate given that smell scores were available for each patient in each arm of the trial.
Effects of interventions
The results are reported with reference to the review's pre‐specified outcome measures.
Metronidazole versus placebo
Primary Outcomes
Malodour
It is unclear if systemic antibiotics are associated with a difference in malodour (MD —2.16, 95% CI —3.60 to —0.72, 1 study, 6 participants, see (Figure 4)). Malodour (smell scores) was graded on a 0 to 3 scale with higher values indicating a more offensive and intolerable smell. The mean smell scores for the metronidazole group was 1.17 (SD 1.60) and the mean for the placebo group was 3.33 (SD 0.82). The results for all six participants from both phases of the cross‐over trial were included in the analysis, rather than the results from the first treatment phase only. We did this because only one study was included, the numbers of participants were small, and the washout period of 14 days was considered clinically sufficient to eliminate any potential effects from 'carry‐over'. The quality of the evidence (GRADE) was assessed as very low for this outcome (summary of findings Table for the main comparison).
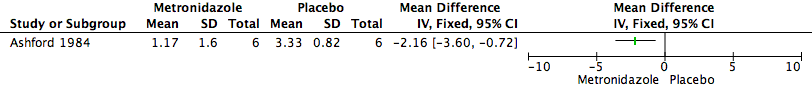
Forest plot of comparison: 1 Metronidazole versus Placebo, outcome: 1.1 Malodour (Smell Score).
Adverse effects
No adverse effects of the treatment were reported in either the intervention or control group by the trial authors.
Secondary Outcomes
The one included study did not report on any of the review's pre‐specified secondary outcomes of health‐related quality of life, pain, exudate/haemorrhage (number of dressing changes), cost, or malignant tumour containment or regression.
Discussion
Summary of main results
Overall, the results of this review highlight the lack of research in this area with very little evidence to direct clinical practice in the treatment of fungating malignant wounds with systemic antibiotics. We identified only one study that met the inclusion criteria for the review, and very few participants (six) were included in this study (Ashford 1984). Due to the very low quality evidence on the primary outcome of malodour, it is uncertain whether systematic metronidazole leads to a reduction in malodour when compared to the use of a placebo. No adverse outcomes of the treatment were reported in either of the groups by the trial authors.
Overall completeness and applicability of evidence
The evidence presented by the one included study is directly relevant to the review question as it addressed the use of systemic antibiotics in the treatment of malignant wounds. The study compared metronidazole with a placebo. Metronidazole is the antibiotic of choice for targeting the anaerobic bacteria commonly found in malignant wounds. The participants had malignant wounds associated with recurrent breast cancer, with this type of cancer being most commonly associated with the development of malignant wounds. Moreover, the study addressed the review's primary outcomes of malodour and adverse effects of the treatment; however, it did not assess any of the review's secondary outcomes. However, while the included study is relevant to answering the review question, the final small sample of six who completed both arms of the trial renders negligible the overall completeness and applicability of the findings of this review for informing clinical practice.
Quality of the evidence
The results of the review show a positive outcome for the use of systemic metronidazole in reducing odour in malignant wounds but the quality of the evidence is very low. We downgraded the evidence for serious limitations as a third of the participants withdrew from the study, resulting in attrition bias. Due to the small sample size, we further downgraded the evidence for very serious imprecision; the lack of research in this area has been highlighted. The body of evidence on the use of systemic antibiotics in the treatment of malignant wounds is currently limited and further high‐quality studies are required. We did not downgrade the evidence for inconsistency or publication bias due to insufficient data. We did not downgrade for indirectness as the evidence is in agreement with the review question.
Potential biases in the review process
A systematic review was undertaken with a predefined and peer‐reviewed research question, search terms, and criteria for inclusion of studies. A comprehensive search strategy was developed and undertaken, including searching of multiple databases. Furthermore, two independent review authors reviewed the results of the search and independently identified studies to be included and excluded. The decision to include both periods of the cross‐over trial may have introduced bias; however, given that the 14‐day washout period in Ashford 1984 was considered a clinically sufficient period of time for washout, this was not considered to be a source of concern. For these reasons, we believe potential biases in the review process are minimal.
Agreements and disagreements with other studies or reviews
A systematic review by De Castro 2015 investigated the effect of metronidazole on the management of odour in patients with fungating wounds, and three trials analysing topical metronidazole preparations met the criteria for inclusion. The authors concluded that these studies supported the use of metronidazole topically but were limited in terms of their methodology. Adderley 2014 additionally systematically reviewed the evidence on topical preparations for the treatment of malignant wounds. No current systematic reviews exist on the use of systemic antibiotics for the treatment of malignant wounds. This review, although providing limited evidence, adds to the overall body of evidence on treatment options in people with malignant wounds.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
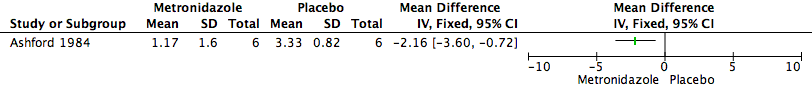
Forest plot of comparison: 1 Metronidazole versus Placebo, outcome: 1.1 Malodour (Smell Score).

Comparison 1 Metronidazole versus Placebo, Outcome 1 Malodour (Smell Score).
| Metronidazole compared to Placebo for treating malignant wounds | ||||||
| Patient or population: treating malignant wounds | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Placebo | Risk with Metronidazole | |||||
| Malodour (smell score measured on a scale of 0 to 3 with higher scores indicating a more offensive smell) | The mean malodour (smell score) was 3.33 (range 2.0 to 4.0) | MD 2.16 lower | ‐ | 6 | ⊕⊝⊝⊝ | It is uncertain whether metronidazole leads to a reduction in malodour because the quality of the evidence is very low |
| Adverse effects | Study population | not estimable | 6 | NA | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 3 levels: serious limitation — insufficient details provided to make clear judgements on random sequence generation and allocation concealment. There was also a 33% loss to follow‐up; very serious imprecision; a small sample size of 6 participants. 2 Smell was independently assessed at each visit by the patient, doctor, and nurse, who graded the smell as "absent" (0), "not offensive" (1), "offensive but tolerable" (2), or "offensive and intolerable" (3), and an amalgamated score calculated. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Malodour (Smell Score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

